Abstract
The BRCA/Fanconi anemia (FA) pathway plays a key role in the repair of DNA double strand breaks. We focused on this pathway to clarify chemoresistance mechanisms in biliary tract cancer (BTC). We also investigated changes in the CD24+/44+ population that may be involved in chemoresistance, as this population likely includes cancer stem cells. We used three BTC cell lines to establish gemcitabine (GEM)-resistant (GR) cells and evaluated the expression of BRCA/FA pathway components, chemoresistance, and the effect of BRCA/FA pathway inhibition on the CD24+/44+ population. FANCD2 and CD24 expression were evaluated in 108 resected BTC specimens. GR cells highly expressed the BRCA/FA components. The BRCA/FA pathway was upregulated by GEM and cisplatin (CDDP) exposure. Inhibition using siRNA and RAD51 inhibitor sensitized GR cells to GEM or CDDP. The CD24+/44+ population was increased in GR and parent BTC cells treated with GEM or CDDP and highly expressed BRCA/FA genes. FANCD2 was related to CD24 expression in resected BTC specimens. Inhibition of the BRCA/FA pathway under GEM reduced the CD24+/44+ population in MzChA1-GR cells. Thus, high expression of the BRCA/FA pathway is one mechanism of chemoresistance against GEM and/or CDDP and is related to the CD24+/44+ population in BTC.
Keywords: BRCA2, CD24, FANCD2, Fanconi anemia, RAD51c
Biliary tract cancer (BTC) is a malignancy with a poor prognosis and increasing incidence worldwide.1 Complete surgical resection is the only potentially curative therapy for patients with BTC;2–4 however, no effective chemotherapy is available for patients with unresectable BTC. Gemcitabine (GEM)-based chemotherapy is an standard treatment, and cisplatin (CDDP) plus GEM can prolong survival.1,2,5 However, no other first-line or second-line chemotherapies are available.6–9 Thus, it is important to explore the mechanism of GEM resistance in BTC.
In the present study, we focused on the DNA damage response (DDR) in several potential mechanisms of chemoresistance. Chemoresistance was recently reported to be associated with the DNA repair genes ERCC1, RAD21, MGMT, PARP1, PARP2, BRCA1, BRCA2, MSH2, MSH6, FAN1 and XRCC1 in various cancers, including lung, colon, breast, ovary and stomach cancer.10–16 GEM is a nucleoside analogue that inhibits DNA elongation and ribonucleotide reductase.17 In addition, GEM may contribute to DNA damage18 and cells may be sensitized by the inhibition of checkpoint kinase 1 (CHK1), which coordinates the DDR,19 suggesting that other DDR proteins are involved in chemoresistance.
The DDR generally protects against genomic instability, which enables cancer development.20,21 Among the DDR-related genes, the BRCA/Fanconi anemia (FA) pathway genes play a role in homologous recombination repair (HRR), particularly the repair of fatal DNA double strand breaks,22 and are related to the development of several cancers. BRCA/FA pathway genes are well known tumor suppressors,21–23 but the percentage of mutations is limited.23 However, the downregulation of BRCA2 may cause radio-sensitization20 and chemosensitization.24,25 We hypothesized that upregulation of BRCA/FA pathway components caused chemoresistance in BTC.
Our objective was to investigate the role of the BRCA/FA pathway in BTC, focusing on several key molecules in the BRCA/FA pathway. In addition to BRCA2, FANCD2 is a central gene in this pathway and associated with cell cycle control at the S/G2 checkpoint. RAD51c is a final factor in this pathway and directly repairs DNA damage in combination with other components.22 We also investigated the relationship between the DDR and CD24+/44+ population, which was reported as a candidate marker for extracting cancer stem cells (CSC) in BTC.26 Enrichment of CSC is a well-known mechanism of chemoresistance.27 The DDR works in stem cells28 and may contribute to the CSC-like population in several cancers.29,30 Our results demonstrate that inhibition of the BRCA/FA pathway not only sensitizes BTC cells to GEM or CDDP, but also reduces the CD24+/44+ population in BTC.
Materials and Methods
Establishment of gemcitabine-resistant biliary tract cancer cells (MzChA1-GR, CCLP1-GR and KMCH1-GR)
Human BTC cell lines (MzChA1, CCLP1 and KMCH1) were kindly provided by Dr Gregory J. Gores of the Mayo Clinic, Rochester, MN, USA.31–34 The GEM-resistant MzChA1 cell line (MzChA1-GR) was recently established in our department.35 The primary MzChA1-GR cell line was developed through exposure to increasing concentrations of GEM (0.2–2.0 ng/mL) with repeated subculturing until the cells became fully resistant. Primary MzChA1-GR cells were cultured in GEM-free medium for 3 weeks prior to the next limiting dilution. After the primary MzChA1-GR cells were confirmed to be significantly more resistant to GEM than the parent cells, a single MzChA1-GR cell was seeded in a 96-well microplate by limiting dilution. Eight MzChA1-GR clones were established from the primary MzChA1-GR cell. To reduce the risk of contamination, we cultured each cell line separately with 6 months interval and each GR cell was also established by different two scientists. The MzChA1-GR cells were cultured under the same conditions as other cell lines, without GEM.35 The concordances of short tandem repeat were 21% in MzChA1 and MzChA1-GR, 81% in CCLP1 and CCLP1-GR, and 90% in KMCH1 and KMCH1-GR (BEX, Tokyo, Japan) like as the previous report included the data of STR changes by DNA damage drug treatment.36
The GEM-resistant CCLP1 cell line (CCLP1-GR) and the GEM-resistant KMCH1 cell line (KMCH1-GR) were developed through exposure to increasing concentrations of GEM (CCLP1, from 5 to 300 ng/mL; KMCH1, from 0.3 to 100 ng/mL) and established using the same method as MzChA1-GR.
Microarray analysis
DNA microarray analysis was performed using a 3D-Gene Human Oligo chip 25k (Toray Industries, Tokyo, Japan). We compared the MzChA1-parent and three MzChA1-GR clones. We determined that RRM1 and dCK mRNA levels were generally upregulated in all GR cells. The normalized data were used to identify genes whose expression appeared to be upregulated or downregulated.
Immunocytochemistry
Immunocytochemistry studies of γH2AX were performed using BTC cells. As a positive control, we also assessed BTC cells 4 h after 6 Gray irradiation using a Gamma Cell 40 Exactor (Nordion International, Ottawa, ON, Canada). Briefly, cells were cultured on six-well chamber slides, fixed with 4% paraformaldehyde, and permeabilized. The cells were then incubated with monoclonal mouse anti-γH2AX (diluted 1:500 [Millipore, Billerica, MA, USA]), followed by Alexa Fluor anti-mouse IgG conjugated to Alexa Fluor 488 (diluted 1:500 [Cell Signaling Technology, Danvers, MA, USA]). The slides were viewed by fluorescence microscopy (BZ-8000 [Keyence, Osaka, Japan]).
Flow cytometry for CD24, CD44 and CD133
The isolation of only CSC is difficult; therefore, we evaluated several populations that have been reported to contain a high concentration of CSC. In BTC, CD24, CD44 and CD133 have been reported to be markers for the selection of CSC-like populations.26,37–40 To analyze the cell surface markers by flow cytometry, cells were resuspended in PBS with 1% FBS at a concentration of 106 cells/100 μL and incubated for 30 min at room temperature with 100-fold dilutions of the following antibodies: anti-CD24-phycoerythrin (BD Biosciences, Mississauga, ON, Canada, 555428), anti-CD44-fluorescein isothiocyanate (BD Biosciences, 559942) and anti-CD133/1-allophycocyanin (Miltenyi Biotec, Bergisch Gladbach, Germany 130-090-826). After incubation, the samples were washed twice with PBS containing 1% FBS and resuspended in PBS containing 1% FBS. Dead cells were eliminated by adding 4′,6-diamidino-2-phenylindole (final concentration 1 μg/mL; Sigma, Tokyo, Japan). Flow cytometric analysis was performed using a FACSAria (BD Immunocytometry System, Franklin Lakes, NJ, USA) after collecting and staining the cells as described earlier. The cells were routinely sorted twice and re-analyzed for purity, which was typically >90%.
Clinical samples
Biliary tract cancer samples (n = 108) were obtained from patients who underwent resection surgery at Osaka University Hospital, Japan between 2004 and 2012. All of the patients were diagnosed with BTC based on clinicopathological findings. Patient characteristics were collected prospectively by the Cancer Board and confirmed by the Clinico-Pathological Conference. The mean patient age was 63 ± 13 years, and the male-to-female ratio was 3:2. The main tumor locations were intrahepatic bile duct (n = 21), extrahepatic bile duct (n = 55), gallbladder (n = 16) and papilla of Vater (n = 16). Fifty-two (48%) patients had pathological lymph node metastasis. Resected specimens were formalin-fixed and preserved in paraffin blocks before immunohistochemistry. The use of resected samples was approved by the Human Ethics Review Committee of the Graduate School of Medicine, Osaka University. Written informed consent was obtained from all patients included in the study.
Classification of immunohistochemistry
FANCD2 and CD24 immunostaining were categorized as described previously.41,42 Briefly, we considered samples to be FANCD2-negative when the staining area in the nucleus was <1% or FANCD2-positive if the staining area was 1–25% (weak), 25–50% (moderate) or ≥50% (strong). We considered samples as CD24-negative when the staining area was 0% or CD24-positive if the staining area was <20% (weak), 20–50% (moderate) or ≥50%. FANCD2 and CD24 immunostaining was classified by two authors (S. N and S. K) with agreement in all cases.
Statistical analysis
All data were expressed as the mean ± standard deviation of at least three independent experiments. Statistical analyses were performed using Student's t-test or Fisher's exact test for categorical data. The unpaired Student's t-test was used to examine differences in the growth inhibitory effects in vitro. P-values < 0.05 were considered significant.
Results
Establishment and characteristics of gemcitabine-resistant biliary tract cancer cells
We confirmed that MzChA1-GR cells maintained GEM-resistance compared to MzChA1-parent cells. Three MzChA1-GR clones were resistant to GEM (IC50 >100 ng/mL; P < 0.001; Fig.1a). MzChA1-GR cells were also resistant to CDDP (IC50, 3.18 μg/mL; P < 0.001; Fig.1b). We evaluated γH2AX expression, a critical event in the mammalian DDR, 72 h after GEM (1.0 ng/mL) or CDDP (0.03 μg/mL) exposure. The positive control for γH2AX expression was irradiated cells. γH2AX was expressed at higher levels, and more cells formed nuclear foci in MzChA1-parent cells compared to GR cells (Fig.1c,d). Thus, DNA damage was reduced in GR cells. Proliferation was more rapid in GR cells (Fig. S1a, P < 0.01) and the cell cycle distribution was not different between MzChA1-parent and GR cells (Fig. S1b). Five CCLP1-GR clones and three KMCH1-GR clones were established and resistant to GEM, as well as CDDP (Fig. S2, P < 0.001). The morphologies of MzChA1-GR, KMCH1-GR and CCLP1-GR cells were spindle-shaped and disconnected (Fig.3).
Fig 1.
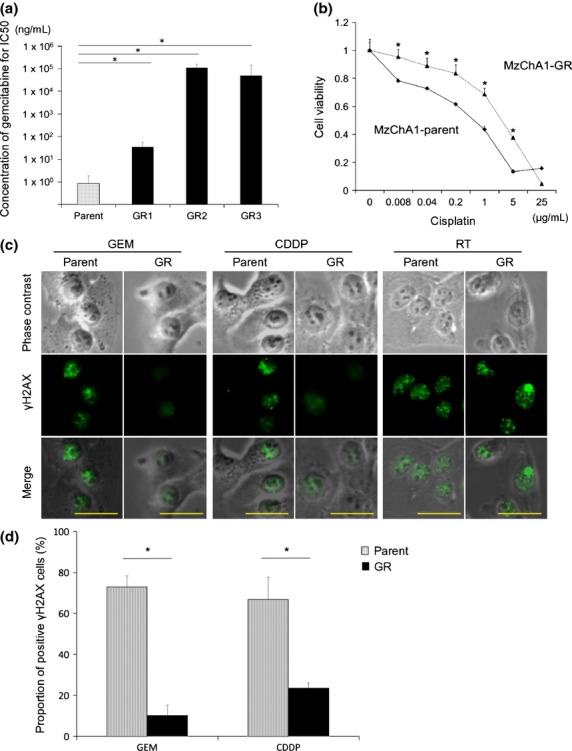
Establishment and characteristics of gemcitabine (GEM)-resistant biliary tract cancer (BTC) cells. (a) IC50 of MzChA1-parent and GEM-resistant (GR) clones for GEM. MTT assays were performed for GEM using MzChA1-parent cells and three GR clones (GR1-GR3). The x-axis indicates the parent cells and GR1-GR3. (b) Chemosensitivity against cisplatin for MzChA1-parent and GR cells. MTT assays were performed for cisplatin using MzChA1-parent and GR cells. The relative ratio of cell viability was calculated by absorption. (c) Immunocytochemistry for γH2AX. MzChA1-parent and GR cells were exposed to GEM (1.0 ng/mL) and cisplatin (0.03 μg/mL) for 72 h. These cells were also irradiated as a positive control. For each treated cell, we show phase contrast, γH2AX staining and a merged image. Scale bar = 50 μm. (d) Quantification of γH2AX expression. The average proportion of γH2AX-positive cells was evaluated in three fields. *P < 0.001.
Fig 3.
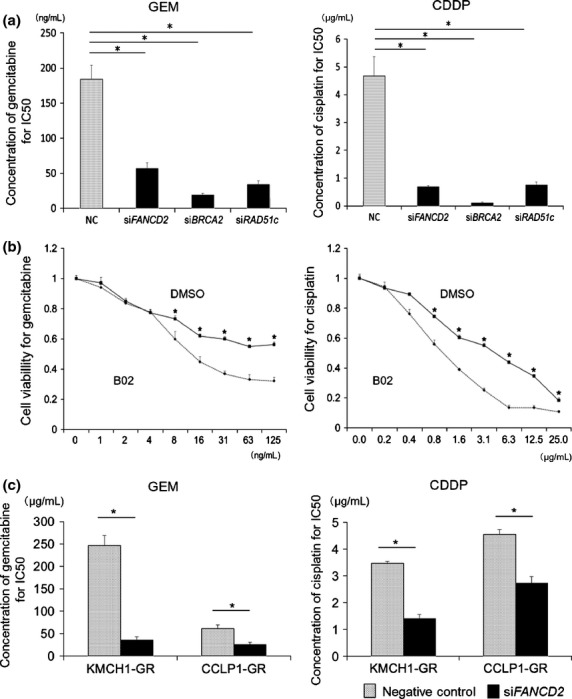
Effect of inhibiting the BRCA/FA pathway on chemoresistance. (a) Change in the IC50 for gemcitabine (GEM) and cisplatin in MzChA1-GR cells transfected with siFANCD2, siBRCA2 and siRAD51c. (b) MTT assays were performed for GEM and cisplatin using MzChA1-GR cells with or without B02 (1.0 μg/mL). (c) KMCH1-GR cells and CCLP1-GR cells were transfected with siFANCD2 and analyzed by western blotting. The IC50 values for GEM and cisplatin were shown in KMCH1-GR and CCLP1-GR cells transfected with siFANCD2. Black bar, GR cells transfected with siFANCD2; dotted bar, negative control; *P < 0.001.
Microarray analysis of MzChA1-parent and gemcitabine-resistant cells
In a microarray analysis of MzChA1-parent and MzChA1-GR cells, we evaluated the expression of genes involved in the major mechanisms of the DNA repair pathway (homologous recombination repair, non-homologous end joining, direct reversal repair, mismatch repair, base excision repair and nucleotide excision repair).43 Almost all components of the BRCA/FA pathway were upregulated in MzChA1-GR cells (Table1).
Table 1.
BRCA/Fanconi anemia genes in microarray
| Parent | GR1 | GR2 | GR3 | Fold change | |
|---|---|---|---|---|---|
| FANCA | 11 | 20 | 19 | 16 | 1.64 |
| FANCB | 8 | 32 | 24 | 31 | 3.53 |
| FANCC | 30 | 40 | 44 | 41 | 1.40 |
| FANCD1 (BRCA2) | 14 | 48 | 37 | 41 | 3.03 |
| FANCD2 | 3 | 10 | 9 | 9 | 3.55 |
| FANCE | 8 | 12 | 12 | 15 | 1.71 |
| FANCF | 95 | 93 | 116 | 125 | 1.17 |
| FANCG | 18 | 55 | 45 | 53 | 2.80 |
| FANCI | 76 | 534 | 401 | 504 | 6.35 |
| FANCJ (BRIP1) | 3 | 11 | 11 | 7 | 3.50 |
| FANCL | 157 | 305 | 239 | 193 | 1.57 |
| FANCM | 2 | 7 | 5 | 5 | 3.54 |
| FANCN (PALB2) | 126 | 167 | 155 | 171 | 1.31 |
| FANCP (SLX4) | 41 | 36 | 34 | 38 | 0.87 |
| FANCO (RAD51c) | 44 | 77 | 74 | 80 | 1.75 |
Expression of BRCA/Fanconi anemia pathway mRNA and proteins in gemcitabine-resistant biliary tract cancer cells
We confirmed the expression of BRCA2, FANCD2, FANCI and RAD51c mRNA in MzChA1-parent and MzChA1-GR cells. We used the gene-specific oligonucleotide primers provided in Table S1. All mRNA were expressed at significantly higher levels in MzChA1-GR cells than MzChA1-parent cells (P < 0.001; Fig.2a). Western blot analysis revealed that the expression of BRCA2, FANCD2 and RAD51c proteins was elevated in MzChA1-GR cells (Fig.2b). We confirmed that these proteins were also elevated in CCLP1-GR and KMCH1-GR cells (Fig.2c). Accordingly, long-term GEM exposure (i.e. in resistant cells) elevated mRNA and protein levels in the BRCA/FA pathway.
Fig 2.
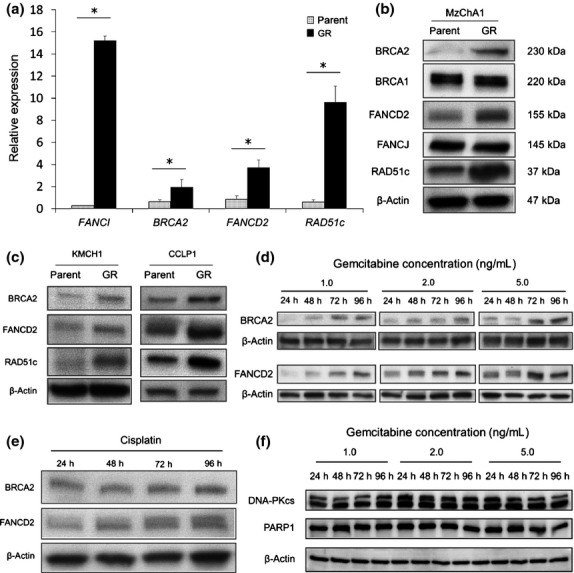
DNA repair pathway in parent and gemcitabine (GEM)-resistant BTC cells. (a) qRT-PCR and (b) western blotting of BRCA/FA pathway proteins in MzChA1-parent and GEM-resistant (GR) cells. (c) Western blotting of BRCA/FA pathway proteins in CCLP1-parent, CCLP1-GR, KMCH1-parent and KMCH1-GR cells. (d) Expression of BRCA/FA proteins in MzChA1 cells as a function of GEM exposure. MzChA1 cells were treated with GEM (1.0, 2.0, or 5.0 ng/mL) for 24, 48, 72 and 96 h. Total proteins were extracted for immunoblotting for FANCD2 and BRCA2. (e) Expression of BRCA/FA proteins in MzChA1 cells exposed to cisplatin (0.1 μg/mL) for 24, 48, 72 and 96 h. (f) Expression of DNA-dependent protein kinase catalytic subunit (DNA-PKc) and PARP1 proteins in MzChA1 cells exposed to GEM. Cells were treated at the indicated concentrations and times. *P < 0.001.
Expression of BRCA/Fanconi anemia pathway components in parent biliary tract cancer cells after gemcitabine or cisplatin exposure
We evaluated changes in BRCA/FA proteins in MzChA1 cells after GEM exposure. The MzChA1 cells were treated with concentrated GEM (1.0, 2.0 or 5.0 ng/mL) for 24, 48, 72 and 96 h. Total proteins were extracted for immunoblotting of BRCA2 and FANCD2. The expression of BRCA2 and FANCD2 protein increased in a time-dependent and dose-dependent manner (Fig.2d). The expression of these two proteins also increased after CDDP treatment (0.03 μg/mL) (Fig.2e). However, we did not observe gradual higher expression of PARP1 and DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which are involved in other DNA repair pathways (Fig.2f). Taken together, the results indicate that short-term GEM or CDDP exposure increases the expression of the BRCA/FA pathway in the DNA repair system.
Effect of inhibiting the BRCA/Fanconi anemia pathway on chemoresistance
Next, we assessed BRCA2, FANCD2 and RAD51c mRNA and protein expression using MzChA1-GR cells transfected with siRNA (siFANCD2, siBRCA2, siRAD51c). The expression of all mRNA and proteins was inhibited in transfected GR cells (Fig. S4). Inhibition of the BRCA/FA pathway sensitized cells to not only GEM but also CDDP (Fig. S5), and reduced the IC50 for GEM (NC, 183.8 ng/mL; siFANCD2, 57.1 ng/mL, P < 0.001; siBRCA2, 38.4 ng/mL, P < 0.001; siRAD51c, 48.8 ng/mL, P < 0.001; Fig.3a, left) and CDDP (NC, 4.67 μg/mL; siFANCD2, 0.69 μg/mL, P < 0.001; siBRCA2, 0.11 μg/mL, P < 0.001; siRAD51c; 0.77 μg/mL, P < 0.001; Fig.3a, right). Inhibition of RAD51c using B02, a specific RAD51 inhibitor binding nucleoprotein filament at the site of damaged DNA,44 also reduced the IC50 for GEM and CDDP (13.0 ng/mL and 1.05 μg/mL, respectively, P < 0.001; Fig.3b). The concentration of the inhibitor was determined as the maximum dose that did not influence proliferation (Fig. S6). We confirmed that inhibition of FANCD2 by siRNA (Fig. S7) reduced the IC50 for GEM and CDDP in KMCH1-GR and CCLP1-GR cells (KMCH1-GR, 35.8 and 1.40 μg/mL, P < 0.001; CCLP1-GR, 25.6 and 2.73 μg/mL, P < 0.001; Fig.3c). Thus, silencing the BRCA/FA pathway sensitized GR cells to GEM or CDDP.
CD24+/44+ population in parent and gemcitabine-resistant cells
Using FACS, the proportion of CD24+/44+ cells was higher in MzChA1-GR cells than MzChA1-parent cells (P = 0.035; Fig.4a). The same results were observed in KMCH1-GR and CCLP1-GR cells (KMCH1-GR, P < 0.001; CCLP1-GR, P = 0.048; Fig.4a, right). CD133+ cells were not detected in MzChA1-parent and MzChA1-GR cells, and few were detected in KMCH1 (0.2%) and KMCH1-GR cells (1.4%) (Fig. S8). The CD24+/44+ population was increased in MzChA1 cells after GEM or CDDP exposure for 72 h compared to no treatment (GEM, P = 0.030; CDDP, P = 0.031; Fig.4b). With short-term and long-term exposure to GEM, the CD24+/44+ population was increased in BTC cells.
Fig 4.
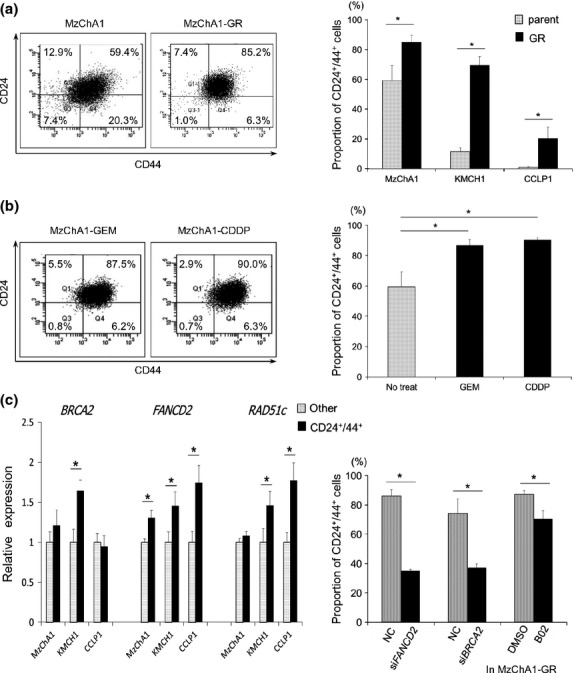
The CD24+/44+ population in biliary tract cancer (BTC) cells. (a) Left: representative figure of CD24+/44+ flow cytometry in MzChA1-parent and MzChA1-GR cells. Right: proportion of CD24+/44+ cells in MzChA1, KMCH1 and CCLP1 parent and GR cells. (b) Change in the CD24+/44+ population in MzChA1 cells treated with gemcitabine (GEM) (1.0 ng/mL) or cisplatin (0.1 μg/mL) for 72 h. (c) Expression of BRCA/FA pathway genes in the CD24+/44+ population of MzChA1, KMCH1 and CCLP1 cells by qRT-PCR. Black bar, CD24+/44+ cells; dotted bar, other cells. (d) Proportion of CD24+/44 MzChA1-GR cells transfected with siFANCD2 or siBRCA2 and MzChA1-GR cells treated with B02 after GEM (100 ng/mL) exposure for 72 h. *P < 0.05.
BRCA/Fanconi anemia pathway in CD24+/44+ biliary tract cancer cells
Next, MzChA1, CCLP1 and KMCH1 cells were sorted by CD24+/44+ expression. BRCA2, FANCD2 and RAD51c mRNA levels were determined in the CD24+/44+ population and other populations (CD24− or CD44−) by qRT-PCR. BRCA2 mRNA was expressed at significantly higher levels in the CD24+/44+ population of KMCH1 cells (P = 0.013; Fig.4c). FANCD2 mRNA levels were also higher in the CD24+/44+ populations of MzChA1 (P = 0.030), CCLP1 (P = 0.027) and KMCH1 (P = 0.045; Fig.4c) cells. RAD51c mRNA levels were higher in the CD24+/44+ population of CCLP1 (P = 0.026) and KMCH1 (P = 0.047; Fig.4c) cells. Therefore, BRCA/FA genes were expressed at higher levels in the CD24+/44+ population compared to the other population.
Effect of inhibiting the BRCA/Fanconi anemia pathway on the CD24+/44+ population
The proportion of CD24+/44+ cells decreased in MzChA1-GR cells transfected with siRNA and treated with GEM compared to MzChA1-GR cells transfected with scrambled oligonucleotide siRNA (siFANCD2, P = 0.001; siBRCA2, P = 0.018; Fig.4d). The CD24+/44+ population was also decreased in MzChA1-GR cells treated with GEM and B02 (P = 0.019; Fig.4d).
Expression of FANCD2 and CD24 in resected specimens
We evaluated FANCD2 staining in the nucleus and CD24 staining in the cytoplasm. Positive reactivity for FANCD2 was observed in 49 (45%) BTC specimens (Fig.5a[i,ii]), whereas 66 (61%) BTC specimens were positive for CD24 (Fig.5a[iii,iv], c and d). FANCD2 expression corresponded with CD24 expression (P = 0.001; Fig.5b).
Fig 5.
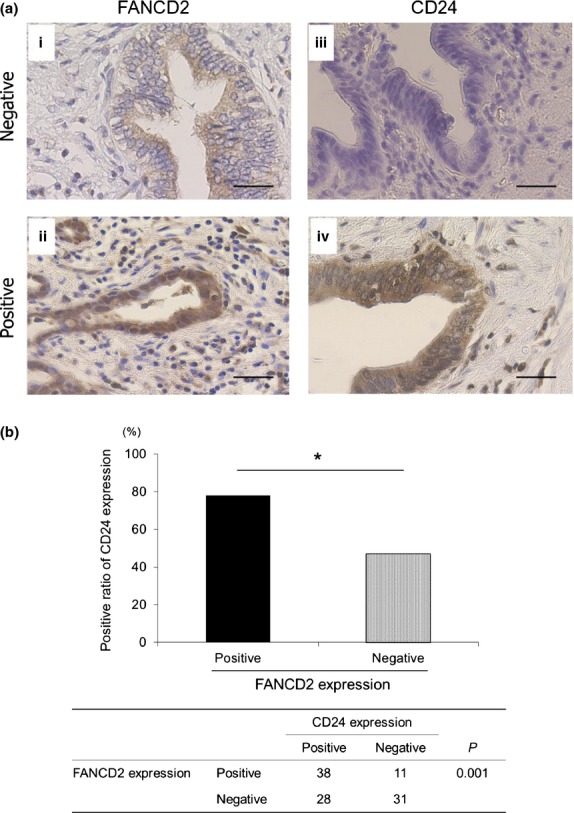
Expression of BRCA/FA proteins in resected biliary tract cancer (BTC) specimens. (a) Immunohistochemistry for FANCD2 and CD24 in resected specimens. (i) Representative figure of negative staining for FANCD2, (ii) positive staining for FANCD2, (iii) negative staining for CD24 and (iv) positive staining for CD24. Magnification ×100, scale bar = 100 μm. (b) Positive ratio of CD24 expression in FANCD2-positive and -negative specimens.
Discussion
Our data show that the BRCA/FA pathway is upregulated by short-term and long-term exposure to GEM, which contributes to GEM and/or CDDP resistance, and is closely related to the CD24+/44+ population. The main mechanism of action of GEM is inhibition of DNA elongation and ribonucleotide reductase,17 and GEM has been reported to cause DNA double strand breaks.18 DNA double strand breaks are also thought to be caused by radiation, topoisomerase inhibitors and DNA interstrand cross-linking (ICL) agents, such as CDDP or mitomycin C.21 Our data on γH2AX expression confirm that GEM, and CDDP, induces DNA double strand breaks.45,46 A recent report showed that CHK1, which coordinates the DDR, is essential for GEM sensitization,19 and our data support GEM being influenced by the DDR system. Two mechanisms are known for the repair of DNA double strand breaks: HRR and non-homologous end joining. The former involves the BRCA/FA pathway and specifically repairs “fatal DNA double strand breaks.”22 As BRCA-silenced cells were reported to be sensitized to not only CDDP, but also GEM,47 the BRCA/FA pathway would be necessary for the DDR after GEM or CDDP exposure, and this was supported by our results of silencing BRCA2, FANCD2 and RAD51c. In contrast, short-term and long-term exposure to GEM induced temporal and/or sustained elevated expression of BRCA/FA pathway genes, which could induce co-resistance against CDDP as in previous reports of lung cancer and pancreatic cancer.48–50 Notably, all of our BTC cells expressed BRCA/FA pathway genes. In BRCA2-mutated cancer cells, our speculation would be inappropriate. Thus, we need to address drug selection for first-line and second-line chemotherapies in cases of GEM-refractory BTC.
In this study, we also investigated the relationship between the BRCA/FA pathway and the CD24+/44+ population in BTC. The DDR should work in stem cells to escape “fatal DNA damage.”28 To address whether the DDR would work in CSC in BTC, we investigated the expression of BRCA/FA pathway genes in the CD24+/44+ population and how silencing BRCA/FA pathway genes affects these cells, as recent reports support the presence of highly concentrated CSC in the CD24+/44+ population in BTC.26,27,39 Our data show that the expression of BRCA/FA pathway components was elevated in the CD24+/44+ population and silencing of the BRCA/FA pathway contributed to a decrease in the CD24+/44+ population. The DDR may contribute to CSC-like populations in several cancers;29,30 thus, HRR may work in the CD24+/44+ population in BTC. Taken together, our results indicate that GEM exposure leads to enrichment of the CD24+/44+ population highly expressing BRCA/FA genes in BTC, which would cause co-resistance to GEM and CDDP.
Does regulation of the DDR contribute to a new strategy for treating BTC? Several phase I studies have started evaluating the DDR.21 Recent reports show that inhibition of the BRCA/FA pathway by siRNA (e.g. FANCF, BRCA1, and BRCA2) is effective in treating breast and ovarian cancers.51,52 Inhibiting the BRCA/FA pathway and decreasing the CD24+/44+ population in GEM-resistant BTC cells could be a new treatment for BTC, although we need to consider the influence on somatic cells and the drug delivery system. In conclusion, high expression of the components of the BRCA/FA pathway is one of the mechanisms of chemoresistance to GEM and/or CDDP and is related to the CD24+/44+ population in BTC.
Acknowledgments
This work was supported by the Mitsui Life Social Welfare Foundation and a Grant-in-Aid for Scientific Research (C).
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Proliferation (a) and cell cycle distribution (b) in MzChA1-parent andGEM-resistant (GR) cells. (a) Cells were seeded and cultured for 72 h. The graph shows the cell growth rate compared to living cells at 0 h. (b) The cell cycle distribution was evaluated by flow cytometry using cells stained with Hoechst 33342. *P < 0.01.
Fig. S2. Chemosensitivity of CCLP1-GR and KMCH1-GR cells to gemcitabine and cisplatin. MTT assays were performed for gemcitabine or cisplatin using CCLP1-parent or KMCH1-parent cells and GR clones. The GR clones are numbered 1–3 for KMCH1-GR and 1–5 for CCLP1-GR. The half maximal (50%) inhibitory concentration (IC50) is indicated on the y-axis. *P < 0.001. GR, GEM-resistant.
Fig. S3. Morphology of parent and GR biliary tract cancer (BTC) cells. Scale bar = 50 μm.
Fig. S4. Inhibition by siFANCD2, siBRCA2 and siRAD51c in MzChA1-GR cells was confirmed by qRT-PCR and western blotting. *P < 0.001.
Fig. S5. Effect of inhibiting the BRCA/FA pathway. MTT assays were performed for gemcitabine or cisplatin using MzChA1-GR cells transfected with siFANCD2, siBRCA2 and siRAD51c. *P < 0.001.
Fig. S6. Sensitivity to B02, a specific RAD51 inhibitor. MTT assays were performed using MzChA1-parent cells and two GR clones.
Fig. S7. Inhibition by siFANCD2 in KMCH1-GR and CCLP1-GR cells was confirmed by western blotting.
Fig. S8. Expression of CD133 in biliary tract cancer (BTC) cells. FACS was performed with MzChA1, MzChA1-GR, KMCH1 and KMCH1-GR cells.
Data S1. Supporting materials and methods.
Table S1. Primers used in qRT-PCR.
References
- Zhu AX, Hezel AF. Development of molecularly targeted therapies in biliary tract cancers: reassessing the challenges and opportunities. Hepatology. 2011;53:695–704. doi: 10.1002/hep.24145. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Nagano H, Marubashi S, et al. Treatment of borderline cases for curative resection of biliary tract cancer. J Surg Oncol. 2011;104:499–503. doi: 10.1002/jso.21971. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Nagano H, Marubashi S, et al. Clinicopathological features of long-term survivors for advanced biliary tract cancer and impact of the number of lymph nodes involved. Int J Surg. 2013;11:145–51. doi: 10.1016/j.ijsu.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Morine Y, Shimada M, Utsunomiya T, et al. Clinical impact of lymph node dissection in surgery for peripheral-type intrahepatic cholangiocarcinoma. Surg Today. 2012;42:147–51. doi: 10.1007/s00595-011-0057-9. [DOI] [PubMed] [Google Scholar]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- Oh SY, Jeong CY, Hong SC, et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs. 2011;29:1066–72. doi: 10.1007/s10637-010-9417-3. [DOI] [PubMed] [Google Scholar]
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–74. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Isayama H, Nakai Y, et al. Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs. 2012;30:708–13. doi: 10.1007/s10637-010-9553-9. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Isayama H, Nakai Y, et al. A retrospective study of gemcitabine and cisplatin combination therapy as second-line treatment for advanced biliary tract cancer. Chemotherapy. 2013;59:106–11. doi: 10.1159/000354209. [DOI] [PubMed] [Google Scholar]
- Deb S, Xu H, Tuynman J, et al. RAD21 cohesin overexpression is a prognostic and predictive marker exacerbating poor prognosis in KRAS mutant colorectal carcinomas. Br J Cancer. 2014;110:1606–13. doi: 10.1038/bjc.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu TP, Shen H, Liu LX, Shu YQ. Association of ERCC1-C118T and -C8092A polymorphisms with lung cancer risk and survival of advanced-stage non-small cell lung cancer patients receiving platinum-based chemotherapy: a pooled analysis based on 39 reports. Gene. 2013;526:265–74. doi: 10.1016/j.gene.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Chang IW, Hsu CT, Lin JW, Hung CH. The prognostic impact of MGMT expression on low-grade gangliogliomas: a clinicopathological and immunohistochemical study. Folia Neuropathol. 2013;51:275–82. doi: 10.5114/fn.2013.39716. [DOI] [PubMed] [Google Scholar]
- Birkbak NJ, Kochupurakkal B, Izarzugaza JM, et al. Tumor mutation burden forecasts outcome in ovarian cancer with BRCA1 or BRCA2 mutations. PLoS ONE. 2013;8:e80023. doi: 10.1371/journal.pone.0080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia L, Iwamoto T, Di Leo A, et al. DNA repair gene patterns as prognostic and predictive factors in molecular breast cancer subtypes. Oncologist. 2013;18:1063–73. doi: 10.1634/theoncologist.2013-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji M, Xu B, Jiang JT, et al. Relationship between glutathione S-transferase P1 (GSTP1), X-ray repair cross complementing group 1 (XRCC1) and 5,10-methylenetetrahydrofolate reductase (5,10-MTHFR) gene polymorphisms and response to chemotherapy in advanced gastric cancer. Onkologie. 2013;36:335–40. doi: 10.1159/000351260. [DOI] [PubMed] [Google Scholar]
- De Dosso S, Zanellato E, Nucifora M, et al. ERCC1 predicts outcome in patients with gastric cancer treated with adjuvant cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2013;72:159–65. doi: 10.1007/s00280-013-2181-2. [DOI] [PubMed] [Google Scholar]
- Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51:6110–7. [PubMed] [Google Scholar]
- Ewald B, Sampath D, Plunkett W. H2AX phosphorylation marks gemcitabine-induced stalled replication forks and their collapse upon S-phase checkpoint abrogation. Mol Cancer Ther. 2007;6:1239–48. doi: 10.1158/1535-7163.MCT-06-0633. [DOI] [PubMed] [Google Scholar]
- Parsels LA, Morgan MA, Tanska DM, et al. Gemcitabine sensitization by checkpoint kinase 1 inhibition correlates with inhibition of a Rad51 DNA damage response in pancreatic cancer cells. Mol Cancer Ther. 2009;8:45–54. doi: 10.1158/1535-7163.MCT-08-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer. 2012;12:801–17. doi: 10.1038/nrc3399. [DOI] [PubMed] [Google Scholar]
- D'Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- Teng DH, Bogden R, Mitchell J, et al. Low incidence of BRCA2 mutations in breast carcinoma and other cancers. Nat Genet. 1996;13:241–4. doi: 10.1038/ng0696-241. [DOI] [PubMed] [Google Scholar]
- Vencken PM, Kriege M, Hoogwerf D, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011;22:1346–52. doi: 10.1093/annonc/mdq628. [DOI] [PubMed] [Google Scholar]
- Lim JJ, Yang K, Taylor-Harding B, Wiedemeyer WR, Buckanovich RJ. VEGFR3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of BRCA1 and BRCA2. Neoplasia. 2014;16:343–53. doi: 10.1016/j.neo.2014.04.003. e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Xiao J, Shen M, et al. Isolation and characterization of tumorigenic extrahepatic cholangiocarcinoma cells with stem cell-like properties. Int J Cancer. 2011;128:72–81. doi: 10.1002/ijc.25317. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Nagaria P, Robert C, Rassool FV. DNA double-strand break response in stem cells: mechanisms to maintain genomic integrity. Biochim Biophys Acta. 2013;1830:2345–53. doi: 10.1016/j.bbagen.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Maugeri-Sacca M, Bartucci M, De Maria R. DNA damage repair pathways in cancer stem cells. Mol Cancer Ther. 2012;11:1627–36. doi: 10.1158/1535-7163.MCT-11-1040. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kang MJ, Cho YM. Low production of reactive oxygen species and high DNA repair: mechanism of radioresistance of prostate cancer stem cells. Anticancer Res. 2013;33:4469–74. [PubMed] [Google Scholar]
- Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–65. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Demetris AJ, Gollin SM, et al. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252–60. doi: 10.1002/ijc.2910520217. [DOI] [PubMed] [Google Scholar]
- Murakami T, Yano H, Maruiwa M, Sugihara S, Kojiro M. Establishment and characterization of a human combined hepatocholangiocarcinoma cell line and its heterologous transplantation in nude mice. Hepatology. 1987;7:551–6. doi: 10.1002/hep.1840070322. [DOI] [PubMed] [Google Scholar]
- Knuth A, Gabbert H, Dippold W, et al. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579–96. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- Yamada D, Kobayashi S, Wada H, et al. Role of crosstalk between interleukin-6 and transforming growth factor-beta 1 in epithelial-mesenchymal transition and chemoresistance in biliary tract cancer. Eur J Cancer. 2013;49:1725–40. doi: 10.1016/j.ejca.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Masters JR, Thomson JA, Daly-Burns B, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci USA. 2001;98:8012–7. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CJ, Gao J, Wang M, et al. CD133(+) gallbladder carcinoma cells exhibit self-renewal ability and tumorigenicity. World J Gastroenterol. 2011;17:2965–71. doi: 10.3748/wjg.v17.i24.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Tian R, Wang M, et al. CD44+CD133+ population exhibits cancer stem cell-like characteristics in human gallbladder carcinoma. Cancer Biol Ther. 2010;10:1182–90. doi: 10.4161/cbt.10.11.13664. [DOI] [PubMed] [Google Scholar]
- Kokuryo T, Yokoyama Y, Nagino M. Recent advances in cancer stem cell research for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:606–13. doi: 10.1007/s00534-012-0542-6. [DOI] [PubMed] [Google Scholar]
- Iwahashi S, Utsunomiya T, Shimada M, et al. High expression of cancer stem cell markers in cholangiolocellular carcinoma. Surg Today. 2013;43:654–60. doi: 10.1007/s00595-012-0437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M, Span SW, Vischioni B, et al. FANCD2 expression in advanced non-small-cell lung cancer and response to platinum-based chemotherapy. Clin Lung Cancer. 2005;6:250–4. doi: 10.3816/CLC.2005.n.005. [DOI] [PubMed] [Google Scholar]
- Kim K, Min HS, Chie EK, et al. CD24 expression predicts distant metastasis in extrahepatic bile duct cancer. World J Gastroenterol. 2013;19:1438–43. doi: 10.3748/wjg.v19.i9.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- Huang F, Motlekar NA, Burgwin CM, Napper AD, Diamond SL, Mazin AV. Identification of specific inhibitors of human RAD51 recombinase using high-throughput screening. ACS Chem Biol. 2011;6:628–35. doi: 10.1021/cb100428c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Lobrich M, Shibata A, Beucher A, et al. gammaH2AX foci analysis for monitoring DNA double-strand break repair: strengths, limitations and optimization. Cell Cycle. 2010;9:662–9. doi: 10.4161/cc.9.4.10764. [DOI] [PubMed] [Google Scholar]
- Alli E, Sharma VB, Hartman AR, Lin PS, McPherson L, Ford JM. Enhanced sensitivity to cisplatin and gemcitabine in Brca1-deficient murine mammary epithelial cells. BMC Pharmacol. 2011;11:7. doi: 10.1186/1471-2210-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maacke H, Jost K, Opitz S, et al. DNA repair and recombination factor Rad51 is over-expressed in human pancreatic adenocarcinoma. Oncogene. 2000;19:2791–5. doi: 10.1038/sj.onc.1203578. [DOI] [PubMed] [Google Scholar]
- Takenaka T, Yoshino I, Kouso H, et al. Combined evaluation of Rad51 and ERCC1 expressions for sensitivity to platinum agents in non-small cell lung cancer. Int J Cancer. 2007;121:895–900. doi: 10.1002/ijc.22738. [DOI] [PubMed] [Google Scholar]
- Taron M, Rosell R, Felip E, et al. BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet. 2004;13:2443–9. doi: 10.1093/hmg/ddh260. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao L, Sun H, et al. Gene silencing of FANCF potentiates the sensitivity to mitoxantrone through activation of JNK and p38 signal pathways in breast cancer cells. PLoS ONE. 2012;7:e44254. doi: 10.1371/journal.pone.0044254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Meng Q, Auborn K, Carter T, Rosen EM. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer. 2006;94:407–26. doi: 10.1038/sj.bjc.6602935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Proliferation (a) and cell cycle distribution (b) in MzChA1-parent andGEM-resistant (GR) cells. (a) Cells were seeded and cultured for 72 h. The graph shows the cell growth rate compared to living cells at 0 h. (b) The cell cycle distribution was evaluated by flow cytometry using cells stained with Hoechst 33342. *P < 0.01.
Fig. S2. Chemosensitivity of CCLP1-GR and KMCH1-GR cells to gemcitabine and cisplatin. MTT assays were performed for gemcitabine or cisplatin using CCLP1-parent or KMCH1-parent cells and GR clones. The GR clones are numbered 1–3 for KMCH1-GR and 1–5 for CCLP1-GR. The half maximal (50%) inhibitory concentration (IC50) is indicated on the y-axis. *P < 0.001. GR, GEM-resistant.
Fig. S3. Morphology of parent and GR biliary tract cancer (BTC) cells. Scale bar = 50 μm.
Fig. S4. Inhibition by siFANCD2, siBRCA2 and siRAD51c in MzChA1-GR cells was confirmed by qRT-PCR and western blotting. *P < 0.001.
Fig. S5. Effect of inhibiting the BRCA/FA pathway. MTT assays were performed for gemcitabine or cisplatin using MzChA1-GR cells transfected with siFANCD2, siBRCA2 and siRAD51c. *P < 0.001.
Fig. S6. Sensitivity to B02, a specific RAD51 inhibitor. MTT assays were performed using MzChA1-parent cells and two GR clones.
Fig. S7. Inhibition by siFANCD2 in KMCH1-GR and CCLP1-GR cells was confirmed by western blotting.
Fig. S8. Expression of CD133 in biliary tract cancer (BTC) cells. FACS was performed with MzChA1, MzChA1-GR, KMCH1 and KMCH1-GR cells.
Data S1. Supporting materials and methods.
Table S1. Primers used in qRT-PCR.


