Abstract
Interleukin-11 (IL-11), a member of the IL-6 family of cytokines, exerts pleiotropic oncogenic activities by stimulating angiogenesis and metastasis in many cancer types. The present study aims to evaluate the impact of IL-11 expression on recurrence and mortality of patients with clear-cell renal cell carcinoma (ccRCC). We retrospectively enrolled 193 ccRCC patients undergoing nephrectomy at a single center. Clinicopathologic features, recurrence-free survival (RFS) and overall survival (OS) were recorded. IL-11 intensity was assessed by immunohistochemistry in tumor specimens. The Kaplan–Meier method was applied to compare survival curves. Cox regression models were used to analyze the impact of prognostic factors on RFS and OS. The concordance index (C-index) was calculated to assess predictive accuracy. High IL-11 expression is associated with increased risk of recurrence and poor survival for ccRCC patients (P < 0.001 and P < 0.001, respectively), especially those with early-stage disease (TNM stage I + II). Multivariate analyses confirmed that IL-11 expression was an independent prognostic factor for RFS and OS (P = 0.006 and P = 0.008, respectively). The predictive accuracy of well-established prognostic models was improved when IL-11 expression was integrated. In conclusion, high IL-11 expression is an independent predictor of poor prognosis in ccRCC patients. It may help identify patients who could benefit from additional treatments and closer follow up.
Keywords: Clear-cell renal cell carcinoma, interleukin-11, overall survival, prognostic biomarker, recurrence-free survival
Renal cell carcinoma (RCC) accounts for 2–3% of all malignancies in adults.1,2 Clear-cell RCC (ccRCC) is the most common histological subtype, representing 70–80% of all RCC cases.3,4 To date, surgical resection remains the only curative therapy for patients with localized RCC. However, approximately 30% of patients have metastatic RCC at diagnosis, and another 25–30% of patients undergoing surgery will experience local or distant recurrence. Once metastasis develops, the prognosis is poor, with the 5-year survival rate ranging between 0 and 10%.5 Following surgery for localized RCC, we still rely solely on models combining clinicopathologic factors for prognostic stratification. The predictive accuracy of these models may be further improved by the addition of novel molecular biomarkers.
Inflammation plays an important role in tumor initiation and progression.6 Cytokines and chemokines are now recognized as key mediators linking the inflammatory tumor microenvironment and cancer cell growth, invasion and metastasis, and, therefore, serve as potential therapeutic targets and prognostic factors.7 The interleukin (IL)-6 family of cytokines is defined by the shared use of the gp130 receptor β-subunit and is considered to be one of the most important pro-tumoral cytokine families.8 IL-11, a member of the IL-6 cytokine family, was initially regarded as a regulator of T cell and macrophage polarization and platelet production.9 In recent years, increasing attention has been paid to the effects of IL-11 on tumor invasion and metastasis in many cancer types. Previous studies uncovered that IL-11 stimulates tumor cell proliferation and invasion via its downstream signaling cascade in colorectal adenocarcinoma10 and that IL-11 overexpression is correlated with poor clinical outcomes in gastric cancer.11 In addition, IL-11 promotes the migration of glioma cells and tumor-associated brain endothelial cells.12 However, the relationship between IL-11 expression and oncologic outcomes after nephrectomy for ccRCC patients has not been well studied.
In this study, we assessed the expression of IL-11 by immunohistochemistry in ccRCC tissue samples and its association with clinicopathologic characteristics and patient outcomes. We further evaluated whether IL-11 expression provides additional information to well-established prognostic models.
Materials and Methods
Patients
This study retrospectively enrolled 193 patients who had experienced radical or partial nephrectomy for ccRCC and had stored tumor tissue available at Zhongshan Hospital, Fudan University (Shanghai, China) between January 2003 and December 2004. The study was approved by the research ethics committee of Zhongshan Hospital and informed consent was obtained from all patients. Patients who received preoperative neoadjuvant therapy were excluded from the current study. For each patient, clinicopathologic information, including age, gender, tumor size, TNM stage, Fuhrman grade, tumor necrosis and Eastern Cooperative Oncology Group performance status (ECOG-PS) were collected. Patients were reassigned according to the 2010 AJCC TNM classification. The Mayo Clinic Stage, Size, Grade and Necrosis (SSIGN) and University of Los Angeles Integrated Staging System (UISS) scores were applied to each patient.13,14
Generally, at our institution, patients were followed up every 3 to 4 months for the first year following surgery, every 6 months from the second through the fifth year, and annually thereafter. Abdominal and chest CT scans were alternated with abdominal ultrasound and chest radiographs. Bone and brain scans were requested only in the case of overt symptoms. When new metastasis occurred, surgical resection was indicated in all cases when feasible. Subsequent metastasectomies were taken into consideration when complete resection was considered achievable. Immunotherapy and targeted therapy were only administered in cases of unresectable metastatic progression. The survival status of each patient was updated in October 2013. The median follow-up period was 104 months (range, 11–120 months). Recurrence-free survival (RFS) was calculated from the date of surgery to the date of recurrence or death from ccRCC, whichever happened first. Overall survival (OS) was measured from the date of surgery to death from all causes. Patients with tumor metastasis (N1 or M1 tumors) at the time of surgery were excluded from the analysis of tumor recurrence as indicated by the end point of RFS.
Immunohistochemistry and evaluation
Tissue microarray construction and immunohistochemistry protocol were described previously.15 The polyclonal anti-IL-11 antibody (NBP1-19815, Littleton, CO, USA [dilution 1:100]) was applied in the procedure. The specificity of the anti-IL-11 antibody was confirmed by western blot using RCC cell lines. Tissue samples processed similarly, except for the omission of the primary antibody, were used as negative controls in the analysis. A semiquantitative immunohistochemistry score on a scale of 0–300 was calculated for each sample by multiplying the staining intensity (0, no staining; 1, weak; 2, moderate; and 3, strong) and the percentage of tumor cells (0–100%) at each intensity level. The immunostaining was evaluated by two experienced pathologists (L. Chen and Q. Fu) blinded to the clinical data.
Statistical analyses
To determinate high and low expression of IL-11, the optimal cutoff value was estimated by X-tile plots v3.6.1 (Yale University, New Haven, CT, USA)16 to achieve the minimum P-value. Correlations between immunohistochemical variables and clinicopathologic characteristics were analyzed using χ2-tests and t-tests. The probability of death or recurrence was determined using the Kaplan–Meier method, with log-rank tests assessing the differences between the groups. All statistical tests were two-sided and performed at a significance level of 0.05. Cox proportional hazard models were used to analyze the impact of prognostic factors on RFS and OS. Harrell's concordance index (C-index) was calculated to measure how well the fitted Cox model distinguishes between patients who die and those who survive.17 The analyses were performed using Stata 12 and Medcalc software.
Results
Patient cohort clinical data and correlations with Interleukin-11 expression
As listed in Table1, of the 193 patients with ccRCC 133 were male (69%) and 60 were female (31%). The mean age at surgery was 55.4 years, and the mean tumor size was 4.4 cm. Tumor necrosis was presented in 22% of cases. The TNM stages were I in 121 (63%) patients, II in 17 (9%), III in 47 (24%) and IV in 8 (4%). Fuhrman grades were 1, 2, 3 and 4 in 16%, 45%, 27% and 12% of cases, respectively. ECOG-PS was evaluated as ≥1 in 24% of cases. During the follow-up period, 61 (32%) patients had disease recurrence or died from ccRCC, including 15 patients with locoregional recurrence only, 28 patients with distant recurrence only and 7 patients with both local and distant recurrence, and 11 patients died before verified recurrence. A total of 48 (25%) patients died due to all causes during the follow-up period.
Table 1.
Correlations between intratumoral IL-11 expression and clinicopathologic characteristics
| Characteristic | Patients (n = 193) | IL-11 expression | P value | ||
|---|---|---|---|---|---|
| Number | % | High (n = 92) | Low (n = 101) | ||
| Mean age (years) | 55.4 | 54.4 | 56.2 | 0.278 | |
| Gender | |||||
| Male | 133 | 68.9 | 63 | 70 | 0.901 |
| Female | 60 | 31.1 | 29 | 31 | |
| Mean tumor size (cm) | 4.4 | 4.7 | 4.2 | 0.126 | |
| TNM stage | |||||
| I | 121 | 62.7 | 48 | 73 | 0.003 |
| II | 17 | 8.8 | 9 | 8 | |
| III | 47 | 24.4 | 27 | 20 | |
| IV | 8 | 4.1 | 8 | 0 | |
| Fuhrman grade | |||||
| 1 | 31 | 16.1 | 13 | 18 | 0.159 |
| 2 | 87 | 45.1 | 40 | 47 | |
| 3 | 52 | 26.9 | 23 | 29 | |
| 4 | 23 | 11.9 | 16 | 7 | |
| Tumor necrosis | |||||
| Absent | 150 | 77.7 | 66 | 84 | 0.057 |
| Present | 43 | 22.3 | 26 | 17 | |
| ECOG-PS | |||||
| 0 | 147 | 76.2 | 70 | 77 | 0.980 |
| ≥1 | 46 | 23.8 | 22 | 24 | |
| UISS score (localized) | |||||
| Low risk | 67 | 34.7 | 25 | 42 | 0.215 |
| Intermediate risk | 106 | 54.9 | 54 | 52 | |
| High risk | 11 | 5.7 | 5 | 6 | |
| SSIGN score | |||||
| Low risk | 116 | 60.1 | 44 | 72 | 0.001 |
| Intermediate risk | 65 | 33.7 | 38 | 27 | |
| High risk | 12 | 6.2 | 10 | 2 | |
ECOG-PS, Eastern Cooperative Oncology Group performance status; IL-11, interleukin-11; SSIGN, The Mayo Clinic Stage, Size, Grade, and Necrosis; UISS, University of Los Angeles Integrated Staging System.
Interleukin-11 positive staining was mainly observed in the cytoplasm of tumor cells. Representative IL-11 immunohistochemical photographs of low expression (score = 13) and high expression (score = 216) are shown in Figure1. According to the results from the “minimum P-value” approach, 104 was determined as the cutoff value; this separated the population into 101 IL-11-low patients and 92 IL-11-high patients (Fig.1c). The associations between the expression of IL-11 and clinicopathologic characteristics are summarized in Table1. High IL-11 expression was associated with advanced tumor stage (P = 0.003) and higher SSIGN scores (P = 0.001). However, IL-11 expression had no statistically significant correlations with age, gender, tumor size, Fuhrman grade, tumor necrosis, performance status or the UISS score.
Fig 1.
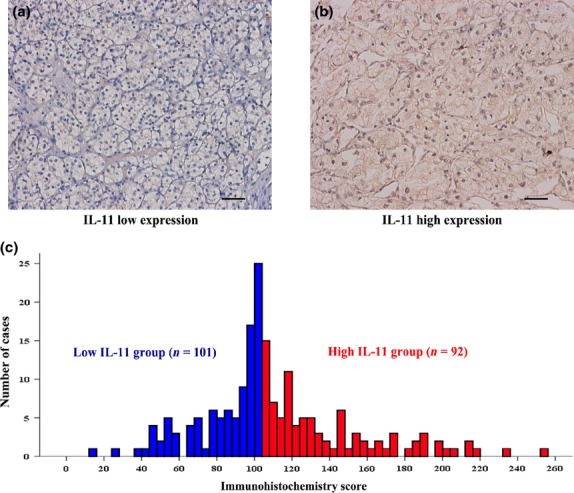
(a, b) Representative photographs of Interl-eukin (IL)-11 immunostaining in clear-cell renal cell carcinoma (ccRCC) tissues (original magnification ×200). (c) Frequency distribution of IL-11 immuno-histochemistry score in 193 ccRCC samples.
High expression of interleukin-11 correlated with poor prognosis
The Kaplan–Meier curves revealed that high IL-11 expression correlated with increased chances of recurrence (P < 0.001, Fig.2a) and decreased survival (P < 0.001, Fig.2b). To evaluate whether this finding was dependent on the extent of the disease, patients with TNM stage I + II and stage III + IV tumors were analyzed separately (Fig.2c–f). In the subgroup with TNM stage I + II, patients with high IL-11 expression tended to have a worse RFS (P = 0.014) and OS (P = 0.007) than these with low IL-11 expression (Fig.2c,d). However, in the TNM stage III + IV subgroup, IL-11 expression could not further refine RFS (P = 0.077) or OS (P = 0.115) (Fig.2e,f).
Fig 2.
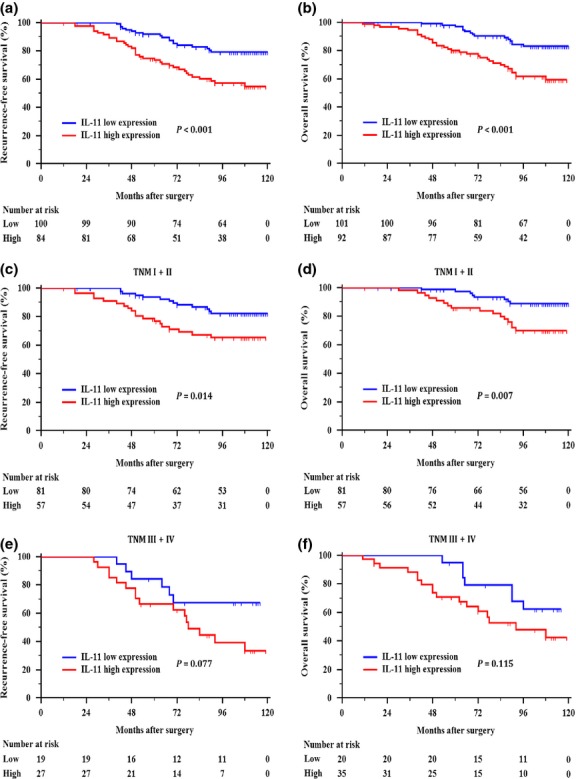
Kaplan–Meier curves showing recurrence-free survival and overall survival based on the Interleukin (IL)-11 expression levels in (a, b) all patients, (c, d) TNM stage I + II, and (e, f) stage III + IV patients with clear-cell renal cell carcinoma.
We then assessed the independent prognostic value of IL-11 expression using the multivariate Cox proportional hazard model. The results showed that the IL-11 expression was independently prognostic of recurrence (hazard ratio [HR], 2.265; 95% CI, 1.269 to 4.044; P = 0.006) and mortality (HR, 2.349; 95% CI, 1.248 to 4.420; P = 0.008) in patients with ccRCC after controlling for conventional clinicopathologic factors (Table2).
Table 2.
Multivariate Cox regression analyses of potential prognostic characteristics for recurrence-free survival and overall survival
| Characteristic | Recurrence-free survival | Overall survival | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Tumor size (cm) | 1.060 (0.948–1.186) | 0.306 | 1.040 (0.917–1.181) | 0.540 |
| TNM stage (III + IV vs I + II†) | 1.928 (1.085–3.426) | 0.025 | 2.461 (1.361–4.449) | 0.003 |
| Fuhrman grade (3 + 4 vs 1 + 2†) | 2.358 (1.358–4.095) | 0.002 | 2.126 (1.186–3.810) | 0.011 |
| Tumor necrosis (present vs absent†) | 1.243 (0.654–2.363) | 0.507 | 1.767 (0.938–3.330) | 0.078 |
| ECOG-PS (≥1 vs 0†) | 2.072 (1.144–3.749) | 0.016 | 2.249 (1.210–4.179) | 0.010 |
| IL-11 (high vs low†) | 2.265 (1.269–4.044) | 0.006 | 2.349 (1.248–4.420) | 0.008 |
Reference group. 95% CI, 95% confidence interval.
Ability of interleukin-11 expression to enhance established prognostic models
As shown above, high IL-11 expression correlated with high probability of recurrence and reduced survival in early-stage ccRCC patients. Thus, we further investigated whether the combination of the UISS or SSIGN scores with IL-11 expression would improve their predictive accuracies. For RFS, the C-index of the UISS improved from 0.620 to 0.678 when IL-11 expression was combined, which was statistically significant (P = 0.001). However, the C-index of the SSIGN increased from 0.662 to 0.686 after the integration of IL-11 expression, which did not reach statistical significance (P = 0.276). Likewise for OS, the C-index of the UISS improved from 0.679 to 0.725 (P = 0.003), whereas the C-index of the SSIGN increased from 0.690 to 0.721 (P = 0.073) when IL-11 expression was added (Table3).
Table 3.
Comparison of the predictive accuracies of prognostic models
| Prognostic model | RFS | OS | ||
|---|---|---|---|---|
| C-index | P-value† | C-index | P-value† | |
| UISS | 0.620 | 0.679 | ||
| UISS + IL-11 | 0.678 | 0.001 | 0.725 | 0.003 |
| SSIGN | 0.662 | 0.690 | ||
| SSIGN + IL-11 | 0.686 | 0.276 | 0.721 | 0.073 |
Compared with the original model without IL-11 expression. C-index, Harrell's concordance index. OS, overall survival; RFS, recurrence-free survival.
Discussion
In this study, we used 193 ccRCC specimens to illustrate the prognostic significance of IL-11 expression. Here, we demonstrated that high expression of IL-11 is an independent predictor of adverse prognosis in patients with ccRCC. Furthermore, the incorporation of IL-11 expression into the established prognostic models, such as the UISS score and the SSIGN score system, improved their predictive accuracy, thereby improving the risk stratification and clinical management of ccRCC patients.
Cytokines have recently emerged as pivotal players in tumorigenesis. The IL-6 family of cytokines, especially IL-6 and IL-11, drives many of the cancer hallmarks through downstream activation of the JAK2/STAT3 signaling pathway.18 Increased production of IL-6 has been implicated in RCC, and serum levels of IL-6 have been established as a prognostic factor for metastatic RCC.19 A previous study confirmed that RCC cells produce IL-11 in vitro.20 However, the functions of IL-11 in RCC remain unclear. Recently, the tumor-promoting roles of IL-11 have been uncovered. In gastrointestinal cancer, IL-11 is a driver of tumor development and progression through activating the downstream gp130/STAT3 signaling pathway.18 Similarly, the maintenance of cancers is dependent on IL-11-mediated STAT3 activation in colitis-associated cancer in mice.21 Although STAT3 is very significant in tumor initiation and progression, direct therapeutic targeting of STAT3 has proved difficult.22 Moreover, systemic inhibition of STAT3 is often correlated with thrombocytopenia.23 Accordingly, more therapeutic interest has been paid to the STAT3-activating cytokines, due to the prevalence of STAT3 activation in diverse human cancer types. Previous studies have demonstrated that a peptide-based IL-11 antagonist is well tolerated with no thrombocytopenia in gastrointestinal cancer.18 Moreover, anti-IL-6 antibody has been used in clinical trials for lymphoma, RCC and ovarian cancer.24–26 Here, our findings showed that the high expression of IL-11 was associated with tumor progression and poor outcome in ccRCC patients. Therefore, targeting IL-11 or its receptor may have potential therapeutic effects for ccRCC.
The tumor microenvironment is increasingly recognized as an important player in tumor promotion and progression. Therefore, cytokines and immune cells in the tumor microenvironment have been identified recently as novel prognostic or predictive biomarkers for RCC patients. Jensen and coworkers found that the presence of intratumoral neutrophils is an unfavorable prognostic factor for patients with localized RCC.27 Data from our team demonstrates that intratumoral balance of M1 and M2 macrophage is associated with the cancer-specific survival of RCC patients after surgery.15 Furthermore, a study by Tran et al.28 reveals that cytokine and angiogenic factor profiling has not only prognostic value in patients with metastatic RCC, but also predicts the response to pazopanib treatment. Collectively, combining the existing models with molecular biomarkers will we improve prognostication to identify patients who could benefit from adjuvant treatment and closer postoperative follow up.
The limitations of the present study need to be stated. First are the limitations inherent the retrospective study design and relatively small sample size. Second, external validation in an independent cohort is necessary to confirm that high IL-11 expression is an indicator of unfavorable outcome in patients with ccRCC. Third, immunohistochemistry analysis is always somewhat subjective. Immunohistochemistry was chosen because it is a widely available and clinically applicable technique, which can be applied to routine formalin-fixed paraffin-embedded tissues. To minimize this influence in our study, duplicate tissue cores from the same tumor were used to construct the tissue microarray, and two experienced urologic pathologists blinded to the clinical data evaluated immunostained slides.
In conclusion, our results suggest that high IL-11 expression independently predicts poor postoperative prognosis for ccRCC patients. Incorporation of IL-11 expression into current prognostic systems could improve the risk stratification and clinical management of ccRCC patients.
Acknowledgments
We thank Ms Haiying Zeng (Department of Pathology, Zhongshan Hospital, Fudan University) for technical assistance.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–32. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–24. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Inman BA, Harrison MR, George DJ. Novel immunotherapeutic strategies in development for renal cell carcinoma. Eur Urol. 2013;63:881–9. doi: 10.1016/j.eururo.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–9. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Ernst M, Putoczki TL. Molecular Pathways: IL11 as a tumor-promoting cytokine-translational implications for cancers. Clin Cancer Res. 2014;20:5579–88. doi: 10.1158/1078-0432.CCR-13-2492. [DOI] [PubMed] [Google Scholar]
- Yoshizaki A, Nakayama T, Yamazumi K, Yakata Y, Taba M, Sekine I. Expression of interleukin (IL)-11 and IL-11 receptor in human colorectal adenocarcinoma: IL-11 up-regulation of the invasive and proliferative activity of human colorectal carcinoma cells. Int J Oncol. 2006;29:869–76. [PubMed] [Google Scholar]
- Howlett M, Giraud AS, Lescesen H, et al. The interleukin-6 family cytokine interleukin-11 regulates homeostatic epithelial cell turnover and promotes gastric tumor development. Gastroenterology. 2009;136:967–77. doi: 10.1053/j.gastro.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Liu Z. 2011. Interleukin-11: a study of its effects in glioblastoma multiforme. University of Southern California Master's Thesis.
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–66. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhu Y, Chen L, et al. Prognostic value of diametrically polarized tumor-associated macrophages in renal cell carcinoma. Ann Surg Oncol. 2014;21:3142–50. doi: 10.1245/s10434-014-3601-1. [DOI] [PubMed] [Google Scholar]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. [PubMed] [Google Scholar]
- Putoczki TL, Thiem S, Loving A, et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24:257–71. doi: 10.1016/j.ccr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Negrier S, Perol D, Menetrier-Caux C, et al. Interleukin-6, interleukin-10, and vascular endothelial growth factor in metastatic renal cell carcinoma: prognostic value of interleukin-6–from the Groupe Francais d'Immunotherapie. J Clin Oncol. 2004;22:2371–8. doi: 10.1200/JCO.2004.06.121. [DOI] [PubMed] [Google Scholar]
- Knoefel B, Nuske K, Steiner T, et al. Renal cell carcinomas produce IL-6, IL-10, IL-11, and TGF-beta 1 in primary cultures and modulate T lymphocyte blast transformation. J Interferon Cytokine Res. 1997;17:95–102. doi: 10.1089/jir.1997.17.95. [DOI] [PubMed] [Google Scholar]
- Ernst M, Putoczki TL. Targeting IL-11 signaling in colon cancer. Oncotarget. 2013;4:1860. doi: 10.18632/oncotarget.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedvat M, Huszar D, Herrmann A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–97. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Najdovska M, Grail D, et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–38. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–10. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Rossi JF, Negrier S, James ND, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103:1154–62. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward J, Kulbe H, Chakravarty P, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–96. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–17. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–37. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]


