Abstract
Docetaxel (Taxotere®) has been one of the most important chemotherapeutic drugs for cancer treatment since 1996. Although a large number of clinical studies have been conducted in various cancer fields, there is a discrepancy in the standard dose between Japan and Western countries. This article reviews the pharmacokinetic, pharmacodynamic and toxicological profiles of docetaxel, and explains why there exists an ethnic difference in dose, and further discusses which direction we should go forward to solve this problem. The original recommended dose was 100 mg/m2 every 3 weeks in US and European populations, while a Japanese phase I study suggested the recommended dose as 60 mg/m2 every 3 weeks. A prospective population pharmacokinetic analysis of docetaxel conducted in both the USA/Europe and Japan, indicated an absence of ethnic difference in the pharmacokinetics. Both analyses demonstrated that docetaxel clearance is related to α1-acid glycoprotein level, hepatic function, age and body surface area. The relationship was observed between increasing docetaxel dose and increased tumor response rates across the dose range of 60 to 100 mg/m2. The area under the serum concentration time curve (AUC) of docetaxel at the first cycle was significantly related to time to progression. Hematological toxicities were well correlated with the AUC of docetaxel, and severe hematological toxicities were more frequently observed in Japanese patients treated with 60 mg/m2, compared to the US/European patients treated with 75–100 mg/m2 dose. The Japanese population seems more susceptible to the toxicity of docetaxel. A docetaxel dose of 75 mg/m2 is now standard not only in global trials but also in recent Japanese trials. Although the optimal dose of docetaxel is still unclear, we need to continue to seek the appropriate dose of docetaxel depending on patient status and the goals of chemotherapy.
Keywords: Docetaxel, ethnic difference, pharmacodynamics, pharmacokinetics, toxicity
Background and Aims of This Article
Docetaxel, a more potent semisynthetic derivative of paclitaxel, derived from extracts of the leaves of the European yew tree (Taxus baccata), was discovered in the 1980s.1 Docetaxel (Taxotere®) was first approved for use by the US Food and Drug Administration in 1996 for locally advanced or metastatic breast cancer after failure of prior chemotherapy, with a dose of 60 to 100 mg/m2 administered intravenously over 1 hour every 3 weeks. Thereafter, additional indications were approved at a dose of 75 mg/m2. In contrast, docetaxel was approved in Japan in 1996 with the recommended dose of 60 mg/m2 every 3 weeks, based on a Japanese phase I study.2 Currently, docetaxel is widely used for treatment of breast, non-small cell lung, gastric, head and neck, ovary, esophageal, uterus body and prostate cancers, and the Japanese approved dose has been extended to 60 to 75 mg/m2. However, there is still discrepancy between Japan and Western countries in the standard doses for pretreated non-small cell lung cancer, with docetaxel typically administered at a dose of 60 mg/m2 every 3 weeks in both clinical practice and clinical trials of Japan.
Docetaxel is one of the most important chemotherapeutic drugs and a number of clinical studies have been conducted to extend its clinical applications. When global or multi-national collaborative studies used the dose of 100 mg/m2, Japan was not able to join these studies because the 100 mg/m2 dose exceeded the Japanese approved dose. Recently, some Japanese patients were treated at a dose of 75 mg/m2 when they participated in global clinical trials of docetaxel67. With this unique history of docetaxel dose discrepancy, Japanese oncologists still feel uncertain about the optimal dose selection.
Needless to say, it is important to determine the optimal dose of docetaxel for Japanese patients in routine clinical settings. However, the central question is why the Japanese standard dose of docetaxel differs from the Western dose. The present article focuses on reviewing pharmacokinetic (PK), pharmacodynamic (PD) and toxicological profiles of docetaxel, and discussing whether there is any evidence showing that a lower dose is more appropriate in the Japanese population. We also discuss which direction we should go forward to solve the current clinical problem.
Pharmacokinetics
Standard pharmacokinetics
Docetaxel PK parameters are summarized in Table1.2–8 In a phase I trial, the PK of docetaxel was linear, determined by 23 patients receiving 20–115 mg/m2. At high doses of docetaxel (85–115 mg/m2), a three-compartment model was found to provide a better fit than a two-compartment model with a terminal half-life of 13.5 ± 7.5 h (mean ± SD), a plasma clearance of 21.1 ± 5.3 L/h/m2 and a distribution volume of 72 ± 40 L/m2.3,9 A PK analysis suggested the presence of nonlinear pathways,10 whereas PK parameters remained linear up to 175 mg/m2 in a phase I and PK study of docetaxel administered with granulocyte colony-stimulating factor.11 Docetaxel exposure as measured by the area under the plasma concentration time curve (AUC) was the only significant predictor of severe toxicity during the first course of chemotherapy.12 It was also reported that docetaxel PK were similar for weekly and 3-weekly regimens.13
Table 1.
Pharmacokinetic features of docetaxel
| Authors | Patients | N | Dose range (mg/m2) | Cmax (μg/mL) | AUC (μg h/mL) | CL (L/h/m2) | Ethnicity |
|---|---|---|---|---|---|---|---|
| Extra et al.3 | Solid tumor (Phase 1) | 65 | 5–115 | 2.41† | 5.93† | NA | |
| Burris et al.4 | Solid tumor (Phase 1) | 58 | 5–115 | 5.9 ± 1.9† | 18.3 ± 5.4† | NA | |
| Taguchi et al.2 | Solid tumor (Phase 1) | 27 | 10–90 | 1.61 ± 0.59‡ | 2.44 ± 0.83‡ | Japanese | |
| Yamamoto et al.5 | NSCLC | 29 | 60 | 1.30―3.82 | 2.66 ± 0.91 | 24.5 ± 6.4 | Japanese |
| Rosing et al.6 | Solid tumor | 24 | 100 | 2.6 ± 0.5 | 3.1 ± 0.9 | 34.8 ± 9.3 | NA |
| ten Tije et al.7 | Solid tumor (<65 years) | 20 | 75 | 4.06 ± 1.38 | 5.69 ± 2.27 | 15.4 ± 6.94 | White/Black |
| Solid tumor (≥65 years) | 20 | 75 | 3.54 ± 1.58 | 6.01 ± 3.23 | 16.6 ± 10.0 | ||
| Minami et al.8 | Solid tumor | 69 | 60 | 1.588 | 2.68 | 29.4 L/h | Japanese |
Pharmacokinetic parameters obtained at 100 mg/m2 dose.
Pharmacokinetic parameters obtained at 60 mg/m2 dose. AUC, area under the curve; CL, total body clearance; Cmax, maximum plasma concentration; NA, not available; NSCLC, non-small cell lung cancer.
Large interindividual variability in docetaxel PK was reported despite intravenous infusion dosing (coefficient of variation 30–40%), and the variability was related to both toxicity and efficacy.5,14,15 Therefore, unique clinical trials were conducted to attempt to optimize drug exposure in individual patients. Engels et al. reported a randomized trial to evaluate the effect of PK-guided individualized dosing of docetaxel in cancer patients. The interindividual variability was decreased by 35% after 1 PK-guided course, and PK-guided dosing also decreased the interindividual variability of the percentage decrease in white blood cell and absolute neutrophil counts by 50%.16 Yamamoto et al. also performed a randomized trial to assess individualized doses of docetaxel calculated from the estimated clearance and the target AUC of 2.66 μg h/mL, and reported that the standard deviation of AUC was significantly smaller in the individualized dosing arm than in the body surface area-based dosing arm.17 Although individualized docetaxel dosing was feasible in both trials, they were not able to evaluate the efficacy of this dosing method due to the small sample size.
It should be noted that docetaxel was the first drug whose population PK were evaluated in a prospective manner from phase I through to phase III clinical trials. The population PK analysis performed in the Western countries found that docetaxel clearance was related to α1-acid glycoprotein (AAG) level, hepatic function, age and body surface area.18 The population PK analysis conducted in Japan analyzed data from 102 Japanese patients with solid tumors who participated into phase I and II clinical trials. Clearance was described by a similar equation to that in the Western population (Table2). The mean values and covariate effects of docetaxel clearance in Japanese patients were comparable to those obtained in the European and US population, suggesting no racial difference in the elimination of docetaxel (Fig.1).19
Table 2.
Equations predicting docetaxel clearance for European/American and Japanese populations
| Authors | Equations predicting docetaxel clearance | ωCL (%) |
|---|---|---|
| Bruno et al.18 | CL = BSA (22.1 − 3.55 AAG − 0.095 AGE + 0.225 ALB) (1 − 0.334 HEP) | 33 |
| Tanigawara et al.19 | CL = BSA (37.6 − 6.41 AAG − 0.191 AGE + 0.0436 ALB) (1 − 0.209 HEP) | 25 |
AAG, α1-acid glycoprotein level (g/L); ALB, albumin level (g/L); AGE (years); BSA, body surface area (m2); CL, total body clearance (L/h); HEP, complication of hepatic dysfunction indicated by HEP = 1 (presence) or HEP = 0 (absence).
Fig 1.
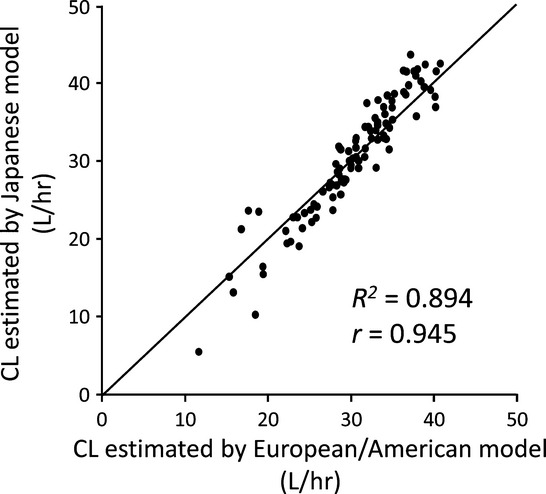
Comparison of clearance estimates predicted by European/US and Japanese population pharmacokinetic models of docetaxel. The model equations are described in Table2, and a correlation analysis is provided: y = 1.178x − 5.454 (R2 = 0.894, r = 0.945). CL, total body clearance (L/h). The solid line shows a unit line.
Metabolism and elimination
Docetaxel is metabolized by the hepatic cytochrome P450 (CYP) 3A isoforms CYP3A4 and CYP3A5, and the predominant route of elimination of parent drug and metabolites is via biliary and intestinal excretion.20,21 CYP3A4 activity was found to be the most significant independent variable for predicting the clearance of docetaxel.15 Docetaxel is excreted mainly in the feces as metabolites. A study of 14C-docetaxel suggested that 6% and 75% of the administered radioactivity is excreted in urine and feces (Taxotere® package insert).
Distribution
Lipoproteins, AAG and albumin were the main carriers of docetaxel in plasma, and owing to the high interindividual variability of AAG plasma concentration, it was reported that AAG should be the main determinant of docetaxel plasma binding variability.22
The fraction of unbound docetaxel was reported to range from 4% to 10% in the plasma of patients treated with docetaxel, and AAG was found to be correlated with fraction unbound and clearance of total docetaxel.23 In a PD analysis of docetaxel monotherapy, greater maximum concentration (Cmax) and AUC of unbound docetaxel, but not total docetaxel, in plasma were associated with greater risk of grade 4 neutropenia.8 These results may reveal that primarily unbound drugs are pharmacologically active because they can distribute into tissues or cells to bind their targets. Also in a clinical study evaluating unbound docetaxel PK, AUC of unbound docetaxel was better associated with severe neutropenia than AUC of total (unbound + bound) docetaxel.24
Drug interactions
Docetaxel is subject to extensive metabolic conversion by CYP3A isoenzymes, which results in several pharmacologically inactive oxidation products.21 Antiepileptic drugs, in particular phenytoin and phenobarbital, possess the potential to interact with docetaxel through induction of the hepatic metabolic activity, and can greatly reduce the potential antitumor effects of docetaxel.21 In contrast, potent inhibitors of CYP3A, such as ketoconazole, were reported to decrease docetaxel clearance (49% decrease in clearance of docetaxel).21,25
Concomitant treatment with doxorubicin was associated with decreased docetaxel clearance (20% decrease in clearance),26 probably because doxorubicin inhibited the CYP3A activity. In a PK and PD study for the combination of docetaxel and topotecan in patients with solid tumors, administration of topotecan on days 1–4 and docetaxel on day 4 resulted in an approximate 50% decrease in docetaxel clearance and was associated with increased neutropenia.27 In designing combination regimens of chemotherapeutic agents, attention should be paid to drug interactions.
Pharmacogenomics
ABCB1 (P-glycoprotein, multidrug resistance 1), ABCC2 (MRP2) and SLCO1B3 (OATP1B3, OATP8) are considered as transporters to carry docetaxel. ABCB1 plays important and crucial roles in intestinal absorption and biliary excretion.28,29 There was no statistically significant effect of genotype on the clearance of docetaxel for ABCB1 genetic variations.30,31 However, the homozygous allele T of C1236T polymorphism in the ABCB1 gene (ABCB1*8) was significantly correlated with a decreased docetaxel clearance (−25%; P = 0.0039).32 Single nucleotide polymorphism (SNP) in ABCB1 may influence toxicity more than the PK of docetaxel.
ABCC2 and SLCO1B3 have cooperative roles in the docetaxel transport process in the liver.33 A Japanese case-control association study indicated a significant association of both rs12762549 in ABCC2 (P = 0.00022) and rs11045585 in SLCO1B3 (P = 0.00017) with docetaxel-induced leukopenia/neutropenia.34
Regarding hepatic metabolism, influences of polymorphism in CYP3A4 and CYP3A5 on docetaxel PK were not demonstrated in several studies.31,35 One study reported that simultaneous presence of the CYP3A4*1B and CYP3A5*1A alleles was associated with a 64% increase in docetaxel clearance (P = 0.0015).36 In addition, rs7761731 of CYP39A1 was found to be the only SNP significantly associated (P = 0.049, OR = 9.0) with the incidence of grade 4 neutropenia among 28 SNP, which were associated with the AUC of docetaxel.37 The pharmacogenomics of docetaxel have not yet been elucidated in a large clinical study. A genome-wide association study is expected to find genomic variants which relate to the efficacy or toxicity of docetaxel.38,39
Special population: Hepatic dysfunction
In a population PK analysis, patients with concomitant elevations of transaminases (ALT or AST > 1.5 × ULN) and alkaline phosphatase (>2.5 × ULN) showed a 27% reduction in docetaxel clearance and are higher risk of toxicity.14 However, in an observational study of docetaxel for patients with metastatic breast cancer and liver dysfunction, PK results suggested that docetaxel at 25 mg/m2 for patients with more severe hepatic dysfunction (serum total bilirubin 1.5–3 × ULN, and ALT or ALT 2.5–5 × ULN) may be underdosed compared with docetaxel at 100 mg/m2 for patients with normal hepatic functions.40 Docetaxel unbound clearance was lower and more variable in patients with hepatic dysfunction compared to those without hepatic dysfunction.41 Compared to patients with normal liver function, patients with grade 2 and 3 elevations of transaminases at baseline in conjunction with elevation of alkaline phosphatase (grade ≥1) showed 22% and 38% lower clearances, respectively. Minami et al. proposed dose reduction by approximately 20% and 40% for patients with grade 2 and 3 elevations of transaminases at baseline in conjunction with elevation of alkaline phosphatase, respectively.42 Caution is warranted and appropriate dose reduction seems advisable for docetaxel when treating patients with liver dysfunction, although the correlation between serum drug exposure and toxicity needs further investigation.
Special population: Renal dysfunction
Preclinical PK studies show that hepatobiliary extraction is the major route of elimination, with similar metabolic pathways in all species.43 No apparent differences were seen in the plasma concentration time curves of docetaxel administered before or after dialysis.44 Because docetaxel is excreted mainly in the feces as metabolites, docetaxel PK may not be altered in patients with renal dysfunction.
Special population: Elderly
A population PK showed that age was one of factors affecting docetaxel clearance (6.7% decrease in mean clearance for a 71-year-old patient).18 In a prospective PK study of 75 mg/m2 docetaxel every 3 weeks for patients with solid tumors, mean docetaxel clearance was not altered in elderly patients (≥65 years) versus younger patients (<65 years). However, the percentage of patients experiencing grade 4 and febrile neutropenia was remarkably higher in elderly (63% and 16%, respectively) versus younger (30% and 0%, respectively) cohort.7
In two phase II studies separately conducted in elderly (≥75 years) and non-elderly (<75 years) using non-small cell lung cancer patients treated with three weekly administration of docetaxel and cisplatin every 4 weeks, there was no difference in the PK of docetaxel or cisplatin between the two groups with regard to clearance and volume of distribution. In the PD analysis, neutropenia was positively correlated with the AUC for docetaxel but not for cisplatin.45 These results suggest that there is no significant difference in the PK of docetaxel between elderly and non-elderly patients, and that docetaxel is more toxic for elderly patients. In a PK study of paclitaxel, clearance of the unbound drug, the pharmacologically active fraction, was significantly different between elderly (≥70 years) and younger (<70 years) patients.46 However, there is no data on the difference in clearance of unbound docetaxel between elderly and non-elderly patients.
Inter-ethnic difference
There are some reports discussing inter-ethnic difference for PK and toxicity of docetaxel. In a prospective study of 106 patients with non-hematologic malignancy, there was no difference in geometric mean docetaxel clearance between Black patients (40.3 L/h) and White patients (41.8 L/h, P = 0.6). There was also no difference between Black and White patients in the percentage decrease in absolute neutrophil count nor docetaxel PK parameters related to the genotypes of CYP3A4, CYP3A5 and ABCB1.47 By using published data of phase II and III clinical trials to investigate single agent docetaxel every 3 weeks, docetaxel-induced grade 3/4 neutropenia was frequently observed in Asian clinical studies compared to non-Asian studies (odds ratio 19.0). However, a major limitation of this study was not evaluating PK and serum AAG level.48 According to results of population PK studies, there may be no significant difference in docetaxel clearance between Japanese and White patients. (Fig.1).18,19 In PK analyses of docetaxel for Asian patients with breast cancer, including Chinese, Malays and Indians, no ethnic difference was observed.49 Therefore, there seems present an inter-ethnic difference in toxicities, but ethnic difference in the PK of docetaxel has not been demonstrated.
Pharmacodynamics
Clinical response
In phase III studies for breast cancer patients previously treated with anthracycline, docetaxel 100 mg/m2 produced significantly higher response rates than other regimens (response rate: 30–36%).50,51 In two randomized phase III studies, docetaxel 100 mg/m2 was significantly superior in overall survival.50,52
Regarding hormone-refractory prostate cancer, two large phase III trials demonstrated that docetaxel showed survival benefits.53,54 Until now, there has been no randomized phase III study to show efficacy of docetaxel monotherapy for Japanese patients with metastatic breast cancer and prostate cancer.
There was a phase III study of docetaxel monotherapy versus best supportive care in patients with non-small cell lung cancer, previously treated with platinum-based chemotherapy. Survival in patients treated with docetaxel 75 mg/m2 was significantly better than in those treated with best supportive care (P = 0.01).55 Table3 summarizes data on the efficacy of docetaxel monotherapy every 3 weeks for pretreated non-small cell lung cancer patients.55–72 Response rates varied from 2.7% to 17.9% for non-small cell lung cancer patients in second-line settings.
Table 3.
Efficacies and toxicities of docetaxel monotherapy (phase III for previously treated non-small cell lung cancer patients)
| Authors | Dose (mg/m2) | N | Gr3-4 ANC (%) | Gr4 ANC (%) | Gr3–4 WBC (%) | Gr4 WBC (%) | Gr3–4 FN (%) | ORR (%) | Median PFS (mo) | Median OS (mo) | Ethnicity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shepherd et al. 200055 | 75 | 55 | 67.3 | 1.8 | 5.5 | 7.5 | NA | ||||
| 100 | 49 | 85.7 | 22.4 | 6.3 | 5.9 | ||||||
| Fossella et al. 200056 | 75 | 121 | 54 | 8 | 6.7 | TTP 8.5w | 5.8 | NA | |||
| 100 | 121 | 77 | 12 | 10.8 | TTP 8.4w | 6.0 | |||||
| Hanna et al. 200457 | 75 | 276 | 40.2 | 12.7 | 8.8 | 2.9 | 7.9 | NA | |||
| Gridelli et al. 200458 | 75 | 110 | 18 | 11 | 10 | 3 | 5 | 2.7 | 7.3 | NA | |
| Schuette et al. 200559 | 75 | 103 | 20.6 | 27.5 | 2 | 12.6 | TTP 3.4 | 6.3 | NA | ||
| Camps et al. 200660 | 75 | 129 | 9.3 | 10.1 | 7.8 | 9.3 | TTP 2.7 | 6.6 | NA | ||
| Ramlau et al. 200668 | 75 | 415 | 60 | 36 | 41 | 11 | 3 | 5 | TTP 13w | 7.8 | White/Oriental/Black |
| Kim et al. 200861 | 75 | 733 | 58.2 | 10.1 | 7.6 | 2.7 | 8.0 | White/Asian/Black | |||
| Maruyama et al. 200862 | 60 | 239 | 73.6 | 39.3 | 7.1 | 12.8 | 2.0 | 14.0 | Japanese | ||
| Paz-Ares et al. 200863 | 75 | 416 | 37 | 2 | 6 | 12 | TTP 2.6 | 6.9 | Caucasian/Black/Asian/Hispanic | ||
| Takeda et al. 200964 | 60 | 65 | 85.9 | 64.1 | 25.0 | 6.8 | 2.1 | 10.1 | Japanese | ||
| Krzakowski et al. 201065 | 75 | 277 | 29.5 | 18.8 | 21.3 | 4.8 | 4.7 | 5.5 | 2.3 | 7.2 | NA |
| Lee et al. 201066 | 75 | 79 | 7.6 | 3.4 | 12.2 | Korean | |||||
| Herbst et al. 201067 | 75 | 697 | 24 | 11 | 6 | 10 | 4.2 | 10.0 | Caucasian/East Asian | ||
| Ramlau et al. 201269 | 75 | 457 | 21.1 | 4.2 | 8.9 | 4.1 | 10.4 | NA | |||
| Garassino et al. 201370 | 75 | 110 | 21 | 12 | 4 | 15.5 | 2.9 | 8.2 | White/Asian | ||
| Kawaguchi et al. 201471 | 60 | 151 | 80.0 | 64.0 | 15.3 | 17.9 | 3.2 | 12.2 | Japanese | ||
| Reck et al. 201472 | 75 | 659 | 29.9 | 21.2 | 2.4 | 0.6 | 4.7 | 3.3 | 2.7 | 9.1 | White/Asian/Black/Indian |
ANC, absolute neutrophil count; FN, febrile neutropenia; Gr, grade; NA, not available; mo, months; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression; w, weeks; WBC, white blood cell.
Exposure-Response relationships
In a phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer, 527 patients were randomly assigned to docetaxel 60, 75 or 100 mg/m2 intravenously every 3 weeks.51 A relationship between an increasing dose of docetaxel and increased tumor response was observed across the dose range of 60 to 100 mg/m2 (response rate: 22.1%, 23.3% and 36.0%, respectively) and toxicities were also related to increasing dose. However, there was no statistically significant relationship between the objective response rate and docetaxel exposure. In a population PK analysis, the AUC of docetaxel in the first cycles was significantly related to time to progression.14 A Japanese population PK study showed that the efficacy of docetaxel was not correlated with the AUC.19
A large scale population PK/PD analysis was successfully implemented during the clinical development of docetaxel.73 Cumulative dose and baseline AAG were significant independent prognostic factors for survival in patients with non-small cell lung cancer. In six phase II studies of docetaxel monotherapy at a dose of 100 mg/m2 for non-small cell lung cancer, baseline AAG was a significant predictor of response and survival.12 A population PK study also showed that AAG was a significant predictor of response.14 However, a predictive biomarker for the efficacy of docetaxel has not yet been established.
Toxicity
Toxicity profiles
The dose-limiting toxicity in phase I studies was neutropenia.2–4 Neutropenia, leukopenia, neurological toxic effects, diarrhea, alopecia, asthenia and nausea were common adverse effects of docetaxel monotherapy every 3 weeks. Toxicities of docetaxel monotherapy every 3 weeks for non-small cell lung cancer patients are shown in Table3.55–72 Despite Japanese patients being treated with lower 60 mg/m2 docetaxel, incidence of severe neutropenia and febrile neutropenia was higher in Japanese trials compared with patients treated with 75 mg/m2 docetaxel in Western country trials. In addition, it was reported that docetaxel-induced grade 3/4 neutropenia was more frequently observed in Asian clinical studies compared to non-Asian studies.48
In a phase III study of docetaxel versus best supportive care in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy, the docetaxel dose was reduced from 100 to 75 mg/m2 based on interim safety-data monitoring which identified a significantly higher toxic death rate in the chemotherapy arm of the study.55 Based on this study, the standard dose of docetaxel for patients with pretreated non-small cell lung cancer has been considered as 75 mg/m2 every 3 weeks. It is also reported that docetaxel at a dose of 100 mg/m2 showed severe toxicities in heavily pretreated breast cancer patients.74 Therefore, an optimal dose of docetaxel potentially depends on treatment line and types of cancer.
Exposure–toxicity relationship
Hematological toxicity of docetaxel correlated with the exposure to docetaxel.14 Based upon a population PK/PD model describing factors responsible for the neutropenia caused by docetaxel, serum AAG levels, level of chemotherapy pretreatment and treatment center were identified as significant covariates of neutropenia.75 In two randomized PK studies of docetaxel to compare PK-guided individual dosing and dosing based on body surface area, the PK-guided individual dosing decreased interindividual variability in hematological toxicity.16,17
Idiosyncratic toxicity
Popular idiosyncratic toxicities of docetaxel include fluid retention, nail changes, tearing and hypersensitivity reactions. Fluid retention was reported to occur in 53% of patients treated with docetaxel monotherapy at a dose of 75 or 100 mg/m2, and median time to onset was 85 days (95% CI 81–92 days). The cumulative dose of docetaxel was the most important predictor for fluid retention.14
In two studies to evaluate nail change in patients treated with docetaxel, 26–58% developed nail changes.76,77 These studies showed that the number of chemotherapy cycles and cumulative docetaxel doses were strongly associated with the development of nail changes.
Clinical Trial Simulations
Virtual computer simulations resembling clinical trials of docetaxel were performed to assess the benefit from dose intensification in patients with high AAG levels, using PK/PD and death/drop-out models describing time to progression.78–80 The simulated phase III trial showed slightly longer median survival in the 125 mg/m2 docetaxel group than in the 100 mg/m2 group, but the results did not show a useful benefit of increasing the dose. Clinical trial simulation was also applied to design the most advantageous combination regimen. Based on modeling techniques using clinical data, the PD interaction was simulated between capecitabine and docetaxel in metastatic breast cancer.79 Bruno et al. conducted simulations to determine how much of a decreased capecitabine dose would be non-inferior to the standard dose in a combination regimen with docetaxel for the second-line treatment of metastatic breast cancer.80
What is the Optimal Dose?
In a phase I study of docetaxel performed in the USA, neutropenia was the dose-limiting toxicity and the maximum tolerated dose for patients receiving two or fewer chemotherapy regimens was 100–115 mg/m2. This study concluded that the recommended doses for phase II trials were 100 mg/m2 for good-risk patients and 80 mg/m2 for poor-risk patients, respectively.4 A European phase I study of docetaxel also reported that the tolerated dose was 115 mg/m2 and the recommended dose for a phase II study was 100 mg/m2.3 In a Japanese phase I study of docetaxel, based on the observation of dose-limiting toxicities including leukopenia and neutropenia, the maximum tolerated dose was determined to be 70–90 mg/m2. The Japanese investigators concluded that a dosage regimen of 60 mg/m2 at 3–4-week intervals was appropriate for the early phase II clinical trial.2 This discrepancy in the Western and Japanese phase I trials created the important difference in the recommended dose of docetaxel between US/Europe and Japan. Prospective population PK analysis of docetaxel for Japanese patients showed similar clearance values to European and US populations, suggesting the absence of ethnic difference in the PK.18,19,73 Although hematological toxicity of docetaxel was reported to be well correlated with exposure to docetaxel, similar PK cannot explain the difference in the recommended dose of docetaxel between US/Europe and Japan.(14).However, based upon the toxicity profiles, the Japanese population seems more susceptible to the toxicity of docetaxel. This difference in toxicity profiles was possibly caused by unknown genetic factors, and differences in unbound docetaxel concentrations or baseline counts of white blood cells. However, mechanistic insights are not yet elucidated for different sensitivity to docetaxel toxicity between Japanese and Western populations.
Nowadays, a docetaxel dose of 75 mg/m2 is used in most of global clinical trials.53–56 The approved recommended doses of docetaxel in Asian countries except Japan (China, Korea, Taiwan and Singapore) are 100 mg/m2 for breast cancer and 75 mg/m2 for non-small lung cancer.66 A docetaxel study in Singapore using doses of 75 or 100 mg/m2 showed similar PK but relatively higher incidence of febrile neutropenia compared to the Western population.31 Recent Japanese clinical trials also used 75 mg/m2 dose for breast cancer and non-small cell lung cancer.81,82 With an increasing number of medical oncologists in Japan with experiences and skills in toxicity management and with the significant progress in supportive care, a docetaxel dose of 75 mg/m2 is likely to become a chemotherapy treatment option with curative intent in Japan.
Conclusion
Oncologists have used docetaxel for almost 20 years, and it is now one of the most important cytotoxic drugs for cancer treatment. A prospective population PK analysis of docetaxel indicated the absence of ethnic difference in PK. However, although so far most Japanese patients have been treated with 60 mg/m2 docetaxel, severe hematological toxicities are more frequently experienced in the Japanese compared to the US and European patients given 75–100 mg/m2 doses. Twenty years ago, there was a difference in the recommended dose of docetaxel for US/Europe and Japan. Today, with the growing experience of medical oncologists and the remarkable progress in supportive care, a 75 mg/m2 dose of docetaxel is a treatment option in Japan. We need to continue to work towards determining the optimal dose of docetaxel, taking into account individual patients' status and the goal of chemotherapy.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83:288–91. doi: 10.1093/jnci/83.4.288. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Furue H, Niitani H, et al. Phase I clinical trial of RP 56976 (docetaxel) a new anticancer drug. Gan To Kagaku Ryoho. 1994;21:1997–2005. [PubMed] [Google Scholar]
- Extra JM, Rousseau F, Bruno R, Clavel M, Le Bail N, Marty M. Phase I and pharmacokinetic study of Taxotere (RP 56976; NSC 628503) given as a short intravenous infusion. Cancer Res. 1993;53:1037–42. [PubMed] [Google Scholar]
- Burris H, Irvin R, Kuhn J, et al. Phase I clinical trial of taxotere administered as either a 2-hour or 6-hour intravenous infusion. J Clin Oncol. 1993;11:950–8. doi: 10.1200/JCO.1993.11.5.950. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tamura T, Kamiya Y, Sekine I, Kunitoh H, Saijo N. Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. J Clin Oncol. 2000;18:2301–8. doi: 10.1200/JCO.2000.18.11.2301. [DOI] [PubMed] [Google Scholar]
- Rosing H, Lustig V, van Warmerdam LJ, et al. Pharmacokinetics and metabolism of docetaxel administered as a 1-h intravenous infusion. Cancer Chemother Pharmacol. 2000;45:213–8. doi: 10.1007/s002800050032. [DOI] [PubMed] [Google Scholar]
- ten Tije AJ, Verweij J, Carducci MA, et al. Prospective evaluation of the pharmacokinetics and toxicity profile of docetaxel in the elderly. J Clin Oncol. 2005;23:1070–7. doi: 10.1200/JCO.2005.03.082. [DOI] [PubMed] [Google Scholar]
- Minami H, Kawada K, Sasaki Y, et al. Pharmacokinetics and pharmacodynamics of protein-unbound docetaxel in cancer patients. Cancer Sci. 2006;97:235–41. doi: 10.1111/j.1349-7006.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SJ, Rivory LP. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet. 1999;36:99–114. doi: 10.2165/00003088-199936020-00002. [DOI] [PubMed] [Google Scholar]
- McLeod HL, Kearns CM, Kuhn JG, Bruno R. Evaluation of the linearity of docetaxel pharmacokinetics. Cancer Chemother Pharmacol. 1998;42:155–9. doi: 10.1007/s002800050799. [DOI] [PubMed] [Google Scholar]
- Goncalves A, Viret F, Ciccolini J, et al. Phase I and pharmacokinetic study of escalating dose of docetaxel administered with granulocyte colony-stimulating factor support in adult advanced solid tumors. Clin Cancer Res. 2003;9:102–8. [PubMed] [Google Scholar]
- Bruno R, Olivares R, Berille J, et al. Alpha-1-acid glycoprotein as an independent predictor for treatment effects and a prognostic factor of survival in patients with non-small cell lung cancer treated with docetaxel. Clin Cancer Res. 2003;9:1077–82. [PubMed] [Google Scholar]
- Baker SD, Zhao M, Lee CK, et al. Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res. 2004;10:1976–83. doi: 10.1158/1078-0432.ccr-0842-03. [DOI] [PubMed] [Google Scholar]
- Bruno R, Hille D, Riva A, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–96. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- Hirth J, Watkins PB, Strawderman M, Schott A, Bruno R, Baker LH. The effect of an individual's cytochrome CYP3A4 activity on docetaxel clearance. Clin Cancer Res. 2000;6:1255–8. [PubMed] [Google Scholar]
- Engels FK, Loos WJ, van der Bol JM, et al. Therapeutic drug monitoring for the individualization of docetaxel dosing: a randomized pharmacokinetic study. Clin Cancer Res. 2011;17:353–62. doi: 10.1158/1078-0432.CCR-10-1636. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tamura T, Murakami H, et al. Randomized pharmacokinetic and pharmacodynamic study of docetaxel: dosing based on body-surface area compared with individualized dosing based on cytochrome P450 activity estimated using a urinary metabolite of exogenous cortisol. J Clin Oncol. 2005;23:1061–9. doi: 10.1200/JCO.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Bruno R, Vivier N, Vergniol JC, De Phillips SL, Montay G, Sheiner LB. A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm. 1996;24:153–72. doi: 10.1007/BF02353487. [DOI] [PubMed] [Google Scholar]
- Tanigawara Y, Sasaki Y, Ohtsu T, et al. Population pharmacokinetics of docetaxel in Japanese patients. ASCO Meeting Abstracts Proc Am Soc Clin Oncol. 1996;15:479a. (abstr 1518);, 1996. [Google Scholar]
- Shou M, Martinet M, Korzekwa KR, Krausz KW, Gonzalez FJ, Gelboin HV. Role of human cytochrome P450 3A4 and 3A5 in the metabolism of taxotere and its derivatives: enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics. 1998;8:391–401. doi: 10.1097/00008571-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel: recent developments. Clin Pharmacokinet. 2006;45:235–52. doi: 10.2165/00003088-200645030-00002. [DOI] [PubMed] [Google Scholar]
- Urien S, Barre J, Morin C, Paccaly A, Montay G, Tillement JP. Docetaxel serum protein binding with high affinity to alpha 1-acid glycoprotein. Invest New Drugs. 1996;14:147–51. doi: 10.1007/BF00210785. [DOI] [PubMed] [Google Scholar]
- Loos WJ, Baker SD, Verweij J, Boonstra JG, Sparreboom A. Clinical pharmacokinetics of unbound docetaxel: role of polysorbate 80 and serum proteins. Clin Pharmacol Ther. 2003;74:364–71. doi: 10.1016/S0009-9236(03)00222-4. [DOI] [PubMed] [Google Scholar]
- Baker SD, Li J, ten Tije AJ, et al. Relationship of systemic exposure to unbound docetaxel and neutropenia. Clin Pharmacol Ther. 2005;77:43–53. doi: 10.1016/j.clpt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Engels FK, Ten Tije AJ, Baker SD, et al. Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther. 2004;75:448–54. doi: 10.1016/j.clpt.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Rudek MA, Sparreboom A, Garrett-Mayer ES, et al. Factors affecting pharmacokinetic variability following doxorubicin and docetaxel-based therapy. Eur J Cancer. 2004;40:1170–8. doi: 10.1016/j.ejca.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Egorin MJ, Van Echo DA, et al. Pharmacokinetic and pharmacodynamic study of the combination of docetaxel and topotecan in patients with solid tumors. J Clin Oncol. 2000;18:3288–94. doi: 10.1200/JCO.2000.18.18.3288. [DOI] [PubMed] [Google Scholar]
- van Zuylen L, Verweij J, Nooter K, Brouwer E, Stoter G, Sparreboom A. Role of intestinal P-glycoprotein in the plasma and fecal disposition of docetaxel in humans. Clin Cancer Res. 2000;6:2598–603. [PubMed] [Google Scholar]
- van Zuylen L, Sparreboom A, van der Gaast A, et al. Disposition of docetaxel in the presence of P-glycoprotein inhibition by intravenous administration of R101933. Eur J Cancer. 2002;38:1090–9. doi: 10.1016/s0959-8049(02)00035-7. [DOI] [PubMed] [Google Scholar]
- Sissung TM, Baum CE, Deeken J, et al. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res. 2008;14:4543–9. doi: 10.1158/1078-0432.CCR-07-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh BC, Lee SC, Wang LZ, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol. 2002;20:3683–90. doi: 10.1200/JCO.2002.01.025. [DOI] [PubMed] [Google Scholar]
- Bosch TM, Huitema AD, Doodeman VD, et al. Pharmacogenetic screening of CYP3A and ABCB1 in relation to population pharmacokinetics of docetaxel. Clin Cancer Res. 2006;12:5786–93. doi: 10.1158/1078-0432.CCR-05-2649. [DOI] [PubMed] [Google Scholar]
- Cui Y, Konig J, Keppler D. Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol Pharmacol. 2001;60:934–43. doi: 10.1124/mol.60.5.934. [DOI] [PubMed] [Google Scholar]
- Kiyotani K, Mushiroda T, Kubo M, Zembutsu H, Sugiyama Y, Nakamura Y. Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci. 2008;99:967–72. doi: 10.1111/j.1349-7006.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran A, Jullien V, Alexandre J, et al. Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther. 2006;79:570–80. doi: 10.1016/j.clpt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Baker SD, Verweij J, Cusatis GA, et al. Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther. 2009;85:155–63. doi: 10.1038/clpt.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T, Kanno H, Ishitani K, et al. An SNP in CYP39A1 is associated with severe neutropenia induced by docetaxel. Cancer Chemother Pharmacol. 2012;69:1617–24. doi: 10.1007/s00280-012-1872-4. [DOI] [PubMed] [Google Scholar]
- Low SK, Chung S, Takahashi A, et al. Genome-wide association study of chemotherapeutic agent-induced severe neutropenia/leucopenia for patients in Biobank Japan. Cancer Sci. 2013;104:1074–82. doi: 10.1111/cas.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu N, Schaid DJ, Abo RP, et al. Genetic association with overall survival of taxane-treated lung cancer patients – a genome-wide association study in human lymphoblastoid cell lines followed by a clinical association study. BMC Cancer. 2012;12:422. doi: 10.1186/1471-2407-12-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann K, Michaud LB, Rivera E, et al. Pilot study to assess toxicity and pharmacokinetics of docetaxel in patients with metastatic breast cancer and impaired liver function secondary to hepatic metastases. J Oncol Pharm Pract. 2014;20:120–9. doi: 10.1177/1078155213480536. [DOI] [PubMed] [Google Scholar]
- Hooker AC, Ten Tije AJ, Carducci MA, et al. Population pharmacokinetic model for docetaxel in patients with varying degrees of liver function: incorporating cytochrome P4503A activity measurements. Clin Pharmacol Ther. 2008;84:111–8. doi: 10.1038/sj.clpt.6100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami H, Kawada K, Sasaki Y, et al. Population pharmacokinetics of docetaxel in patients with hepatic dysfunction treated in an oncology practice. Cancer Sci. 2009;100:144–9. doi: 10.1111/j.1349-7006.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R, Sanderink GJ. Pharmacokinetics and metabolism of Taxotere (docetaxel) Cancer Surv. 1993;17:305–13. [PubMed] [Google Scholar]
- Mencoboni M, Olivieri R, Vannozzi MO, Schettini G, Viazzi F, Ghio R. Docetaxel pharmacokinetics with pre- and post-dialysis administration in a hemodyalized patient. Chemotherapy. 2006;52:147–50. doi: 10.1159/000092903. [DOI] [PubMed] [Google Scholar]
- Minami H, Ohe Y, Niho S, et al. Comparison of pharmacokinetics and pharmacodynamics of docetaxel and Cisplatin in elderly and non-elderly patients: why is toxicity increased in elderly patients? J Clin Oncol. 2004;22:2901–8. doi: 10.1200/JCO.2004.10.163. [DOI] [PubMed] [Google Scholar]
- Smorenburg CH, ten Tije AJ, Verweij J, et al. Altered clearance of unbound paclitaxel in elderly patients with metastatic breast cancer. Eur J Cancer. 2003;39:196–202. doi: 10.1016/s0959-8049(02)00611-1. [DOI] [PubMed] [Google Scholar]
- Lewis LD, Miller AA, Rosner GL, et al. A comparison of the pharmacokinetics and pharmacodynamics of docetaxel between African-American and Caucasian cancer patients: CALGB 9871. Clin Cancer Res. 2007;13:3302–11. doi: 10.1158/1078-0432.CCR-06-2345. [DOI] [PubMed] [Google Scholar]
- Yano R, Konno A, Watanabe K, et al. Pharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trials. Int J Clin Oncol. 2013;18:96–104. doi: 10.1007/s10147-011-0349-5. [DOI] [PubMed] [Google Scholar]
- Hor SY, Lee SC, Wong CI, et al. PXR, CAR and HNF4alpha genotypes and their association with pharmacokinetics and pharmacodynamics of docetaxel and doxorubicin in Asian patients. Pharmacogenomics J. 2008;8:139–46. doi: 10.1038/sj.tpj.6500478. [DOI] [PubMed] [Google Scholar]
- Nabholtz JM, Senn HJ, Bezwoda WR, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. J Clin Oncol. 1999;17:1413–24. doi: 10.1200/JCO.1999.17.5.1413. [DOI] [PubMed] [Google Scholar]
- Harvey V, Mouridsen H, Semiglazov V, et al. Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol. 2006;24:4963–70. doi: 10.1200/JCO.2005.05.0294. [DOI] [PubMed] [Google Scholar]
- Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–51. doi: 10.1200/JCO.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- Gridelli C, Gallo C, Di Maio M, et al. A randomised clinical trial of two docetaxel regimens (weekly vs 3 week) in the second-line treatment of non-small-cell lung cancer. The DISTAL 01 study. Br J Cancer. 2004;91:1996–2004. doi: 10.1038/sj.bjc.6602241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuette W, Nagel S, Blankenburg T, et al. Phase III study of second-line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3-weekly docetaxel. J Clin Oncol. 2005;23:8389–95. doi: 10.1200/JCO.2005.02.3739. [DOI] [PubMed] [Google Scholar]
- Camps C, Massuti B, Jimenez A, et al. Randomized phase III study of 3-weekly versus weekly docetaxel in pretreated advanced non-small-cell lung cancer: a Spanish Lung Cancer Group trial. Ann Oncol. 2006;17:467–72. doi: 10.1093/annonc/mdj115. [DOI] [PubMed] [Google Scholar]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008;26:4244–52. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L, Ross H, O'Brien M, et al. Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. Br J Cancer. 2008;98:1608–13. doi: 10.1038/sj.bjc.6604372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Negoro S, Tamura T, et al. Phase III trial of docetaxel plus gemcitabine versus docetaxel in second-line treatment for non-small-cell lung cancer: results of a Japan Clinical Oncology Group trial (JCOG0104) Ann Oncol. 2009;20:835–41. doi: 10.1093/annonc/mdn705. [DOI] [PubMed] [Google Scholar]
- Krzakowski M, Ramlau R, Jassem J, et al. Phase III trial comparing vinflunine with docetaxel in second-line advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy. J Clin Oncol. 2010;28:2167–73. doi: 10.1200/JCO.2009.23.4146. [DOI] [PubMed] [Google Scholar]
- Lee DH, Park K, Kim JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16:1307–14. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–26. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlau R, Gervais R, Krzakowski M, et al. Phase III study comparing oral topotecan to intravenous docetaxel in patients with pretreated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:2800–7. doi: 10.1200/JCO.2005.03.6491. [DOI] [PubMed] [Google Scholar]
- Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol. 2012;30:3640–7. doi: 10.1200/JCO.2012.42.6932. [DOI] [PubMed] [Google Scholar]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14:981–8. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: docetaxel and Erlotinib Lung Cancer Trial (DELTA) J Clin Oncol. 2014;32:1902–8. doi: 10.1200/JCO.2013.52.4694. [DOI] [PubMed] [Google Scholar]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–55. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- Bruno R, Vivier N, Veyrat-Follet C, Montay G, Rhodes GR. Population pharmacokinetics and pharmacokinetic-pharmacodynamic relationships for docetaxel. Invest New Drugs. 2001;19:163–9. doi: 10.1023/a:1010687017717. [DOI] [PubMed] [Google Scholar]
- Salminen E, Bergman M, Huhtala S, Ekholm E. Docetaxel: standard recommended dose of 100 mg/m(2) is effective but not feasible for some metastatic breast cancer patients heavily pretreated with chemotherapy-A phase II single-center study. J Clin Oncol. 1999;17:1127. doi: 10.1200/JCO.1999.17.4.1127. [DOI] [PubMed] [Google Scholar]
- Puisset F, Alexandre J, Treluyer JM, et al. Clinical pharmacodynamic factors in docetaxel toxicity. Br J Cancer. 2007;97:290–6. doi: 10.1038/sj.bjc.6603872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther D, Saunte DM, Knap M, Haahr V, Jensen AB. Nail changes due to docetaxel – a neglected side effect and nuisance for the patient. Support Care Cancer. 2007;15:1191–7. doi: 10.1007/s00520-007-0232-0. [DOI] [PubMed] [Google Scholar]
- Hong J, Park SH, Choi SJ, et al. Nail toxicity after treatment with docetaxel: a prospective analysis in patients with advanced non-small cell lung cancer. Jpn J Clin Oncol. 2007;37:424–8. doi: 10.1093/jjco/hym042. [DOI] [PubMed] [Google Scholar]
- Veyrat-Follet C, Bruno R, Olivares R, Rhodes GR, Chaikin P. Clinical trial simulation of docetaxel in patients with cancer as a tool for dosage optimization. Clin Pharmacol Ther. 2000;68:677–87. doi: 10.1067/mcp.2000.111948. [DOI] [PubMed] [Google Scholar]
- Frances N, Claret L, Bruno R, Iliadis A. Tumor growth modeling from clinical trials reveals synergistic anticancer effect of the capecitabine and docetaxel combination in metastatic breast cancer. Cancer Chemother Pharmacol. 2011;68:1413–9. doi: 10.1007/s00280-011-1628-6. [DOI] [PubMed] [Google Scholar]
- Bruno R, Lindbom L, Schaedeli Stark F, et al. Simulations to assess phase ii noninferiority trials of different doses of capecitabine in combination with docetaxel for metastatic breast cancer. CPT Pharmacometrics Syst Pharmacol. 2012;1:e19. doi: 10.1038/psp.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Ando M, Masuda N, et al. Randomized phase II study of primary systemic chemotherapy and trastuzumab for operable HER2 positive breast cancer. Clin Breast Cancer. 2012;12:49–56. doi: 10.1016/j.clbc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Yamada K, Takeoka H, Mizoguchi Y, et al. Feasibility re-evaluation of 75 mg/m(2) docetaxel in Japanese patients with previously treated non-small cell lung cancer. Jpn J Clin Oncol. 2014;44:338–45. doi: 10.1093/jjco/hyt236. [DOI] [PubMed] [Google Scholar]


