Abstract
Our previous studies demonstrated that specific inhibition of the BIG3-PHB2 complex, which is a critical modulator in estrogen (E2) signaling, using ERAP, a dominant negative peptide inhibitor, leads to suppression of E2-dependent estrogen receptor (ER) alpha activation through the reactivation of the tumor suppressive activity of PHB2. Here, we report that ERAP has significant suppressive effects against synergistic activation caused by the crosstalk between E2 and growth factors associated with intrinsic or acquired resistance to anti-estrogen tamoxifen in breast cancer cells. Intrinsic PHB2 released from BIG3 by ERAP effectively disrupted each interaction of membrane-associated ERα and insulin-like growth factor 1 receptor beta (IGF-1Rβ), EGFR, PI3K or human epidermal growth factor 2 (HER2) in the presence of E2 and the growth factors IGF or EGF, followed by inhibited the activation of IGF-1Rβ, EGFR or HER2, and reduced Akt, MAPK and ERα phosphorylation levels, resulting in significant suppression of proliferation of ERα-positive breast cancer cells in vitro and in vivo. More importantly, combined treatment with ERAP and tamoxifen led to a synergistic suppression of signaling that was activated by crosstalk between E2 and growth factors or HER2 amplification. Taken together, our findings suggest that the specific inhibition of BIG3-PHB2 is a novel potential therapeutic approach for the treatment of tamoxifen-resistant breast cancers activated by the crosstalk between E2 and growth factor signaling, especially in premenopausal women.
Keywords: Crosstalk, estrogen, growth factor, PHB2, tamoxifen-resistance
Activation of estrogen-receptor-α (ERα) by estrogen (E2) plays a pivotal role in the development and progression of breast cancer.1 The biological actions of the E2–ERα complex are mediated by a genomic pathway acting directly as a transcription factor in the nucleus and a non-genomic pathway interacting with adjacent growth factor receptors, such as insulin-like growth factor 1 receptor (IGF-1R) and epidermal growth factor receptor (EGFR).2–4 Accumulating evidence suggests a mechanistic crosstalk between the membrane-associated ERα and IGF-1R or EGFR signaling pathways whereby ERα forms a ternary complex of IGF-1R or EGFR, and Shc and activates its downstream signaling pathway, such as the MAPK and Akt cascade.4–8 An interdependence between E2 and growth factor responses was confirmed in breast cancer cells in which the knockdown of ERα using small interfering RNA or inhibition by anti-estrogens prevented E2 and EGF stimulation of DNA synthesis.9 Therefore, ERα is required for growth factor-mediated biological function, which suggests an important therapeutic target for breast cancer.
Current endocrine therapies for breast cancer are primarily based on targeting the ERα signaling pathways using anti-estrogens, such as tamoxifen, or aromatase inhibitors that prevent E2 synthesis.10,11 However, up to 50% of patients with ERα-positive tumors either initially do not respond or become resistant to these drugs.12,13 Endocrine-resistance is a major clinical problem and leading cause of treatment failure and mortality.14,15 Mounting evidence suggests that the crosstalk between membrane-associated ERα and growth factor receptor pathways is one mechanism of the development of endocrine resistance.16 Notably, increased ERα localization to the cytoplasm/membrane correlated with increased cytoplasmic signaling in endocrine-resistant MCF-7 cells.17 Endocrine resistance in many tumors is associated with an overexpression or hyperactivation of proteins involved in ERα and IGF-1R/EGFR signaling.18,19 Therefore, one logical therapeutic approach in endocrine-resistant breast cancer is the combination of target therapies to block both ERα and growth factor signaling.
We previously reported that the oncoprotein brefeldin A inhibited the guanine nucleotide-exchange protein 3 (BIG3) and tumor suppressor prohibitin 2 (PHB2) complex, which plays a critical role in E2 signaling modulation in ERα-positive breast cancer, and resulted in constitutive ERα activation.20,21 We further demonstrated that a dominant-negative peptide, ERAP, specifically disrupted the BIG3-PHB2 interaction, and released the PHB2 tumor suppressive activity to inhibit both nuclear-associated and membrane-associated ERα activation. Intrinsic PHB2 binding to ERα completely inhibited ERα-IGF-1Rβ and/or ERα-PI3K interactions and ERα phosphorylation at multiple sites in the presence of E2 in ERα-positive breast cancer cells, which may have suppressed acquired tamoxifen resistance in E2-dependent ERα-positive breast cancer cells.21 However, the antitumor effects of ERAP on endocrine resistance that are associated with crosstalk between E2 and growth factor ligands and ERα and human epidermal growth factor 2 (HER2) signaling, which have been observed in primary breast cancers, are unclear.
Here, we report that ERAP has significant antitumor effects against the synergistic activation of crosstalk between E2 and several growth factor ligands and/or HER2 signaling in breast cancer cells.
Materials and Methods
Materials
A dominant-negative peptide (ERAP; 11R-GGG-QMLSDLTLQLRQR) designed to specifically inhibit BIG3-PHB2 interaction was synthesized as previously described.21 Tamoxifen, IGF-1 and EGF were purchased from Sigma (St. Louis, MO, USA), Cell Signaling Technology (Danvers, MA, USA) and R&D Systems (Minneapolis, MN, USA), respectively. All chemicals were of analytical grade.
Cell lines and culture conditions
Human breast cancer cell lines (MCF-7 and BT-474) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The KPL-3C cells were kindly provided by Dr Jun-ichi Kurebayashi (Kawasaki Medical School, Kurashiki, Japan).22 All of the cell lines were cultured according to previously described methods.22 For the experiments using ERAP, the cells were incubated for 24 h and treated with 10 nM 17-estradiol (E2, Sigma) ± peptides (e.g. ERAP) as previously described.22
Antibodies and immunoblot analyses
SDS-PAGE and immunoblot analyses were performed as described previously,21 with antibodies against the following proteins: PHB2 (1:1000), ERα (phospho Y537; 1:500), ErbB2/HER2 and phosphor-HER2 (pY877) (Abcam, Cambridge, UK); ERα (SP-1, 1:500; Thermo Fisher Scientific, Fremont, CA, USA); Akt, phospho-Akt (S473) (587F11, 1:1000), p44/42 MAPK, phospho-p44/42 MAPK (T202/Y204) (1:1,000), Shc (1:500), phospho-Shc (Tyr239/240) (1H12, 1:500), phospho-ERα (S104/S106; 1:500), IGF-1Rβ (1:500), phospho-IGF-1Rβ (Y1135/1136) (19H7, 1:500), EGFR (1:1000) and phospho-EGFR (Tyr1068) (1:500) (Cell Signaling Technology); PI3-kinase p85α (U13; 1:500) and phospho-ERα (S118; 1:500) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); phospho-ERα (S167) (1:500) and phospho-ERα (S305) (1:500) (Merck Millipore, Billerica, MA, USA); phosphotyrosine (1:500) (Life Technologies, Rockville, MD, USA); and β-actin (AC-15; 1:5000) (Sigma). All of the experiments were performed in triplicate at minimum.
Immunoprecipitation
Immunoprecipitation was performed with 5 μg of antibodies against ERα, IGF-1Rβ, EGFR and HER2 as described previously.21 Subsequent SDS-PAGE and immunoblot analyses were performed as described above.
Luciferase reporter assay
Transfections of an ERE-luciferase reporter into MCF-7 cells were performed using an ERE reporter assay kit (Qiagen, Hilden, Germany) as previously described.21 Briefly, the culture medium was changed to assay medium (Opti-MEM, 10% FBS, 0.1 mM NEAA, 1 mM sodium pyruvate and 10 μg/mL insulin) 16 h post-transfection. The cells were incubated for 8 h and exposed to E2 and/or IGF-1 or EGF in the presence of 10 μM ERAP for 24 h. Cells were harvested and analyzed for luciferase and Renilla-luciferase activities using the Promega dual luciferase reporter assay (Promega KK, Tokyo, Japan) as previously described.21
Cell proliferation assay
Cell proliferation assays were performed using the Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) as previously described.21 The data represent the mean ± SD of three independent experiments.
Cell cycle assay
The cell cycle assay was performed by flow cytometry using a FACSCalibur with CellQuest software (BD, Franklin Lakes, NJ, USA) as described previously.21
In vivo tumor growth inhibition
We established an orthotropic xenograft tumor model in nude mice using the KPL-3C cells as previously described.21 Tumors developed after a few days and reached sizes of approximately 100 mm3 [calculated as 1/2 × (width × length2)]. The mice were randomized into 13 treatment groups (five animals per group): (1) no treatment; (2) 6 μg E2 per day; (3) E2 + 14 mg/kg/day ERAP; (4) E2 + 4 mg/kg/day tamoxifen; (5) E2 + ERAP + tamoxifen; (6) 10 μg/day IGF-1; (7) IGF-1 + ERAP; (8) IGF-1 + tamoxifen; (9) IGF-1 + ERAP + tamoxifen; (10) E2 + IGF-1; (11) E2 + IGF-1 + ERAP; (12) E2 + IGF-1 + tamoxifen; and (13) E2 + IGF-1 + ERAP + tamoxifen. E2 was delivered via the application of a solution to the neck skin. The other treatments were delivered via i.p. injection. Tumor volume was measured with calipers for 21 days, after which time the animals were killed, and the tumors were excised. All of the experiments were performed in accordance with the guidelines of the animal facility at the University of Tokushima.
Immunohistochemical staining of xenografts
We stained 3-μm sections of paraffin-embedded tumors with an anti-Ki-67 antibody (MIB-1, 1:50; Dako Japan, Tokyo, Japan) to examine Ki-67 expression in KPL-3C xenograft tumors.
TUNEL assay
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assays were performed on paraffin sections from KPL-3C xenograft tumors using an in situ apoptosis detection kit (Takara, Shiga, Japan) as described previously.23
Statistical analyses
Student's t-test was used to determine the significance of differences between the experimental groups. Values of P < 0.05 were considered significant.
Results
ERAP suppresses genomic and non-genomic estrogen receptor alpha signaling that is activated by crosstalk between E2 and insulin-like growth factor 1
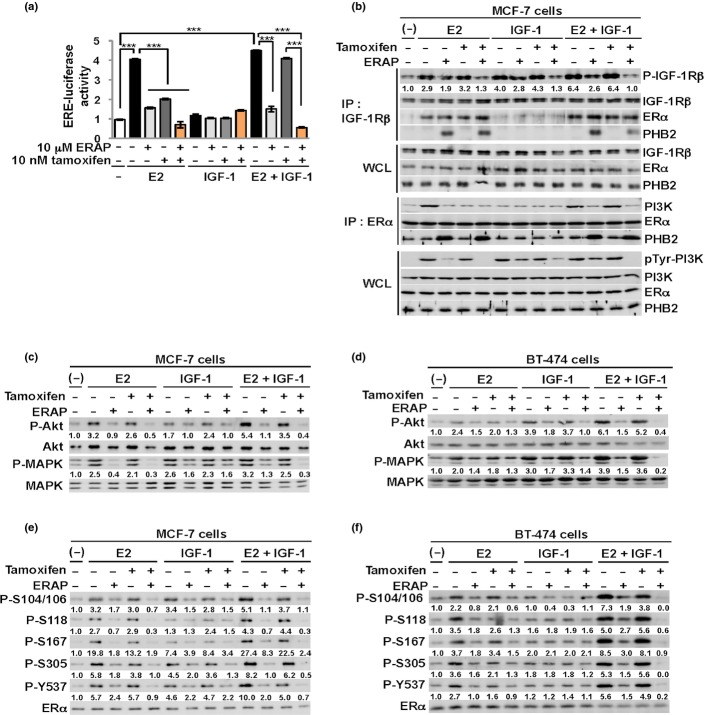
We first investigated whether the dominant-negative peptide ERAP suppressed E2-dependent ERα transcriptional activity in the presence of the growth factor stimulator IGF-1 in breast cancer cells using a luciferase assay with an ERE reporter. The results showed that IGF-1 stimulation enhanced ERα transcriptional activity in the presence but not the absence of E2 in MCF-7 cells (Fig.1a), indicating that IGF-1 enhances E2-dependent ERα genomic action in cancer cells. Notably, ERAP treatment but not 10 nM tamoxifen significantly reduced the E2-dependent ERα transcriptional activity of MCF-7 cells after stimulation with IGF-1 (Fig.1a). Treatment with a combination of ERAP and tamoxifen significantly suppressed the crosstalk induced by E2 and IGF-1 compared with ERAP or tamoxifen alone (Fig.1a). These results suggest that treatment with a combination of ERAP and tamoxifen may additively suppress the ERα transcriptional activity stimulated by the crosstalk between E2 and IGF-1 in breast cancer cells.
Fig 1.
ERAP suppresses non-genomic signaling by the crosstalk between E2 and insulin-like growth factor 1 (IGF-1). (a) Luciferase assays showing the inhibitory effect of ERAP on the estrogen receptor alpha (ERα) transcriptional activity of MCF-7 cells by 10 nM E2 and 50 ng/mL IGF-1. These data represent the mean ± SD of three independent experiments (***P < 0.001). (b) The inhibitory effects of ERAP on the interactions of ERα with IGF-1Rβ and PI3K in the presence of E2 and IGF-1. (c–f) The inhibitory effects of ERAP on Akt (c, d), MAPK (c, d), and multiple ERα phosphorylations (e, f) induced by E2 and IGF-1 stimulation in MCF-7 (c, e) and BT-474 (d, f) cells. The data are expressed as the fold increase over untreated cells at 0 h. The above all blot were cropped, and the full-length blots are included in Supplementary Figure S4.
We next investigated the effect of ERAP on the formation of ERα and IGF-1Rβ or PI3K complexes in the presence of E2 and IGF-1 stimulation in MCF-7 cells. E2 and IGF-1 stimulation markedly enhanced IGF-1Rβ tyrosine phosphorylation and the interaction of ERα and IGF-1Rβ, which was similar to E2 stimulation alone (Fig.1b, IGF-1Rβ: IP). In contrast, stimulation of IGF-1 alone enhanced the tyrosine phosphorylation levels of both IGF-1Rβ and PI3K but did not enhance ERα and IGF-1Rβ or PI3K interactions. Notably, ERAP treatment stimulated the formation of a new complex consisting of IGF-1Rβ, ERα and PHB2 released from BIG3, which suppressed IGF-1Rβ and PI3K tyrosine phosphorylation levels (Fig.1b, IGF-1Rβ, ERα: IP) in the presence of E2 and IGF-1 in MCF-7 cells, respectively. An inhibitory effect of ERAP on IGF-1Rβ tyrosine phosphorylation was observed in the presence of E2 and IGF-1 in KPL-3C cells, another ERα-positive breast cancer cell line (Suppl. Fig. S1). ERAP treatment also interfered with the E2-induced and IGF-1-induced interaction of ERα and PI3K in MCF-7 cells (Fig.1b, ERα:IP).
We then examined the effects of ERAP on the phosphorylation status of Akt and MAPK, which are the downstream signaling molecules of IGF-1Rβ and PI3K, respectively, in MCF-7 and BT474 cells, which strongly express IGF-1Rβ (Suppl. Fig. S2). Crosstalk stimulation of E2 and IGF-1 clearly enhanced Akt (S473) and p42/44 MAPK (T202/Y204) phosphorylation levels compared with E2 or IGF-1 alone, whereas treatment with ERAP but not tamoxifen completely suppressed both ligand-induced phosphorylation status in breast cancer cells (Fig.1c,d).
We also demonstrated that ERAP but not tamoxifen treatment abrogated ERα phosphorylation levels at five sites (S104/S106, S118, S167, S305 and Y537) associated with ERα transcriptional activity, DNA-binding, co-activator binding, protein stability and cell proliferation in ERα-positive breast cancer cells after the combined stimulation of E2 and IGF-1 in MCF-7 and BT474 cells, respectively (Fig.1e,f).24–31 Notably, combination treatment with ERAP and tamoxifen completely suppressed ERα phosphorylation levels at all five sites in MCF-7 and BT474 cells (Fig.1e,f). Collectively, these results strongly suggest that ERAP interfered with E2-induced and IGF-1-induced signal pathways, and the combination ERAP and tamoxifen treatment caused an additive inhibitory effect of E2 and IGF-1-induced signaling pathways in breast cancer cells.
ERAP inhibits the estrogen receptor alpha-positive breast cancer cell growth activated by the crosstalk between E2 and insulin-like growth factor 1
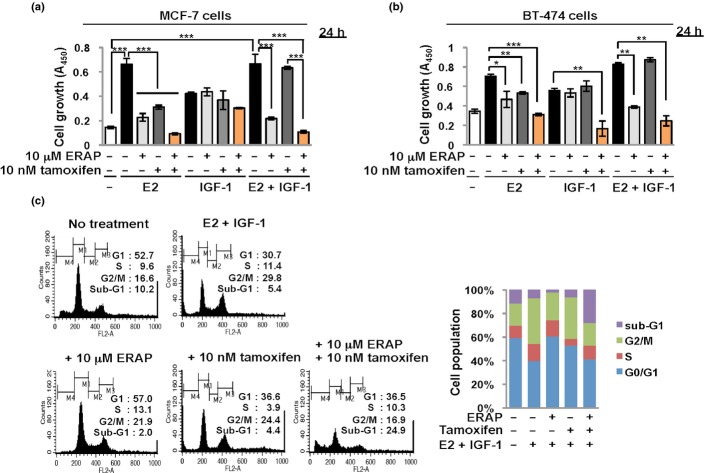
We next elucidated the inhibitory effect of ERAP on E2-dependent cell proliferation in the presence of IGF-1 stimulation in MCF-7 and BT474 cells using the MTT assay. IGF-1 stimulation significantly enhanced proliferation in the presence of E2 and in the absence of E2 in both cancer cell lines (Fig.2a,b). Figure1a shows that treatment with IGF-1 alone did not enhance ERα transcriptional activity in MCF-7 cells. These findings suggest that IGF-1 stimulation enhances an E2-independent proliferation of ERα-positive breast cancer cells. Notably, ERAP treatment but not 10 nM tamoxifen treatment significantly reduced the growth of MCF-7 cells after E2 and IGF-1 stimulation (Fig.2a,b). In contrast, ERAP treatment had no effect on IGF-1-induced cell growth in the absence of E2 (Fig.2a,b), indicating that ERAP suppressed E2-dependent IGF-1 signaling in breast cancer cells. Furthermore, treatment with a combination of ERAP and tamoxifen synergistically suppressed the cell growth activated by the crosstalk between E2 and IGF-1 compared with ERAP or tamoxifen alone (Fig.2a,b). These results suggest that ERAP suppressed endocrine-resistant breast cancer, which is associated with the crosstalk between the E2 and IGF-1 signaling pathways.
Fig 2.
ERAP suppresses the cell growth induced by the crosstalk between E2 and insulin-like growth factor 1 (IGF-1). (a, b) MTT assays evaluating the inhibitory effect of ERAP on the growth of MCF-7 (a) and BT-474 (b) cells by 10 nM E2 and/or 50 ng/mL IGF-1 stimulation. These data represent the mean ± SD of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001). (c) Flow cytometric analyses showing the effect of ERAP and tamoxifen on the cell cycle in the presence of E2 and IGF-1 in MCF-7 cells.
We examined the effects of ERAP on cell cycle distribution in response to E2 and IGF-1 crosstalk using flow cytometry. The population of cells in the G2/M phase markedly increased after a 24 h crosstalk stimulation, whereas the population in the G1 phase increased after ERAP or tamoxifen treatment. These results indicate growth suppression via the induction of G1 arrest (ERAP; 57.0%, tamoxifen; 36.6%) (Fig.2c). Notably, a remarkable increase in the sub-G1 cell population (apoptotic cell) was observed after treatment with a combination of ERAP and tamoxifen (24.9%) (Fig.2c). These findings strongly suggest that ERAP treatment suppressed cell growth of endocrine-resistant breast cancer cells due to crosstalk between E2 and IGF-1, which induced a G1 arrest, and the combination treatment of ERAP and tamoxifen synergistically suppressed cell growth by inducing rapid apoptosis.
ERAP inhibits the breast tumor growth activated by the crosstalk between E2 and insulin-like growth factor 1 in vivo
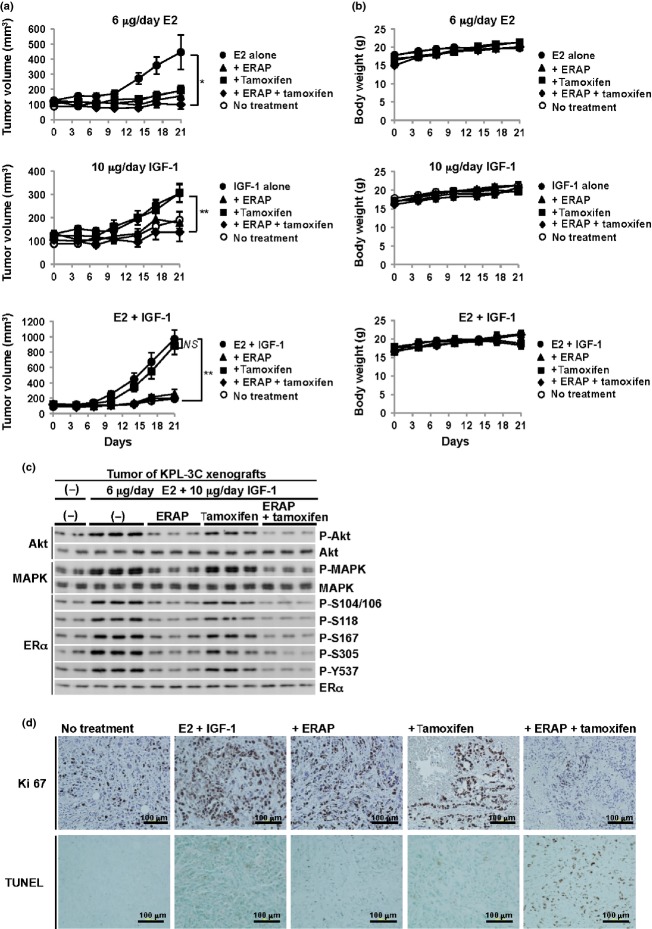
KPL-3C orthotropic breast cancer xenografts were developed in nude mice to determine the anti-tumor activity of ERAP against E2 and IGF-1 crosstalk in vivo. Once the tumors were fully established, ERAP (14 mg/kg/day), tamoxifen (4 mg/kg/day), a combination of ERAP and tamoxifen, or vehicle alone were administered daily via i.p. injection for 21 days. The animals also received daily treatments of E2 (6 μg/day), IGF-1 (10 ng/day), or a combination of E2 and IGF-1. Daily combined E2 and IGF-1 treatment resulted in a robust growth of KPL-3C tumors (971 ± 119 mm3 at 21 days) compared with E2 alone (446 ± 113 mm3) or IGF-1 alone (304 ± 36 mm3) (Fig.3a, Suppl. Fig. S3a–c). In contrast, ERAP treatment but not tamoxifen treatment significantly inhibited the tumor growth induced by the co-stimulation of E2 and IGF-1 (Fig.3a, Suppl. Fig. S3c; n = 5; P < 0.01 in two-sided Student's t-test). Unexpectedly, ERAP significantly inhibited IGF-1 alone-induced tumor growth in vivo, in contrast to in vitro, suggesting that the suppression of IGF-1-induced signaling pathways is associated with in vivo tumor formation by ERAP (Fig.3a, middle). No toxicity or significant body weight losses were observed with any treatments throughout these experiments (Fig.3b).
Fig 3.
ERAP suppresses in vivo tumor growth induced by the crosstalk between E2 and insulin-like growth factor 1 (IGF-1). (a) ERAP inhibits tumor growth in a human breast cancer KPL-3C xenograft mouse model. The tumor volume represents the mean ± SE of each group (n = 5) (*P < 0.05; ** P < 0.01; NS, no significance). (b) The body weights of the KPL-3C xenograft mice. The body weight represents the mean ± SD of each group (n = 5). (c) The effects of ERAP on the phosphorylation levels of Akt, MAPK and estrogen receptor alpha (ERα) proteins in tumors. The blots were cropped, and the full-length blots are included in Supplementary Figure S4. (d) Representative immunohistochemical staining of nuclear Ki67 (upper) and TUNEL staining (lower) in tumors at day 21.
We next examined the in vivo inhibitory effects of ERAP on the activation of the non-genomic ERα-signaling pathway using co-stimulation with E2 and IGF-1. As expected, ERAP treatment considerably suppressed both Akt and MAPK phosphorylation levels in tumors, and this suppression was comparable to combined treatment with ERAP and tamoxifen (Fig.3c), which is consistent with the in vitro inhibitory effect (Fig.1c,d). We further investigated the expression of the proliferative markers Ki-67 using immunohistochemistry. The results showed that the number of cells expressing Ki-67 was extremely increased in tumors treated with E2 and IGF-1 (Fig.3d, upper panels) and drastically decreased after the combined treatment of ERAP and tamoxifen (Fig.3d, upper panels). Furthermore, a significant induction of TUNEL-positive apoptotic tumor cells were observed after the combined treatment of ERAP and tamoxifen (Fig.3d; lower panels), but only a slight induction of TUNEL-positive apoptotic tumors were observed after ERAP or tamoxifen alone (Fig.3d; lower panels). Consistent with this result, treatment with ERAP or tamoxifen alone showed lower Ki-67 expression than the crosstalk between E2 and IGF-1. These results suggest a slight induction of apoptosis in addition to G1 arrest by treatment with ERAP or tamoxifen alone.
ERAP regulates the signaling crosstalk of E2 and epidermal growth factor or human epidermal growth factor 2
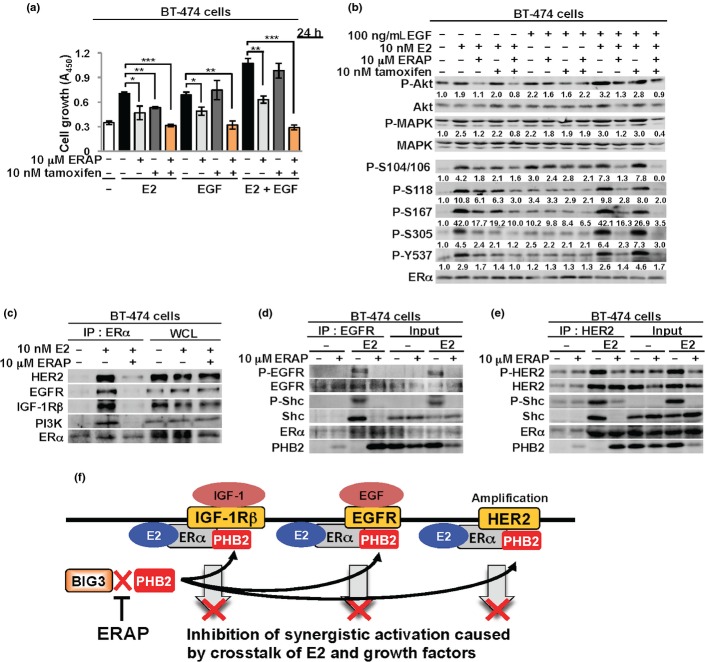
Endogenous membrane-associated ERα has been shown to exhibit crosstalk to the trans activation of EGF and HER2 in breast cancer cells.32,33 We first measured the proliferation of BT-474 cells overexpressing EGFR and HER2 using the MTT assay at 24 h after ERAP treatment to examine the inhibitory effects of ERAP on the activation of signal pathways stimulated by the crosstalk of E2 and EGF. The results showed that ERAP treatment but not tamoxifen treatment significantly inhibited cell growth that was enhanced by the crosstalk of both E2 and EGF in BT-474 cells (Fig.4a). Notably, the combined treatment of ERAP and tamoxifen significantly suppressed each crosstalk-induced cell proliferation. This combination showed a synergistic inhibitory effect on EGF-induced cell growth (Fig.4a), which was unlike the effect in MCF-7 cells (Fig.2a). These results suggest the possibility that this difference was due to different expression levels of EGFR between BT-474 and MCF-7 cells (Suppl. Fig. S2). Furthermore, ERAP treatment remarkably suppressed Akt (S473) and p42/44 MAPK (T202/Y204) and ERα phosphorylation levels at five sites (S104/S106, S118, S167, S305 and Y537) activated by the crosstalk between E2 and EGF, which was comparable to the combined treatment of ERAP and tamoxifen (Fig.4b).
Fig 4.
ERAP regulates signaling pathways by the crosstalk of E2 and a variety of growth factors. (a) An MTT assay showing the inhibitory effect of ERAP on the growth of BT-474 cells by E2 and/or EGF stimulation. These data represent the mean ± SD of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001). (b) The inhibitory effects of ERAP on Akt, MAPK, and estrogen receptor alpha (ERα) phosphorylation induced by E2 and/or EGF stimulation in BT-474 cells. The data are expressed as the fold increase over untreated cells at 0 h. (c) Inhibitory effects of ERAP on the interactions of estrogen receptor alpha (ERα) with human epidermal growth factor 2 (HER2), epidermal growth factor receptor (EGFR), insulin-like growth factor 1 receptor beta (IGF-1Rβ) or PI3K. (d, e) The inhibitory effects of ERAP on the interactions of EGFR (d) or HER2 (e) with ERα and Shc in BT-474 cells. The above all blot were cropped, and the full-length blots are included in Supplementary Figure S4. (f) Schematic illustrations of inhibitory effects of ERAP on cell functions caused by the crosstalk of ERα and IGF-1R, EGFR or HER2.
Amplification of the HER2 oncogene occurs in approximately 15% of invasive breast cancers and the mechanism of tamoxifen resistance for which clinical data exist, is the overexpression of HER2.34–38 We performed experiments to clarify the inhibitory effects of the interactions of ERα with HER2, EGFR or IGF-1Rβ in BT-474 cells to further elucidate the potential role of ERAP against the crosstalk responsible for endocrine resistance. Co-immunoprecipitation experiments revealed that ERAP completely inhibited all of the complex formation of endogenous ERα with EGFR (Fig.4c,d), HER2 (Fig.4c,e), IGF-1Rβ (Fig.4c) or PI3K (Fig.4c) in the presence of E2 in BT474 cells. Recently, we demonstrated that ERAP removed Shc from a ternary complex of ERα, IGF-1Rβ and Shc in the cell membrane of MCF-7 and KPL-3C cells and formed a new ternary complex consisting of ERα, IGF-1Rβ and PHB2.21 We also demonstrated that ERAP removed Shc from a ternary complex of ERα, Shc and EGFR or HER2 in the cell membrane and formed a new complex consisting of EGFR, ERα and PHB2 or HER2, ERα and PHB2 (Fig.4d,e). These new complexes suppressed E2-induced tyrosine phosphorylation of EGFR or HER2 by ERAP treatment (Fig.4d,e). Therefore, our data suggest that PHB2, which is released from BIG3 by ERAP, removed Shc and inhibited the E2-dependent interactions of ERα and EGFR, HER2 and IGF-1Rβ. This inhibition may have suppressed the signaling pathways associated with endocrine-resistant breast cancers (Fig.4f).
Discussion
This study demonstrated that ERAP had significant inhibitory effects against signaling crosstalk between E2 and growth factors, such as IGF and EGF, and HER2 amplification through its complete inhibition of ERα-IGF-1Rβ, ERα-EGFR, ERα-HER2 and/or ERα-PI3K interactions. This mechanism of the inhibitory effect of ERAP was possibly due to the removal of the Shc adaptor protein from each complex and the formation of new complexes that included PHB2 released from BIG3. ERAP considerably suppressed the activation of signaling pathways, including Akt and MAPK, which are activated by the crosstalk between E2 and IGF or EGF and associated with tamoxifen resistance. We also demonstrated that ERAP suppressed E2-dependent ERα transcriptional activity in the presence of IGF-1 in breast cancer cells (Fig.1a). Moreover, we previously reported that PHB2 released from BIG3 by ERAP treatment directly binds to ERα and acts as a corepressor by recruiting HDAC1 and NcoR, thereby leading to an almost complete suppression of the ERα target gene expression.21 Accordingly, these findings suggest the possibility that ERAP also represses ERα transcriptional activity stimulated by crosstalk with E2 and IGF-1 in breast cancer cells via the same mechanism. More interestingly, accumulating evidence indicates that PHB2 also represses other transcriptional regulators such as MyoD, MEF2, EZH2 and RNF2-dependent transcriptional activities.39 This evidence suggests the possibility that ERAP may represses several transcriptional activities, including ERα by PHB2, although further analyses are needed to elucidate the detailed mechanisms of the inhibitory effects.
ERAP also remarkably reduced the phosphorylation levels at all five sites within ERα (S104/S106, S118, S167, S305 and Y537) activated by E2 and IGF-1 or EGF stimulation. Considerable data indicate that ERα is activated by a variety of kinase growth factor signaling pathways, including ERK1/2, p38MAPK, p21-activated kinase (PAK-1), Akt, c-Src and protein kinase A (PKA). For example, phosphorylation of ERα S118 by Erk1/2 and S167 by Akt are involved in acquired tamoxifen resistance.40–42 Both PAK-1- and PKA-mediated phosphorylation of S305 are associated with tamoxifen responsiveness.43,44 Our findings suggest that ERAP downregulated ERα phosphorylation levels due to a direct or indirect suppression of these kinase activities, and further elucidation of the mechanisms of the inhibitory effects of ERα phosphorylation is required.
The biological actions of E2 have been shown to require the activation of E2-responsive genes and cell membrane-initiated events operated via Src, adaptor protein Shc, and growth factor receptors, such as IGF-1R, EGFR and HER2.45,46 Membrane-associated ERα transduces E2 rapid signals, which lead to the synergistic activation of IGF-1R and EGFR in breast cancer.47 Interestingly, long-term blockade of ERα function with tamoxifen irreversibly causes the overexpression of EGRF or HER2, which results in functional EGFR pathways instead of ERα-signaling.48 Clinical evidence has shown that ERα, IGF-1R and EGFR are co-expressed in breast cancer at the time tumors are diagnosed.49,50 The overexpression of HER2 and EGFR is associated with poorer outcome in breast cancer patients treated with endocrine therapy.34–36
Based on the above findings, clinical trials were conducted and demonstrated that the combination of gefitinib (Iressa), a potent EGFR inhibitor, with an anti-estrogen agent was more efficient at inhibiting ERα-positive breast cancer growth than either agent alone.51 More importantly, we revealed that the combination of ERAP and tamoxifen induced rapid apoptosis in vivo and in vitro against breast cancer cells exposed to the crosstalk of E2 and growth factors compared with either treatment alone. This combined effect suggests that the release of intrinsic PHB2 could modulate multiple aspects of the synergistic signaling network through E2 and growth factors, in addition to the transcriptional repressor for ERα. Therefore, a combination of the current endocrine therapies and BIG3-PHB2 interaction inhibitors may lead to more effective combined effects on intrinsic and acquired endocrine-resistant breast cancer, especially in premenopausal women. These molecular findings provide the rationale for clinical studies of endocrine therapy to block growth factor signaling pathways and restore endocrine responsiveness.
Acknowledgments
This work was supported by the Project Future of Relay For Life Japan, a grant/research support from Tokushima Breast Care Clinic, a Grant-in-Aid for Scientific Research on Innovative Areas (MEXT KAKENHI Grant Number 251347212), Grants-in-Aid for Scientific Research ((B) (MEXT KAKENHI Grant Number 25293079) and (C) (MEXT KAKENHI Grant Number 26461948)), an IMSUT Joint Research Project, and an Extramural Collaborative Research Grant of Cancer Research Institute, Kanazawa University.
Disclosure statement
Toyomasa Katagiri is a stockholder and an external board member of OncoTherapy Science, Inc. The other authors have declared that no conflicts of interest exist.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. ERAP suppresses the insulin-like growth factor 1 receptor beta (IGF-1Rβ) phosphorylation by the crosstalk between E2 and IGF-1.
Fig. S2. Expression of ErbB2/human epidermal growth factor 2 (HER2), epidermal growth factor receptor (EGFR), and insulin-like growth factor 1 receptor beta (IGF-1Rβ) proteins in MCF-7, KPL-3C and BT-474 cells.
Fig. S3. KPL-3C xenograft tumors.
Fig. S4. Full-length of images of all immunoblots in Figures and Supplementary Figures.
References
- Ali S, Coombes RC. Estrogen receptor a in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia. 2000;5:271–81. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor α rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–53. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- Pietras RJ. Interactions between estrogen and growth factor receptors in human breast cancers and the tumor-associated vasculature. Breast J. 2003;9:361–73. doi: 10.1046/j.1524-4741.2003.09510.x. [DOI] [PubMed] [Google Scholar]
- Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor a to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:2076–81. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santen RJ, Song RX, Zhang Z, Yue W, Kumar R. Adaptive hypersensitivity to estrogen: mechanism for sequential responses to hormonal therapy in breast cancer. Clin Cancer Res. 2004;10:337S–45S. doi: 10.1158/1078-0432.ccr-031207. [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37:S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–7. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–18. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Di Domenico M, Castoria G. Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res. 2005;65:10585–93. doi: 10.1158/0008-5472.CAN-05-0912. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2:205–13. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Jones HE, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–44. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- Macedo LF, Sabnis G, Brodie A. Preclinical modeling of endocrine response and resistance: focus on aromatase inhibitors. Cancer. 2008;112:679–88. doi: 10.1002/cncr.23191. [DOI] [PubMed] [Google Scholar]
- Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001;53:25–71. [PubMed] [Google Scholar]
- Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–90. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Johnston SR. Endocrine therapy–Current benefits and limitations. Breast Cancer Res Treat. 2005;93:S3–10. doi: 10.1007/s10549-005-9036-4. [DOI] [PubMed] [Google Scholar]
- Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–60. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–9. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- Gee JM, Robertson JF, Gutteridge E, et al. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12:S99–111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- Kim JW, Akiyama M, Park JH, et al. Activation of an estrogen/estrogen receptor signaling by BIG3 through its inhibitory effect on nuclear transport of PHB2/REA in breast cancer. Cancer Sci. 2009;100:1468–78. doi: 10.1111/j.1349-7006.2009.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimaru T, Komatsu M, Matsuo T, et al. Targeting BIG3-PHB2 interaction to overcome tamoxifen resistance in breast cancer cells. Nat Commun. 2013;4:2443. doi: 10.1038/ncomms3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi J, Kurosumi M, Sonoo H. A new human breast cancer cell line, KPL-3C, secretes parathyroid hormone-related protein and produces tumours associated with microcalcifications in nude mice. Br J Cancer. 1996;74:200–7. doi: 10.1038/bjc.1996.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimaru T, Komatsu M, Tashiro E, et al. Xanthohumol suppresses oestrogen-signalling in breast cancer through the inhibition of BIG3-PHB2 interactions. Sci Rep. 2014;4:7355. doi: 10.1038/srep07355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LC, Seekallu SV, Watson PH. Clinical significance of estrogen receptor phosphorylation. Endocr Relat Cancer. 2011;18:R1–14. doi: 10.1677/ERC-10-0070. [DOI] [PubMed] [Google Scholar]
- Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2008;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- Chen D, Riedl T, Washbrook E, et al. Activation of estrogen receptor alpha by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol Cell. 2000;6:127–37. [PubMed] [Google Scholar]
- Chen D, Pace PE, Coombes RC, Ali S. Phosphorylation of human estrogen receptor alpha by protein kinase A regulates dimerization. Mol Cell Biol. 1999;19:1002–15. doi: 10.1128/mcb.19.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel PB, Smith J, Sturgill TW, Fisher TL, Blenis J, Lannigan DA. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol Cell Biol. 1998;18:1978–84. doi: 10.1128/mcb.18.4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Trowbridge JM, Garabedian MJ. Potentiation of human estrogen receptor alpha transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J Biol Chem. 1999;274:22296–302. doi: 10.1074/jbc.274.32.22296. [DOI] [PubMed] [Google Scholar]
- Arnold SF, Melamed M, Vorojeikina DP, Notides AC, Sasson S. Estradiol-binding mechanism and binding capacity of the human estrogen receptor is regulated by tyrosine phosphorylation. Mol Endocrinol. 1997;11:48–53. doi: 10.1210/mend.11.1.9876. [DOI] [PubMed] [Google Scholar]
- Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J. 2001;21:5437–47. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–8. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol. 2005;23:1616–22. doi: 10.1200/JCO.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of theHER2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Berry DA, Muss HB, Thor AD, et al. Her2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. J Clin Oncol. 2000;18:3471–9. doi: 10.1200/JCO.2000.18.20.3471. [DOI] [PubMed] [Google Scholar]
- De Laurentiis M, Arpino G, Massarelli E, et al. A meta-analysis on the interaction between HER2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–8. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Houghton J, Iden C, et al. Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and Her2 status. Ann Oncol. 2006;17:818–26. doi: 10.1093/annonc/mdl016. [DOI] [PubMed] [Google Scholar]
- Frédéric T, Nigel R, Canan GN, Laurent D. Prohibitin ligands in cell death and survival: mode of action and therapeutic potential. Chem Biol. 2013;20:316–31. doi: 10.1016/j.chembiol.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Han SX, Bai E, et al. Dose-dependent effect of tamoxifen in tamoxifen-resistant breast cancer cells via stimulation by the ERK1/2 and AKT signaling pathways. Oncol Rep. 2013;29:1563–9. doi: 10.3892/or.2013.2245. [DOI] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Sun M, Paciga JE, Feldman RI, et al. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor α (ERα) via interaction between ERα and PI3K. Cancer Res. 2001;61:5985–91. [PubMed] [Google Scholar]
- Bostner J, Skoog L, Fornander T, Nordenskjöld B, Stål O. Estrogen receptor-alpha phosphorylation at serine 305, nuclear p21-activated kinase 1 expression, and response to tamoxifen in postmenopausal breast cancer. Clin Cancer Res. 2010;16:1624–33. doi: 10.1158/1078-0432.CCR-09-1733. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Flach K, Bentin Toaldo C, et al. PKA phosphorylation redirects ERα to promoters of a unique gene set to induce tamoxifen resistance. Oncogene. 2013;32:3543–51. doi: 10.1038/onc.2012.361. [DOI] [PubMed] [Google Scholar]
- Cheskis BJ. Regulation of cell signalling cascades by steroid hormones. J Cell Biochem. 2004;93:20–7. doi: 10.1002/jcb.20180. [DOI] [PubMed] [Google Scholar]
- Levin ER. Integration of the extra-nuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–9. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RX, Zhang Z, Santen RJ. Estrogen rapid action via protein complex formation involving ERalpha andSrc. Trends Endocrinol Metab. 2005;16:347–53. doi: 10.1016/j.tem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH. Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer. 2002;97:306–12. doi: 10.1002/ijc.1614. [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Hutcheson IR, Harper ME, et al. Modulation of epidermal growth factor receptor in endocrine-resistant, estrogen-receptor-positive breast cancer. Ann N Y Acad Sci. 2002;63:104–15. doi: 10.1111/j.1749-6632.2002.tb04101.x. [DOI] [PubMed] [Google Scholar]
- Shimizu C, Hasegawa T, Tani Y, et al. Expression of insulin-like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Hum Pathol. 2004;35:1537–42. doi: 10.1016/j.humpath.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kurebayashi J, Okubo S, Yamamoto Y. Sonoo H Inhibition of HER1 signaling pathway enhances antitumor effect of endocrine therapy in breast cancer. Breast Cancer. 2004;11:38–41. doi: 10.1007/BF02968000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. ERAP suppresses the insulin-like growth factor 1 receptor beta (IGF-1Rβ) phosphorylation by the crosstalk between E2 and IGF-1.
Fig. S2. Expression of ErbB2/human epidermal growth factor 2 (HER2), epidermal growth factor receptor (EGFR), and insulin-like growth factor 1 receptor beta (IGF-1Rβ) proteins in MCF-7, KPL-3C and BT-474 cells.
Fig. S3. KPL-3C xenograft tumors.
Fig. S4. Full-length of images of all immunoblots in Figures and Supplementary Figures.