Abstract
Catechol-O-methyltransferase (COMT) is an important molecule in different types of cancers. Its biological effect and therapeutic significance, however, rarely been investigated fully in pancreatic cancer. Immunohistologically, high COMT expression was significantly correlated with the longer overall survival of patients (P < 0.05), indicating its protective nature. The effects of COMT on cell growth, apoptosis, and invasion were evaluated using overexpression and silencing methods. In detail, we carried out experiments using one stably transduced and two transiently transfected pancreatic cancer cell lines in vitro, and one stably transduced cell line in vivo mice xenograft models. In vitro experiments showed that COMT inhibited cell proliferation, enhanced gemcitabine-induced apoptosis, and inhibited cell invasion in stably transduced and transiently transfected cell lines by regulating the PI3K/Akt pathway, p53, and E-cadherin. The COMT overexpressed and silenced cell lines showed significantly inhibited and enhanced growth capacities in in vivo xenograft models, respectively. In conclusion, COMT suppressed pancreatic cancer and its high expression predicted longer survival time. The interaction of COMT with the PI3K/Akt pathway makes it a potential target for therapy.
Keywords: Catechol-O-methyltransferase, invasion, pancreas, RNAi, survival
ancreatic cancer is a dismal disease with an extremely poor prognosis. The 5-year survival rate is approximately 6.7%, and the majority of patients die within the first year of diagnosis. The prognosis is better for localized disease, with a 5-year survival of 25.8%.1 Patients with early-stage disease respond well to surgical interventions; however, only 15–20% of patients are suitable for resection due to the lack of sensitive serum biomarkers for early diagnosis. The sensitivity and specificity of CA19-9 was limited, when diagnosing early stage disease.2,3 Therefore, most patients present with unresectable or metastatic disease at the time of diagnosis. Pancreatic cancer (PC) is resistant to chemotherapy.4 Although 5-fluorouracil and gemcitabine have long been the mainstays of chemotherapy, the objective response was limited.5,6 Combination therapies, such as oxaliplatin, irinotecan, fluorouracil and leucovorin, and nab-paclitaxel plus gemcitabine, have recently made some progressions. However, the objective response rates were no more than 40%.7,8 Thus, new therapeutic targets are needed to overcome chemotherapy resistance.
Catechol-O-methyltransferase (COMT) is widely distributed in various organs, including the brain, liver, kidney, endometrium and breast tissue.9 It consists of two isoenzymes in humans, namely membrane-bound (MB-COMT) and soluble (S-COMT). Deactivates neurotransmitters such as dopamine, norepinephrine, and epinephrine.10 Importantly, S-COMT is mainly distributed in peripheral tissues and is a key enzyme in estrogen catabolism.11 The metabolic products of estrogen, such as 2-OH and 4-OH catechol estrogens, can induce DNA damage and malignant cell transformation.12,13 Catechol-O-methyltransferase can catalyze the methylation and detoxification of those 2-OH and 4-OH catechol estrogens to the less harmful methoxy estrogens.14
The relationship between COMT and cancer risk has long been a focus of research.15–17 Some studies have suggested that low COMT activity would increase breast cancer risk and endometrial cancer.17,18 Our previous studies showed COMT to be involved in colorectal cancer: COMT is overexpressed in colorectal cancer tissue,19 and inhibits colorectal cancer growth.20 We found that COMT is a candidate immunogenic membrane antigen for PC,21 and its expression is higher in PC tissue compared with non-cancerous tissue.22 However, to our knowledge, the biological function of COMT in PC has not been investigated. We aimed to evaluate the role of COMT in PC.
Materials and Methods
Cell lines
Three human PC cell lines, namely BxPC-3, Panc-1, and MIAPaCa-2, were kindly donated by Professor Helmut Freiss from the Technical University of Munich (Munich, Germany) and preserved in the Laboratory of the Department of General Surgery, PUMCH (Beijing, China). One cell line, BxPC-3, underwent lentiviral transduction to stably overexpress or silence COMT. The other two cell lines, Panc-1 and MIAPaCa-2, underwent plasmid transfection to transiently overexpress or silence COMT.
Cell culture
The cell line BxPC-3 was cultured in RPMI-1640 medium (HyClone, Logan, UT, USA) supplemented with 10% FBS (HyClone). The cell lines Panc-1 and MIAPaCa-2 were cultured in DMEM (Life Technologies, Frederick, MD, USA) with 4 mM glutamine. All cell lines were cultured in a humidified incubator with 5% CO2 at 37°C.
Transient transfection
The cDNA of S-COMT was cloned into the pcDNA3.1(+) vector then together transfected into the cell lines. The resulting cell lines were named COMT-OE cell lines. The pcDNA3.1(+) vector was transfected alone into the cell lines to serve as a control (Control-OE).
A specific COMT siRNA (GCCCUUGACUUGGGCACCAAACAUU) based on the cDNA sequence of COMT was transfected into the cell lines, named COMT-miR. A negative control siRNA (UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT) was transfected into the cell lines, named Control-miR. All the transfections were carried out with Lipofectamine 2000 (Life Technologies). After 24 h, the transfected cells were analyzed in following assays, and Western blot analysis was carried out to verify COMT expression.
Stable transduction
Lentiviral transduction was used to generate the COMT overexpression (COMT-OE), normal control (Control-OE), COMT miRNA-interfering (COMT-miR), and normal control (Control-miR) cell lines. In detail, COMT-OE was transduced with lentivirus containing the COMT cDNA sequence; and COMT-miR was transduced with lentivirus containing an oligo antisense DNA that targeted the COMT mRNA. Lentiviral particles were produced by cotransfection of Packaging Mix with Opti-MEM and Lipofectamine 2000 (Life Technologies). The medium was exchanged 6 h after transfection, and lentiviral supernatant was collected 48 h later. The lentiviral supernatants were concentrated using ultracentrifugation at 50 000 g for 2 h, and infection of BxPC-3 cells was carried out in 12-well plates in the presence of 8 mg/mL Polybrene (Sigma-Aldrich, St. Louis, MO, USA). The transduced cells were selected using blasticidin (0.5–10 μg/mL).
Cell proliferation assay
Cell proliferation was determined by the cell count kit cell counting kit-8 (CCK-8, Dojindo, Shanghai, China). Briefly, cells were seeded in 96-well plates, and after 4 h of incubation with culture medium containing the CCK-8 reagent, the absorbance was read at 450 nm using a Spectrum MAX 190 (Molecular Devices, U.S.A.)
Flow cytometry assay of cell cycle
Cells were harvested using trypsinization and collected by centrifugation, washed once with PBS and fixed in 1 mL of 75% ethanol at 4°C. The cells were then washed once with PBS and incubated with 1 mL PBS solution containing 30 g/mL PI and 0.25 mg/mL RNase A for 30 min at room temperature. The DNA content of the cells was analyzed using the fluorescence-activated cell sorter Cytomics FC 500 (Beckman Coulter, Miami, FL, USA), and the data were analyzed using Multicycle AV for Windows (Beckman Coulter).
Cell apoptosis assay
Cell apoptosis was measured using annexin V/PI double staining. Briefly, the cells were seeded in six-well plates, and after 48 h of incubation, the cells were harvested and centrifuged at 400 g for 5 min, the cell apoptosis assay was repeated three times. The cells were then stained with an annexin V–FITC Kit (Beckman Coulter), and the cell number was assayed using a flow cytometer. The wavelengths were set at 515 nm for FITC fluorescence and at 560 nm for PI fluorescence. We used gemcitabine to induce apoptosis, with a concentration of 1.86 nmol/L.
Invasion assays
Invasion was measured using Transwell assays. Briefly, cells were seeded in six-well plates and cultured in BD BioCoat Matrigel Invasion Chambers (Becton Dickinson, Bedford, MA, USA) at 37°C in 5% CO2 for 48 h according to the manufacturer's instructions. Three parallel cultures were measured to determine the cell invasion ratios of five randomly selected microscopic fields at ×200.
Antibodies
Antibodies against phospho-PTEN (cat. #9559S), phospho-PI3K (cat. #4228S), AKT (cat. #4685S), phospho-AKT (cat. #4060S), phospho-GSK3 (cat. #9331S), Bax (cat. #2772S), p21 (cat. #2946S), p27 (cat. #2559P), phospho-Bad (cat. #4366S), cyclin D1 (cat. #2926P), Bad (cat. #9292S), CDK4 (cat. #2906), β-actin (cat. #4970S), Bcl-2 (cat. #2870S), E-cadherin (cat. #3195S), and Bim (cat. #2819S) were from Cell Signaling Technologies (Danvers, MA, USA). Antibodies against COMT (sc-25844) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against p53 (ZM-0408) and HRP-conjugated antibodies were from ZhongShan Golden-Bridge Biotechnology (Beijing, China).
Western blot analysis
Cells were harvested using trypsinization and pelleted by centrifugation. Cell pellets were lysed in lysis buffer, which was supplemented with protease inhibitors, and the proteins were separated using SDS-PAGE and transferred electrically to a PVDF membrane (Millipore, Billerica, MA, USA). The membrane was probed with the primary antibody and an HRP-conjugated secondary antibody. The blots were visualized using Immobilon Western (Millipore), and the intensity of the blots was analyzed using a Gel-Pro Analyzer version 4.0 (Media Cybernetics, Bethesda, MD, USA). The details for antibodies were listed above.
Xenograft studies
This study was approved and monitored by the Institutional Review Board, PUMCH. BALB/c nu/nu female mice were raised in a specific pathogen-free (SPF) room in the Animal Model Center, Chinese Academy of Medical Science (Beijing, China). Human PC cells were injected s.c. into the right axillary area of the abdomens of 4-week-old mice. The mice were killed 30 days after cell injection, and the tumor size and weight were measured. Hematoxylin–eosin and Ki-67 staining of tumor specimens were carried out according to standard protocol.
Clinical samples
This study was approved and monitored by the Institutional Review Board, PUMCH. Eighty-five formalin-fixed, paraffin-embedded surgical specimens of pancreatic adenocarcinoma of the T1–T3/N0–N1/M0 stages from the Department of General Surgery, PUMCH were included for Immunohistochemistry (IHC) assays. Informed consent was obtained from all patients.
Immunohistochemical assay
IHC was stained for all surgical specimens according to the following standard protocol. Two pathologists who specialized in PC independently rated the staining intensity and percentage of stained cells. The final COMT score was equal to the staining intensity multiplied by the cell percentage. A high COMT level was defined if the COMT score was ≥4, otherwise it was defined as low COMT.
Statistical analysis
Statistical analyses were carried out using the spss 13.0 software (SPSS, Chicago, IL, USA). Continuous data were presented as the mean ± SD, and significant differences were determined using Student's t-test for independent samples. A χ2-test was used to assess the correlation between COMT expression and the clinicopathological parameters of the patients. Univariate analyses were used to compare the clinicopathological parameters with the overall survival of the patients using the Kaplan–Meier method, and the significance was detected using a log–rank test (P < 0.05). For those parameters showing a significant relationship with patients' overall survival, multivariate analyses were used with Cox proportional hazards modelling (P < 0.05). Statistical significance was considered if the P-value was <0.05.
Results
Expression of COMT correlated with patient survival
The IHC revealed that COMT expression was significantly higher in PC tissue compared with adjacent normal pancreatic ductal tissue. Representative illustrations of COMT-high, COMT-low, and normal pancreatic ductal tissue are available as Figure1(a–c), respectively. The mean survival time of patients in the COMT-high group was significantly longer than that in the COMT-low group (log–rank test, P < 0.05), and COMT expression was significantly correlated with the overall survival of patients using univariate and multivariate analyses (Table1). The COMT expression was not correlated with the clinicopathological parameters in this cohort of patients (see Table S1, which illustrates the relationship between COMT expression and clinicopathological parameters in PC). This result indicated that COMT may protect patients and predicted a good prognosis.
Fig 1.
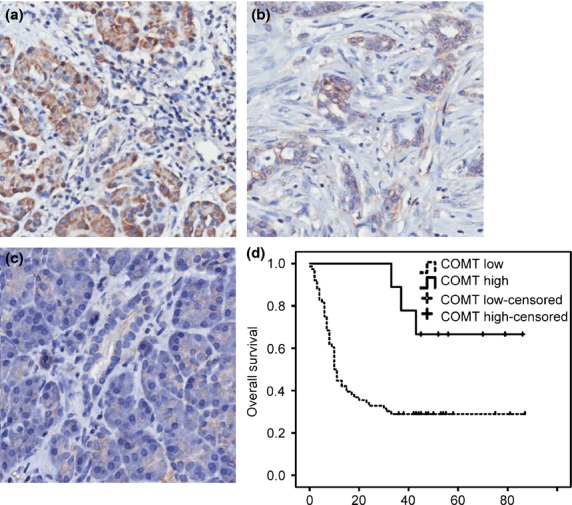
Catechol-O-methyltransferase (COMT) expression level is associated with pancreatic cancer patient survival. Immunohistochemistry of tumor tissues from pancreatic cancer patients with high (a) (Magnification 400×) and low (b) COMT expression, and adjacent normal pancreatic ductal cells with no COMT staining (c). (Magnification 400×) (d) Kaplan–Meier curve for the overall survival of patients with high and low COMT expression. COMT high: COMT staining score is higher high than or equal to 4; COMT low: COMT staining is lower than 4; COMT high-censored: patients with high COMT staining who was alive or loss of follow-ups. COMT low-censored: patients with low COMT staining who was alive or loss of follow-ups.
Table 1.
Univariate and multivariate analyses of the overall survival of pancreatic cancer patients
| Variables | Patient number, N = 85 | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SE (month) | 95% CI | P † | HR | 95% CI | P ‡ | ||
| Gender | 0.033 | 0.06 | |||||
| Male | 54 | 29.4 ± 4.5 | 20.4–38.3 | 1.797 | 0.996–3.344 | ||
| Female | 31 | 42.0 ± 5.9 | 30.5–53.5 | 1 | |||
| Age | 0.940 | ||||||
| ≥65 years | 33 | 37.2 ± 6.5 | 24.3–50.0 | NA | NA | NA | |
| <65 years | 52 | 35.2 ± 4.9 | 25.6–44.8 | NA | NA | NA | |
| Tumour size | 0.685 | ||||||
| >5 cm | 32 | 36.7 ± 6.2 | 24.6–48.8 | NA | NA | NA | |
| ≤5 cm | 53 | 35.9 ± 5.1 | 26.0–45.8 | NA | NA | NA | |
| Histological grade | 0.018 | 0.02 | |||||
| G1-2 | 54 | 43.2 ± 5.0 | 32.4–52.0 | 1 | |||
| G3-4 | 23 | 23.1 ± 6.2 | 10.9–35.3 | 1.963 | 1.095–3.518 | ||
| PNI | 0.370 | ||||||
| Present | 36 | 29.6 ± 5.1 | 19.5–39.7 | NA | NA | NA | |
| Absent | 49 | 39.7 ± 5.4 | 29.0–50.4 | NA | NA | NA | |
| T stage | 0.356 | ||||||
| T1-2 | 51 | 40.2 ± 5.3 | 29.7–50.6 | NA | NA | NA | |
| T3 | 33 | 29.3 ± 5.3 | 18.9–39.6 | NA | NA | NA | |
| N stage | 0.102 | ||||||
| N0 | 48 | 43.0 ± 4.5 | 32.3–53.8 | NA | NA | NA | |
| N1 | 30 | 27.7 ± 6.0 | 16.0–39.4 | NA | NA | NA | |
| COMT level | 0.015 | 0.02 | |||||
| High | 9 | 69.9 ± 7.6 | 54.9–84.9 | 0.243 | 0.075–0.789 | ||
| Low | 76 | 32.1 ± 4.0 | 24.2–40.2 | 1 | |||
Log-rank test. ‡Cox regression test. NA, not applicable; SE, standard error; CI, confidence interval; HR, hazard ratio; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; G4, undifferentiated; PNI, peri-neural invasion; T, tumour; N, lymph node; COMT, Catechol-O-Methyltransferase.
Overexpression of COMT inhibited cell growth, reduced S-phase cell ratio, increased apoptosis, and inhibited invasion in vitro
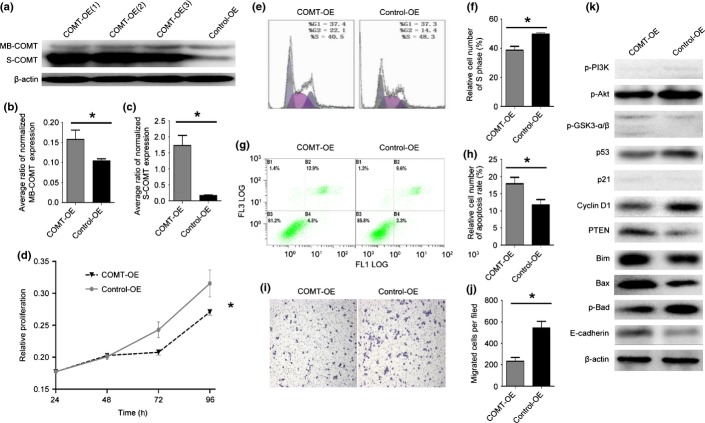
We carried out transient transfections of two PC cell lines (Panc-1 and MIAPaCa-2) and stable transduction of one PC cell line (BxPC-3) with S-COMT cDNA. All three cell lines showed similar relationships between COMT overexpression and cell growth, S-phase ratio, apoptosis rate, and invasion. For illustration purpose, the following paragraph focuses on the stably transduced cell line BxPC-3. The growth and S-phase results of the two transiently transfected cell lines are illustrated in Figures S1 and S2.
After stable transduction, the BxPC-3 cell line showed a 10-fold overexpression of S-COMT and a 1.5-fold overexpression of MB-COMT (Fig.2a–c), and the proliferative capacity was significantly reduced (Fig.2d). Overexpression of COMT also significantly reduced the proportion of cells in S phase (Fig.2e,f). We then speculated whether S-COMT overexpression was associated with alteration in apoptosis. We used flow cytometry to detect apoptosis after 72 h of gemcitabine treatment using annexin V/PI double staining. Overexpression of COMT significantly increased cell apoptosis (Fig.2g,h).
Fig 2.
Catechol-O-methyltransferase (COMT) upregulation inhibits cell growth, reduces the S-phase cell ratio in vitro, inhibits apoptosis, and promotes invasion in vitro. (a) Western blot for COMT proteins for transduction. (b) Average ratio of normalized membrane-binding COMT (MB-COMT) expression. *P < 0.05. COMT-OE, COMT protein overexpression cell lines; Control-OE, normal COMT protein expression. (c) Average ratio of normalized soluble COMT (S-COMT) expression. *P < 0.01. (d) Relative proliferation at 24, 48, 72, 96 h. *P < 0.05. (e) Flow cytometry assay for the cell cycle. (f) Relative number of cells in S phase. *P < 0.005. (g) Flow cytometry assay for apoptosis. (h) Relative cell number of the apoptosis rate. *P < 0.05. (i) Transwell assay for cell invasion. (j) Migrated cells per field. *P < 0.005. (k) Western blotting for molecules critical for cell proliferation, apoptosis, and invasion. Akt, protein kinase B; GSK3, glycogen synthase kinase 3; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog. (b–d, f, h, j) Error bars indicate SD.
Next, we used a Transwell assay to investigate the role of COMT in tumor cell invasion. Significantly decreased invasion ability was observed when COMT was overexpressed (Fig.2i,j).
To illustrate the underlying molecular mechanism for these observations, we used Western blot to measure the expression levels of related molecules (Fig.2k). COMT overexpression was related to downregulation of p-Akt and upregulation of p-GSK3-β. However, no significant difference was observed in the levels of p-PI3K or p-GSK3-α. Mutant p53 was downregulated. Cyclin D1 was downregulated, whereas PTEN was upregulated; p21 was not significantly altered. Pro-apoptotic molecules (Bim and Bax) were upregulated, whereas the anti-apoptotic indicator p-Bad was downregulated in response to COMT overexpression. The invasion suppressor molecule E-cadherin was upregulated when COMT was overexpressed, which rendered the reduced invasion capacity of the COMT-OE cells.
Similarly, both transiently transfected cell lines showed reduced proliferative capacity and S-phase cell proportions (Figs S1,S2), increased gemcitabine-induced apoptosis, and limited ability for invasion (data not shown). The detailed Western blot of two transiently transfected cell lines are shown in Figure3.
Fig 3.
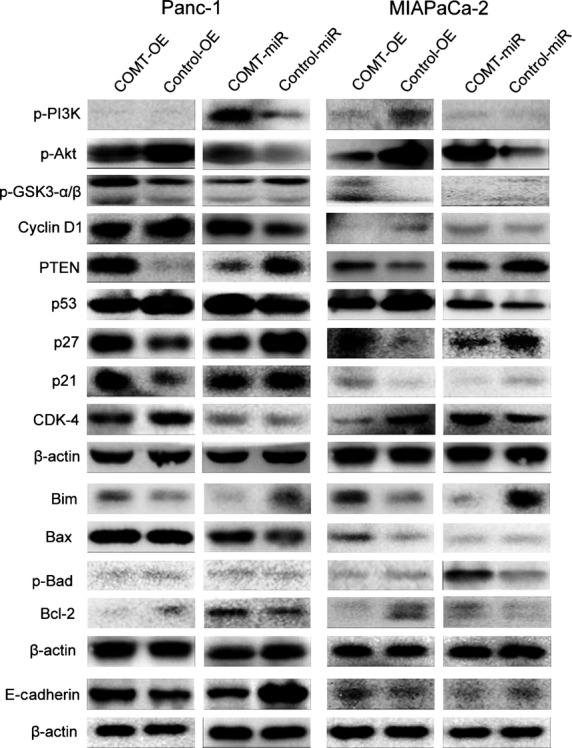
Western blot analysis of two transiently transfected prostate cancer cell lines, Panc-1 and MIAPaCa-2. Akt, protein kinase B; COMT, catechol-O-methyltransferase; COMT-OE, COMT protein overexpression cell lines; COMT-miR, miRNA-interfering COMT cell line; Control-OE, normal COMT protein expression; Control-miR Control-COMT cell line, without miRNA-interference of COMT expression; GSK3, glycogen synthase kinase 3; MB-COMT, membrane-binding COMT; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog.
Silencing of COMT promoted cell growth, increased S-phase cell ratio, inhibited apoptosis, and promoted invasion in vitro
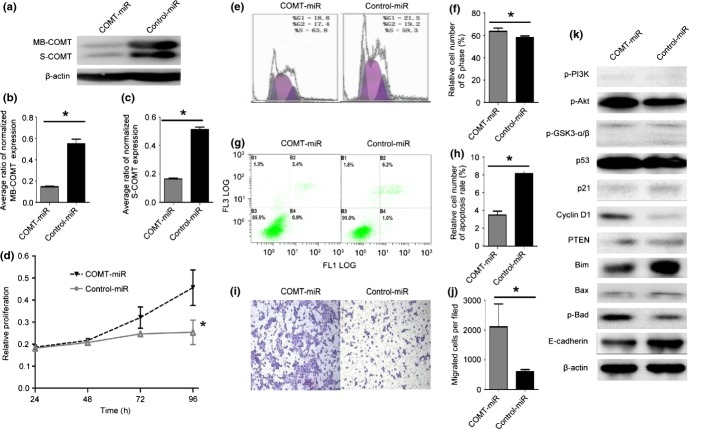
To further confirm that COMT suppressed growth and invasion and induced apoptosis, we silenced COMT expression by transiently transfecting two PC cell lines (Panc-1 and MIAPaCa-2) and stably transducing one PC cell line (BxPC-3) with siRNA targeting COMT.
In the stably transduced BxPC-3 cell line, the S-COMT level was only one-third of the original level, while MB-COMT was only one quarter of the original level (Fig.4a–c) and silencing of COMT significantly increased the proliferative capacity (Fig.4d). As expected, silencing of COMT significantly increased the proportions of cells in S phase, and significantly reduced gemcitabine-induced apoptosis (Fig.4e–h). The Transwell assay revealed that COMT silencing was associated with increased invasion capacity (Fig.4i,j). These changes also occurred in the two transiently transfected PC cell lines (data not shown).
Fig 4.
Catechol-O-methyltransferase (COMT) downregulation promotes cell growth, increases the S-phase cell ratio, reduces apoptosis, and promotes invasion in vitro. (a) Western blot for COMT proteins for transduction. (b) Average ratio of normalized membrane-binding COMT (MB-COMT) expression. *P < 0.0001. COMT-miR, cell lines stably transfected with siRNA; Control-miR, normal COMT protein expression. (c) Average ratio of normalized soluble COMT (S-COMT) expression. *P < 0.0001. (d) Relative proliferation at 24, 48, 72, and 96 h. *P < 0.05. (e) Flow cytometry assay for the cell cycle. (f) Relative number of cells in S phase. *P < 0.05. (g) Flow cytometry assay for apoptosis. (h) Relative cell number of the apoptosis rate. *P < 0.001. (i) Transwell assay for cell invasion. (j) Migrated cells per field. *P < 0.05. (k) Western blotting for molecules critical for cell proliferation, apoptosis, and invasion. Akt, protein kinase B; GSK3, glycogen synthase kinase 3; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog. (b–d, f, h, j) Error bars indicate SD.
We also used Western blot to measure the expression levels of related molecules (Fig.4k). Downregulation of COMT was related to upregulation of p-Akt and downregulation of p-GSK3-β. However, no significant change was observed in p-PI3K, p-GSK3-α or mutant p53. Downregulation was observed for p21 and PTEN, and upregulation was observed for cyclin D1. Two pro-apoptotic molecules (Bim and Bax) were downregulated, whereas the anti-apoptotic indicator p-Bad was upregulated in response to COMT downregulation. The invasion suppressor molecule E-cadherin was downregulated in response to COMT downregulation, which resulted in the increased capacity of COMT-miR cells for invasion. The detailed Western blot results for the two transiently transfected PC cell lines are shown in Figure3.
Pancreatic cancer cell tumor growth suppressed by COMT in vivo in nude mice
To further investigate whether COMT suppresses pancreatic tumor growth in vivo, we s.c. injected four groups of stably transduced BxPC-3 pancreatic cell lines into the subaxillary regions of BALB/c nude mice (Fig.5a). All tumor specimens were stained with Ki-67 to illustrate the “active proliferation zone,” which was present in the center of, or next to, the necrotic lumen in all mice specimens. Figure5(b) shows three typical patterns of active proliferation zone with different necrotic lumen diameters. Thus, to evaluate the proliferation speed of each group, we used tumor volume and weight. Significantly reduced tumor volume and weight were observed when the COMT protein was overexpressed in COMT-OE cells compared with Control-OE cells. Conversely, significantly increased tumor volume and non-significantly increased weight were observed when the COMT protein was silenced in COMT-miR cells compared with Control-miR cells (Fig.5). These results indicated that COMT protein could suppress pancreatic tumor growth in vivo.
Fig 5.

Catechol-O-methyltransferase (COMT) suppresses pancreatic tumor growth in BALB/c nude mice. (a) Gross appearance of pancreatic tumors from BALB/c nude mice in four groups. COMT-miR, COMT protein silencing cell lines; COMT-OE, COMT protein overexpression cell lines; Control-miR, control for COMT-miR cell lines with normal COMT protein expression; Control-OE, control for COMT-OE cell lines with normal COMT protein expression. (b) Illustration of Ki-67-positive cells in specimens of the xenograft model with different central necrotic lumen diameters. (c) Volume of tumors in each group. *P < 0.01; **P < 0.01. (d) Weight of tumors in each group. *P < 0.05; **P = 0.0585. (c, d) Error bars indicate SD.
Discussion
As an enzyme involved estrogen catabolism, COMT has long been a focus of research due to its biological functions in many types of estrogen-dependent cancers, such as breast cancer, endometrial carcinoma, and colorectal cancer. However, the prognostic value and biological function of COMT in PC has rarely been addressed. The COMT expression predicted a longer overall survival time, based on the survival analysis of the 85 patients whose samples were submitted to the IHC assay, suggesting that COMT could serve as a potential prognostic marker for patient survival. Therefore, more work was needed to explain this phenomenon.
We proposed that COMT suppresses PC progression. We carried out experiments to overexpress or silence the COMT level in two human PC cell lines (Panc-1 and MIAPaCa-2) by transient transfection and in one PC cell line (BxPC-3) by stable transduction. Cell growth, apoptosis, and invasion were influenced by COMT in in vitro experiments, and growth capacity was influenced in in vivo xenograft experiments. These results indicated that COMT could induce G1 cell cycle arrest, reduce cell proliferation and invasion capacity, and increase gemcitabine-induced apoptosis. Thus, COMT would slow the progression of PC and prolong survival time.
The initiation and progression of PC involves abnormal activation or inhibition of multiple intracellular signaling pathways. Single-target therapy has minimal effects on reversing the progression of this disease. Thus, the combination of multiple-target therapeutic agents with traditional chemotherapeutic agents, such as gemcitabine and 5-fluorouracil, will be a promising direction for prolonging the lives of patients.
The PI3K/Akt/mTOR pathway is well known for its important role in the regulation of cell growth and apoptosis, and its abnormal activation is present in the majority of PC.23,24 However, mTOR inhibitor alone or together with epidermal growth factor receptor inhibitor failed in phase II clinical trials, possibly due to the loss of feedback inhibition of upstream molecules such as PI3K and Akt.25–27 To improve the efficacy of mTOR inhibition agents, it is necessary to suppress the activation of upstream molecules such as PI3K or Akt. Our results showed that COMT suppressed the activation of Akt and activated PTEN, an upstream suppressor of the PI3K/Akt/mTOR pathway. Although the mechanism of this regulation is unknown, we observed similar regulation between COMT and the PI3K/mTOR pathway in colorectal cancer cells.20
In addition to the PI3K/Akt/mTOR pathway, COMT regulates other molecules such as p53, cyclin D1, and p21. The p53 gene is frequently mutated in PC, and mutant p53 promotes growth and metastasis.28 Cyclin D1 is required for the G1/S transition. Therefore, COMT could downregulate mutant p53 and cyclin D1 expression.
We also measured pro-apoptotic molecules (Bim and Bax), which were upregulated. The anti-apoptotic indicator p-Bad was downregulated in response to COMT overexpression. E-cadherin is a member of a transmembrane receptor family that is involved in tumor metastasis.29 We found that overexpression of COMT significantly impaired tumor invasion by increasing E-cadherin expression.
Catechol-O-methyltransferase is a well-known enzyme for estrogen catabolism. We admit that the present study only focused on the regulation of COMT with mTOR signaling; the antitumor effect of COMT could possibly be due to the alteration of metabolic products of estrogen.
In conclusion, COMT could serve as a candidate for targeted therapy. The interaction between COMT and upstream members of the PI3K/Akt/mTOR pathway should raise consideration for combining COMT with mTOR inhibitors as a new regimen to treat PC.
Acknowledgments
We thank Prof. Helmut Freiss from the Technical University of Munich (Munich, Germany) for providing us with the pancreatic cancer cell lines used in this study. This research was funded by the National Natural Sciences Foundation of China (Grant No. 81272768).
Glossary
Abbreviations
- AKT
protein kinase B
- COMT
catechol-O-methyltransferase
- COMT-OE
COMT-overexpression cell line
- COMT-miR
miRNA-interfering COMT cell line
- Control-OE
control-COMT cell line, without COMT overexpression
- Control-miR
control-COMT cell line, without miRNA-interference of COMT expression
- GSK3
glycogen synthase kinase 3
- IHC
immunohistochemistry
- MB-COMT
membrane-binding COMT
- mTOR
mammalian target of rapamycin
- PC
pancreatic cancer
- PI
propidium iodide
- PI3K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homolog
- PUMCH
Peking Union Medical College Hospital
- S-COMT
soluble COMT
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1. Relationship between catechol-O-methyltransferase (COMT) expression and clinicopathological parameters in pancreatic cancer.
Fig. S1. Cell growth of transiently transfected Panc-1 and MIAPaCa-2 pancreatic cancer cell lines.
Fig. S2. S-phase cell ratio of transiently transfected Panc-1 and MIAPaCa-2 pancreatic cancer cell lines.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Chang CY, Huang SP, Chiu HM, Lee YC, Chen MF, Lin JT. Low efficacy of serum levels of CA 19-9 in prediction of malignant diseases in asymptomatic population in Taiwan. Hepatogastroenterology. 2006;53:1–4. [PubMed] [Google Scholar]
- Tessler DA, Catanzaro A, Velanovich V, Havstad S, Goel S. Predictors of cancer in patients with suspected pancreatic malignancy without a tissue diagnosis. Am J Surg. 2006;191:191–7. doi: 10.1016/j.amjsurg.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Sergeant G, Vankelecom H, Gremeaux L, Topal B. Role of cancer stem cells in pancreatic ductal adenocarcinoma. Nat Rev Clin Oncol. 2009;6:580–6. doi: 10.1038/nrclinonc.2009.127. [DOI] [PubMed] [Google Scholar]
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- Pasetto LM, Jirillo A, Stefani M, Monfardini S. Old and new drugs in systemic therapy of pancreatic cancer. Crit Rev Oncol Hematol. 2004;49:135–51. doi: 10.1016/S1040-8428(03)00170-7. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- Amin AM, Creveling CR, Lowe MC. Immunohistochemical localization of catechol methyltransferase in normal and cancerous breast tissues of mice and rats. J Natl Cancer Inst. 1983;70:337–42. [PubMed] [Google Scholar]
- Raftogianis R, Creveling C, Weinshilboum R, Weisz J. Estrogen metabolism by conjugation. J Natl Cancer Inst Monogr. 2000;27:113–24. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- Andersen S, Skorpen F. Variation in the COMT gene: implications for pain perception and pain treatment. Pharmacogenomics. 2009;10:669–84. doi: 10.2217/pgs.09.13. [DOI] [PubMed] [Google Scholar]
- Service RF. New role for estrogen in cancer? Science. 1998;279:1631–3. doi: 10.1126/science.279.5357.1631. [DOI] [PubMed] [Google Scholar]
- Akanni A, Abul-Hajj YJ. Estrogen-nucleic acid adducts: reaction of 3,4-estrone-o-quinone radical anion with deoxyribonucleosides. Chem Res Toxicol. 1997;10:760–6. doi: 10.1021/tx970026c. [DOI] [PubMed] [Google Scholar]
- Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–22. [PubMed] [Google Scholar]
- Thompson PA, Shields PG, Freudenheim JL, et al. Genetic polymorphisms in catechol-O-methyltransferase, menopausal status, and breast cancer risk. Cancer Res. 1998;58:2107–10. [PubMed] [Google Scholar]
- Mao C, Wang XW, Qiu LX, Liao RY, Ding H, Chen Q. Lack of association between catechol-O-methyltransferase Val108/158Met polymorphism and breast cancer risk: a meta-analysis of 25,627 cases and 34,222 controls. Breast Cancer Res Treat. 2010;121:719–25. doi: 10.1007/s10549-009-0650-4. [DOI] [PubMed] [Google Scholar]
- Huang CS, Chern HD, Chang KJ, Cheng CW, Hsu SM, Shen CY. Breast cancer risk associated with genotype polymorphism of the estrogen-metabolizing genes CYP17, CYP1A1, and COMT: a multigenic study on cancer susceptibility. Cancer Res. 1999;59:4870–5. [PubMed] [Google Scholar]
- Sasaki M, Kaneuchi M, Sakuragi N, Dahiya R. Multiple promoters of catechol-O-methyltransferase gene are selectively inactivated by CpG hypermethylation in endometrial cancer. Cancer Res. 2003;63:3101–6. [PubMed] [Google Scholar]
- Wu WM, Zhao YP, Liao Q, Zhang TP. The expression of catechol O-methyltransferase gene in colorectal cancer. Zhonghua Wai Ke Za Zhi. 2010;48:535–8. [PubMed] [Google Scholar]
- Wu W, Wu Q, Hong X, et al. Catechol-O-methyltransferase inhibits colorectal cancer cell proliferation and invasion. Arch Med Res. 2014 doi: 10.1016/j.arcmed.2014.12.004. ; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wang WB, Zhao YP, Liao Q, Zhang TP, Wu WM, Wu YD. Candidate immunogenic membrane antigens of human pancreatic cancer. Zhonghua Wai Ke Za Zhi. 2010;48:1412–5. [PubMed] [Google Scholar]
- Wu W, Zhang J, Zhou L, You L, Zhao Y, Li J. Increased COMT expression in pancreatic cancer and correlation with clinicopathologic parameters. Sci China Life Sci. 2012;55:747–52. doi: 10.1007/s11427-012-4375-y. [DOI] [PubMed] [Google Scholar]
- Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–76. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- Arlt A, Gehrz A, Muerkoster S, et al. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–51. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- Kordes S, Richel DJ, Klumpen HJ, Weterman MJ, Stevens AJ, Wilmink JW. A phase I/II, non-randomized, feasibility/safety and efficacy study of the combination of everolimus, cetuximab and capecitabine in patients with advanced pancreatic cancer. Invest New Drugs. 2013;31:85–91. doi: 10.1007/s10637-012-9802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javle MM, Shroff RT, Xiong H, et al. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer. 2010;10:368. doi: 10.1186/1471-2407-10-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpin BM, Hezel AF, Abrams T, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–8. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JP, Timpson P, Karim SA, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:246–51. doi: 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci U S A. 1995;92:7416–9. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relationship between catechol-O-methyltransferase (COMT) expression and clinicopathological parameters in pancreatic cancer.
Fig. S1. Cell growth of transiently transfected Panc-1 and MIAPaCa-2 pancreatic cancer cell lines.
Fig. S2. S-phase cell ratio of transiently transfected Panc-1 and MIAPaCa-2 pancreatic cancer cell lines.