Abstract
Cadmium is a toxic pollutant with occupational and environmental significance, due to its diverse toxic effects. Supramolecules that conjugate and decontaminate toxic metals have potential for use in treatment of cadmium intoxication. In addition, metal-coordinating ability has been postulated to contribute to the cytotoxic effects of anti-tumor agents such as cisplatin or bleomycin. Thiacalixarenes, cyclic oligomers of p-alkylphenol bridged by sulfur atoms, are supramolecules known to have potent coordinating ability to metal ions. In this study, we show that cadmium-coordinated thiacalix[4]arene tetrasulfate (TC4ATS-Cd) exhibits an anti-proliferative effect against T-cell leukemia cells. Cadmium exhibited cytotoxicity with IC50 values ranging from 36 to 129 μM against epithelia-derived cancer cell lines, while TC4ATS-Cd elicited no significant cytotoxicity (IC50 > 947 μM). However, a number of T-cell leukemia cell lines exhibited marked sensitivity to TC4ATS-Cd. In Jurkat cells, toxicity of TC4ATS-Cd occurred with an IC50 of 6.9 μM, which is comparable to that of 6.5 μM observed for cadmium alone. TC4ATS-Cd induced apoptotic cell death through activation of caspase-3 in Jurkat cells. In a xenograft model, TC4ATS-Cd (13 mg/kg) treatment significantly suppressed the tumor growth of Jurkat cells in mice. In addition, TC4ATS-Cd-treated mice exhibited significantly less cadmium accumulation in liver and kidney compared to equimolar cadmium-treated mice. These results suggest that cadmium-coordinated supramolecules may have therapeutic potential for treatment of T-cell leukemia.
Keywords: Cadmium, leukemia, supramolecule, T-cell receptor, thiacalixarene
Cadmium is a nonessential transition metal and a toxic pollutant with occupational and environmental significance. It has been classified as a human carcinogen by the International Agency for Research on Cancer (IARC) and the International Programme on Chemical Safety (IPCS).1,2 Post-mortem analysis of cadmium level in autopsied human organs shows that most of the body cadmium burden is retained in the kidneys and liver. The biological half-life in the kidneys was estimated to be 12–20 years, while in the liver it may be somewhat shorter.2,3 ln Japan, the ingestion of cadmium-contaminated rice resulted in Itai-itai disease, characterized by kidney dysfunction, osteomalacia, osteoporosis and painful bone fractures.4
Chelating therapy is one of the most effective strategies for removing accumulated toxic metals such as cadmium from biological systems. So far, a number of chelating agents of different types have been reported as effective means of countering cadmium toxicity.5–8 Recently, we have focused on the ability of one particular supramolecule to elicit this effect. Calixarenes are cyclic oligomers of p-alkylphenol bridged by a methylene group.9 These compounds are recognized as important host molecules in supramolecular chemistry because of their ability to conjugate small molecules in hydrophobic cavities. Thiacalixarenes, newly developed calixarenes in which the methylene groups are replaced with sulfur atoms, were reported to exhibit improved ability to coordinate with metal ions, as compared to calixarenes.10,11 Due to their coordination ability, thiacalixarenes may be capable of decontaminating cadmium and, therefore, could have therapeutic potential for use in cadmium intoxication. When we evaluated the removal of cadmium in an in vitro cellular system using water-soluble p-tetrasulfonated thiacalix[4]arene (TC4ATS, Fig.1), cadmium toxicity against liver-derived and stomach-derived epithelial cell lines was found to be overcome by this supramolecule (Fig. S1). However, only slight decontamination activity was observed in T-cell leukemia cell lines (Fig. S1). On the basis of these data, we hypothesize that the coordination complex of TC4ATS with cadmium (TC4ATS-Cd) exerts anti-proliferative effects selectively against leukemia cells.
Fig 1.
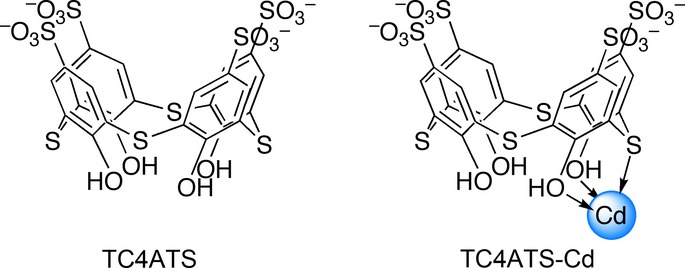
Structures of TC4ATS and TC4ATS-Cd. Structure of TC4ATS-Cd was deduced from mass spectral data and elemental analysis.
In this study, we synthesized TC4ATS-Cd complex (Fig.1) and assessed whether this metal complex elicits cytotoxic anti-tumor effects against various cell lines. TC4ATS-Cd complex exhibited an anti-proliferative effect against leukemia cells in vitro and suppressed tumor growth in a mouse xenograft model using Jurkat T-cell leukemia cell lines, without eliciting obvious side effects.
Materials and Methods
Reagents
Cadmium chloride and cadmium acetate were obtained from Wako (Osaka, Japan). MTT, NADH and sodium pyruvate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ac-DEVD-MCA was obtained from Peptide Institute (Osaka, Japan).
Instrumental analysis
1H-NMR spectra were measured at 300 MHz using a DPX-300 spectrometer (Bruker, Billerica, MA, USA). ESI-MS spectra were analyzed by API4000 (Applied Biosystems, Forester City, CA, USA). Elemental analysis and quantitation of metals were performed using a graphite furnace atomic absorption spectrometer (GFAAS) AA-6300 and GFA-EX7i (Shimadzu, Kyoto, Japan).
Synthesis of TC4ATS-Cd complex
TC4ATS was synthesized as TC4ATS sodium salt (TC4ATS-Na), as described previously.12,13 TC4ATS-Cd complex was synthesized by coordination of cadmium ion to TC4ATS. Briefly, an equimolar mixture of TC4ATS-Na and cadmium acetate was stirred in water. The resulting precipitate was recovered by filtration, washed with isopropanol, and dried to yield TC4ATS-Cd complex as a white powder. The molecular formula for TC4ATS-Cd was determined by NMR, ESI-MS and elemental analysis. 1H-NMR (300 MHz, D2O) δ 8.09 ppm (s, ArH, 8H); ESI-MS for TC4ATS-Cd·2Na (H2O/MeOH) m/z: 970.9 (M-H)−; elemental analysis for C24H12O16S8Na2Cd, calcd: Cd 11.6, Na 4.7, found: Cd 12.4, Na 4.3.
Cell culture
Hepatoma cell lines FLC-4 were provided by Dr S. Nagamori (Kyorin University, Japan). Breast adenocarcinoma cell lines, MCF7, and gastric adenocarcinoma cell lines, AGS, were provided by the Department of Chest, Breast and Endocrine Surgery, Akita University, Japan. Colon carcinoma cell lines, HCT116, and T-cell leukemia cell lines, Jurkat, were provided by Dr H. Tomoda (Kitasato University, Japan). Esophageal carcinoma cell lines, TE4, and T-cell leukemia cell lines, HPB-ALL, HUT78 and PEER, were obtained from Riken Bioresource Center, Japan. Embryonic kidney cell lines, HEK293, were provided by the Department of Hematology, Nephrology and Rheumatology, Akita University. Promyelocytic leukemia cell lines, HL-60, and T-cell leukemia cell lines, JKT-beta-del, were obtained from the Japanese Collection of Research Bioresources, Japan. FLC-4, MCF7 and HCT116 cells were cultured in DMEM/F12, Eagle's MEM containing 10 μg/mL insulin and McCoy's 5A, respectively. AGS and HEK293 cells were cultured in DMEM. TE4, Jurkat, HPB-ALL, HUT78, PEER, HL-60 and JKT-beta-del cells were cultured in PRMI-1460. Each medium was supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin and 2.5 μg/mL amphotericin B. Cells were cultured in a humidified 5% CO2 atmosphere at 37°C.
MTT assay
Cytotoxicity was evaluated using MTT assay. Epithelia-derived cell lines were seeded in 96-well plates 24 h prior to the addition of test compounds, and then incubated at 37°C for an additional 48 h. Leukemia cell lines were cultured in 96-well plates with test compounds at 37°C for 48 h. After the treatment, MTT was added and incubated for 4 h. After incubation, extraction solution (40% dimethylformamide, 2% CH3COOH, 0.03 M HCl and 20% SDS) was added and mixed thoroughly by agitation overnight at room temperature. Cytotoxicity was determined by measuring optical density at 570 nm using a microplate reader (Model 550; Bio-Rad, Hercules, CA, USA).
Lactate dehydrogenase assay
Jurkat cells were incubated in 96-well plates with TC4ATS-Cd at 37°C for 48 h. After treatment, cell-free media were recovered by centrifugation at 3,000 rpm for 5 min. Cell-free media were mixed with 500 μM NADH in 96-well plates. After a 10 min-agitation, lactate dehydrogenase (LDH) reaction was started by the addition of 4 mM sodium pyruvate. Oxidation of NADH to NAD+, accompanied by the conversion of pyruvate to lactate, was monitored by measuring the decrease in absorbance at 340 nm using Infinite M200 microplate reader (TECAN, Männedorf, Switzerland). LDH activity was calculated as the rate of decrease in NADH.
Western blot analysis
Leukemia cell lines were cultured with test compounds at the indicated concentration for 24 h. After treatment, cell lysates were prepared in lysis buffer (25 mM HEPES (pH 7.5), 5 mM EDTA, 2 mM DTT and 0.1% CHAPS) and clarified by centrifugation. After determination of protein concentration, equal amounts of protein were subjected to SDS-PAGE under reducing conditions. After electrophoresis, proteins were transferred to PVDF membranes. Membranes were blocked with 1% skim milk and then incubated with antibodies specific for activated caspase-3 (Cell Signaling Technology, Danvers, MA, USA), cleaved PARP (Cell Signaling Technology) or β-actin (Sigma-Aldrich). After washing, the membranes were incubated with peroxidase-conjugated secondary antibody (Sigma-Aldrich). Subsequently, targeted proteins were detected using the ECL system (GE Healthcare, Buckinghamshire, UK).
Caspase-3 assay
The activity of caspase-3 in TC4ATS-Cd-treated cell lysates was measured by quantifying caspase-specific cleavage of a fluorogenic substrate. Cell lysates were prepared using the same procedure described above for western blot analysis. After determination of protein concentration, equal amounts of proteins were incubated with 20 μM Ac-DEVD-MCA in reaction buffer (25 mM HEPES [pH 7.5]), 10% glycerol, 5 mM EDTA and 10 mM DTT) in 96-well black plates at 37°C for 2 h. Caspase-3 activity was determined by measuring fluorescence intensity using a microplate fluorometer Fluoroskan Ascent (Excitation 355 nm/emission 460 nm; Thermo Fisher Scientific, Waltham, MA, USA).
Xenograft models
All animal experiments were conducted in accordance with the guidelines for animal experiments in Akita University. Jurkat cells (5 × 107 cells) were inoculated s.c. into 6-week-old female SCID mice (CLEA Japan, Tokyo, Japan). Twenty days after inoculation, vehicle (saline), 3 mg/kg (13 μmol/kg) CdCl2 and 13 mg/kg (13 μmol/kg) TC4ATS-Cd were administered by i.p. injections three times weekly for 2 weeks (all groups, n = 5). Body weight and tumor size were measured three times weekly. Tumor volume was calculated as follows: Tumor volume = (Long length) × (Short length)2 × 1/2. Thirty-five days after inoculation, mice were killed, and organs (liver, kidney, spleen and lung) and tumors were excised and weighed.
Quantitation of cadmium levels in tumors and organs
Excised organs and tumors were digested by 1 M HNO3 for 2 h, and then added to 30% H2O2 to digest at 60°C. After centrifugation, cadmium contents in the supernatant were measured using GFAAS.
Statistical analysis
All data were expressed as means ± SD. Student's t-test was used to evaluate statistical differences between groups. A P-value of <0.05 was considered statistically significant.
Results
Attenuated effects of TC4ATS in cadmium toxicity against epithelia-derived cell lines in vitro
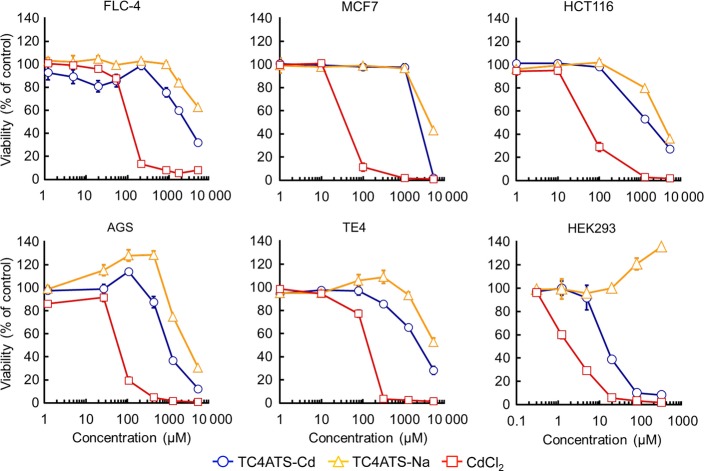
We synthesized the TC4ATS-Cd complex via conjugation of cadmium ion with TC4ATS-Na. Based on the results of instrumental analysis, the molecular composition of TC4ATS-Cd was determined to be TC4ATS-Cd?2Na (Fig.1). We evaluated the cytotoxicity of TC4ATS-Cd using the MTT assay and compared its effect with that of CdCl2 in a variety of human epithelia-derived cancer cell lines, including FLC-4, MCF7, HCT116, AGS and TE4, and embryonic kidney HEK293 cell lines (Fig.2). CdCl2 elicited cytotoxicity with IC50 values of 106, 36, 47, 60, 129 and 1.9 μM against FLC-4, MCF7, HCT116, AGS, TE4 and HEK293 cells, respectively (Fig.2). Results for HEK293 cells were consistent with the fact that the kidney is among the organs with high sensitivity to cadmium.2,3 Compared to CdCl2, TC4ATS-Cd exhibited low cytotoxicity against any of the tested epithelia-derived cell lines (IC50 values of 2,453, 2,208, 1,497, 947, 2,128 and 15 μM against FLC-4, MCF7, HCT116, AGS, TE4 and HEK293 cells, respectively [Fig.2]). In addition, TC4ATS-Na was not associated with any detectable cytotoxicity (IC50 value >2 mM [Fig.2]). In addition, maximum tolerated concentration (=IC20 value) of TC4ATS-Cd against FLC-4, MCF7, HCT116, AGS, TE4 and HEK293 cells were 667, 1,326, 280, 505, 454 and 6.8 μM, respectively (Fig.2). These data indicate that the cytotoxic effect of the cadmium ion is attenuated by its coordination with TC4ATS in cells of epithelial origin.
Fig 2.
TC4ATS-Cd attenuates cadmium toxicity against epithelia-derived cells in vitro. FLC-4, MCF7, HCT116, AGS, TE4 and HEK293 cells were treated with CdCl2, TC4ATS-Na or TC4ATS-Cd at indicated doses for 48 h. Cell viabilities were analyzed using MTT assay to determine the cytotoxicity of tested agents. Data are presented as means ± SD (n = 3).
TC4ATS-Cd inhibits proliferation of leukemia cell lines in vitro
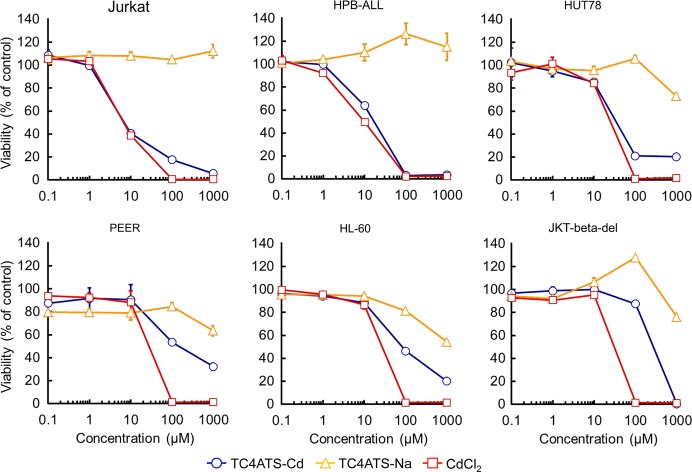
We next evaluated the cytotoxicity of TC4ATS-Cd in a variety of human T-cell leukemia cell lines, including Jurkat, HPB-ALL, HUT78, PEER and JKT-beta-del cell lines, and human promyelocytic leukemia HL-60 cell line (Fig.3). CdCl2 exhibited cytotoxicity with IC50 values of 6.5, 9.8, 25, 28, 27 and 30 μM against Jurkat, HPB-ALL, HUT78, PEER, HL-60 and JKT-beta-del cells, respectively (Fig.3). The sensitivities of leukemia cells for CdCl2 were slightly higher than those of epithelia-derived cancer cell lines (Figs2 and 3). In contrast to the results obtained with epithelia-derived cells (Fig.2), some leukemia cell lines showed remarkable sensitivity to TC4ATS-Cd. IC50 values for TC4ATS-Cd in Jurkat, HPB-ALL and HUT78 were determined to be 6.9, 17 and 35 μM, respectively (Fig.3). In contrast, PEER, HL-60 and JKT-beta-del cells had low sensitivity to TC4ATS-Cd (IC50 values of 144, 81 and 275 μM, respectively [Fig.3]). Taken together, our findings show that a kind of T-cell leukemia cell lines is highly susceptible to TC4ATS-Cd in vitro.
Fig 3.
TC4ATS-Cd inhibits proliferation of leukemia cells in vitro. Jurkat, HPB-ALL, HUT78, PEER, HL-60 and JKT-beta-del cells were treated with CdCl2, TC4ATS-Na or TC4ATS-Cd at indicated doses for 48 h. Cell viabilities were analyzed using MTT assay to determine the cytotoxicity of tested agents. Data are presented as means ± SD (n = 3).
TC4ATS-Cd induces apoptosis in T-cell leukemia cell lines
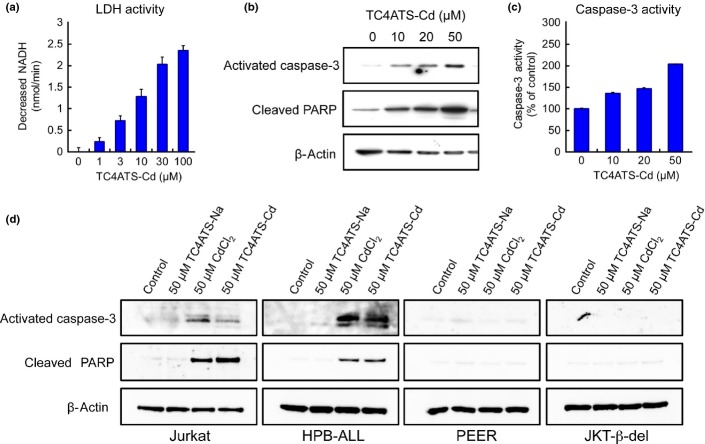
We further investigated the cytotoxic effects of TC4ATS-Cd in human T-cell leukemia Jurkat cells using the release of LDH into the culture medium as an indicator of the loss of cellular membrane integrity. TC4ATS-Cd increased LDH release from Jurkat cells in a dose-dependent manner (Fig.4a), suggesting that TC4ATS-Cd induces apoptosis or necrosis. Apoptosis is a proteolytic cascade involving several cysteine proteases that culminates in cell death. Because caspase-3 is a key protease in the execution of apoptosis and is activated by proteolytic cleavage, we investigated its activation by measuring the expression of the cleaved fragment of caspase-3 (activated caspase-3). TC4ATS-Cd upregulated the levels of activated caspase-3 protein in Jurkat cells (Fig.4b,d). When caspase-3 activity was measured by fluorogenic substrate assay, incubation with TC4ATS-Cd increased its proteolytic activity in Jurkat cells (Fig.4c). The cleavage of PARP, an event in the proteolytic cascade downstream of caspase-3 activation in apoptosis, was found to be consistently and markedly upregulated in TC4ATS-Cd-treated cells (Fig.4b). Similar to Jurkat cells, the caspase-3 activation and the cleavage of PARP in HPB-ALL cells were induced by treatment with 50 μM of CdCl2 and TC4ATS-Cd (Fig.4d). In contrast, apoptotic events in low TC4ATS-Cd-sensitive leukemia cell lines and epithelia-derived cell lines except for HEK293 were not elicited with CdCl2 and TC4ATS-Cd at 50 μM (Figs4d and S2). Thus, TC4ATS-Cd induces cell death by activating the apoptotic machinery in the TC4ATS-Cd-sensitive type of T-cell leukemia cell lines.
Fig 4.
TC4ATS-Cd induces apoptotic cell death in T-cell leukemia cells. (a) Lactate dehydrogenase (LDH) activity. Activities of LDH released into the media of TC4ATS-Cd-trerated Jurkat cells were measured. LDH activities were calculated as the rate of decrease in NADH levels. Data are presented as means ± SD (n = 3). (b) Western blot analysis of activated caspase-3 and cleaved PARP in Jurkat cells. The levels of activated caspase-3, cleaved PARP and β-actin in TC4ATS-Cd-treated Jurkat cells were determined. (c) Caspase-3 activity. Amidolytic activity of caspase-3 in TC4ATS-Cd-treated Jurkat cells were measured using a fluorogenic substrate. Data are presented as means ± SD (n = 3). (d) Western blot analysis of activated caspase-3 and cleaved PARP in T-cell leukemia cells. The levels of activated caspase-3, cleaved PARP and β-actin in TC4ATS-Na, CdCl2 or TC4ATS-Cd-treated T-cell leukemia cells were determined.
In vivo anti-tumor effects of TC4ATS-Cd against T-cell leukemia cell lines
Prior to testing the anti-tumor activity of TC4ATS-Cd in vivo, we first conducted a preliminary experiment to determine the maximum tolerated dose (MTD) of TC4ATS-Cd and CdCl2. As a result of i.p. injection three times weekly for 2 weeks into mice, MTD of TC4ATS-Cd and CdCl2 were determined to be 13 μmol/kg (13 and 3 mg/kg, respectively) (Table S1). Next, we evaluated the effects of TC4ATS-Cd on tumor growth from Jurkat cells transplanted into SCID mice. Twenty days after s.c. implantation of Jurkat cells, TC4ATS-Cd or CdCl2 were administrated by i.p. injections three times weekly for 2 weeks, and body weight and tumor volume were measured (Fig. S3). There was no significant difference in body weight changes between vehicle-treated and TC4ATS-Cd-treated groups (Fig. S3b). Survival rates during the experimental period were 4/5 for animals administered vehicle, 5/5 for TC4ATS-Cd and 3/5 for CdCl2. (Figs5c and S3a). Tumor weights were significantly lower in TC4ATS-Cd-treated mice, as compared to those in vehicle-treated mice, while CdCl2 administration induced a non-significant decrease in tumor weight (Fig.5a,b). Cadmium accumulation in the liver and kidney of TC4ATS-Cd-treated mice was significantly lower than in CdCl2-treated mice, despite the administration at an equimolar dose (Fig.5c). Consistent with the above results, no significant atrophy was observed in the kidney tissue of TC4ATS-Cd-treated mice, while administration of CdCl2 notably induced renal atrophy (Fig. S4). Therefore, TC4ATS-Cd suppresses the tumor growth of T-cell leukemia cells in a xenograft model, without any apparent side effects.
Fig 5.
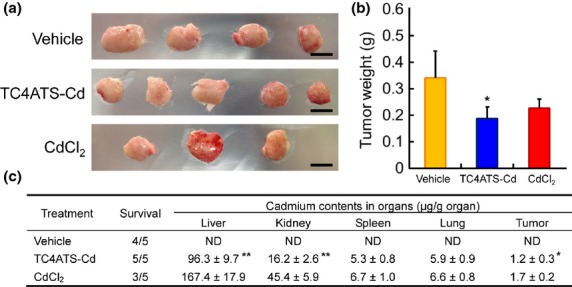
TC4ATS-Cd suppresses tumor growth of Jurkat cells in vivo. SCID mice were inoculated s.c. with Jurkat cells. Twenty days after inoculation, saline vehicle, TC4ATS-Cd (13 μmol/kg), or CdCl2 (13 μmol/kg) were administered by i.p. injection three times weekly for 2 weeks (all groups, n = 5). Thirty-five days after inoculation, mice were killed and tissues of interest and tumors were dissected out. Tumor and organ weights and their cadmium content were measured. (a) Images of excised tumors. Scale bars represent 1 cm. (b) Weights of excised tumor. Data are presented as means ± SD (vehicle, n = 4; TC4ATS-Cd, n = 5; CdCl2, n = 3). *P < 0.05, TC4ATS-Cd versus vehicle. (c) Mouse survival rates and cadmium levels in each organ. Cadmium contents were measured using a graphite furnace atomic absorption spectrometer. Data are presented as means ± SD (vehicle, n = 4; TC4ATS-Cd, n = 5; CdCl2, n = 3). ND, not detected. *P < 0.05, **P < 0.01, versus CdCl2.
Discussion
Cadmium is a well-known, highly toxic environmental and industrial pollutant, which causes a number of adverse health effects and diseases in humans, including renal dysfunction, hepatotoxicity, osteoporosis and cancer.4,14 At the cellular level, cadmium affects cell proliferation, differentiation, apoptosis and other cellular activities.15–17 In developing a new chelating approach for removing the accumulated cadmium from biological systems, we focused on thiacalixarene supramolecule. Recently, TC4ATS has been reported to remove cadmium from cadmium-contaminated soil through extraction methods.18 We found that TC4ATS effectively abrogates the anti-proliferative effects of cadmium against FLC-4 and AGS cells (Fig. S1). In contrast to its effect on epithelia-derived cells, TC4ATS showed only a slight neutralizing action in Jurkat cells (Fig. S1). These preliminary studies suggest that the complex of cadmium with TC4ATS may exhibit selective anti-proliferative activity against leukemia cells.
Recently, a lot of metal complexes have been evaluated as anti-tumor agents, similar to the classical drugs such as cisplatin or bleomycin. Two ruthenium-containing complexes, NAMI-A and KP1019, have reached human clinical testing.19–22 A gold-based antirheumatic agent, auranofin, exhibits anti-tumor activity in vitro and in vivo.23,24 Au(DMDT)Br2, a gold(III) complex, inhibits tumor growth by inhibiting proteasome activity.25,26 Zn(PyDTC)2 and Cu(PyDTC)2, complexes coupled with zinc(II) and copper(II), respectively, also have been shown to induce cell death by inhibiting proteasome activity.27 Cu(8-OHQ)2, a cupper(II) complex, has been shown to reduce cell viability in Jurkat cells.28 Bjelogrlić et al. have reported that a cadmium complex, CdCl2(Hfpsesc), exhibits anti-proliferative effects against a variety of cancer cell lines.29 Synthesized TC4ATS-Cd complex showed anti-proliferative effects elicited by induction of apoptosis in T-cell leukemia cell lines (Figs3 and 4), indicating that TC4ATS is a new type of host molecule that may be useful in the development of metal-coordinated anti-cancer drugs.
In contrast to the cadmium complexes, the cytotoxicity of host molecule TC4ATS-Na was not observed at doses below 1 mM in any of the tested cell lines (Figs2 and 3). The toxicity and adverse effects of TC4ATS are important issues that need to be considered in the development of its potential clinical applications as a host molecule. To our knowledge, the bioactivity and the toxicity of TC4ATS itself have not been reported until now. However, a large number of studies evaluating the bioactivity of p-tetrasulfonated calix[4]arene (C4ATS), a parent compound of TC4ATS, have been reported in the literature, with the findings reviewed by Perret et al.30 Evaluation of a number of toxic effects, including hemolytic activity, immune response and cytotoxicity towards various cell lines, demonstrated that C4ATS is not associated with any detectable toxicity.31–33 In an acute toxicity test in vivo, no toxic effects were observed at doses up to 100 mg/kg.34 Previous studies suggest that TC4ATS could be used as a host molecule, despite the different recognition capability of C4ATS for metal ions or organic molecules.35 However, further investigations are warranted to further determine the toxicity of TC4ATS.
TC4ATS-Cd showed no anti-proliferative effects in epithelia-derived cell lines except for HEK293 cells, even though CdCl2 reduced cell viability in all tested epithelia-derived cell lines (Fig.2). In addition, cadmium accumulation in organs, including kidney and liver, of TC4ATS-Cd-treated mice were lower than those of CdCl2-treated mice (Fig.5c). These results may be accounted for by slower TC4ATS-Cd incorporation into epithelia cells and/or faster TC4ATS-Cd elimination from epithelia cells. Although the mechanism underlying the difference in sensitivity between the TC4ATS-Cd and CdCl2 against epithelia-derived cells remains unclear, administration of TC4ATS-Cd may have fewer adverse effects.
In addition, leukemia cell lines were distinguishable in two groups: high sensitivity and low sensitivity to TC4ATS-Cd (Figs3 and 4d). Interestingly, JKT-beta-del cells in the low-sensitivity group are clone cells that lack the rearranged T-cell receptor (TCR) β gene and, thus, have impaired cell surface expression of TCR/CD3 complex in Jurkat cells in the high-sensitivity group.36 TCR recognizes agonist peptides associated with major histocompatibility complex proteins, and transduces signals controlling both T-cell activation and activation-induced apoptosis across the plasma membrane.37,38 The difference in TC4ATS-Cd-sensitivity determined by the expression levels of TCR suggests a novel mode of action for the cadmium complex. However, the detailed mechanism underlying the action of the TC4ATS-Cd is still under investigation.
In conclusion, TC4ATS-Cd complex exhibits anti-tumor activity in mouse xenograft models of human T-cell leukemia Jurkat cell lines. Treatment with TC4ATS-Cd showed anti-proliferative effects against any kind of leukemia cell lines, accompanied by induction of apoptosis. Furthermore, cadmium contents in liver, kidney and tumors in TC4ATS-Cd-treated mice were lower than in CdCl2-treated mice. These results suggest that TC4ATS-Cd has considerable therapeutic potential for use as an anti-cancer agent in the treatment of T-cell leukemia.
Acknowledgments
We thank Professor K. Kuba for helpful comments in the preparation of this manuscript. We thank Dr M. Kawagoe for assistance. This work was supported in part by JSPS KAKENHI Grant Numbers 22700913 and 24701019 to Y. K. and 25640087 to M. N.; The Tokyo Biochemical Research Foundation to M. Z. and T. S.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Neutralizing effect of TC4ATS-Na against cadmium toxicity.
Fig. S2. Western blot analysis of activated caspase-3 and cleaved PARP in epithelia-derived cell lines.
Fig. S3. TC4ATS-Cd elicits no adverse effects on tumor-bearing mice.
Fig. S4. Relative organ weights in tumor-bearing mice.
Table S1. In vivo toxicity tests of TC4ATS-Cd and CdCl2.
References
- International Agency for Research on Cancer. Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: IARC Scientific Publications; 1993. pp. 119–237. 58: [PMC free article] [PubMed] [Google Scholar]
- International Programme on Chemical Safety, Cadmium. Environmental Health Criteria. Geneva, Switzerland: WHO IPCS; 1992. p. 134. [Google Scholar]
- Tsuchiya K, Seki Y, Sugita M. Organ and tissue cadmium concentrations of cadavers from accidental deaths. Keio J Med. 1976;25:83–90. doi: 10.2302/kjm.25.83. [DOI] [PubMed] [Google Scholar]
- Inaba T, Kobayashi E, Suwazono Y, et al. Estimation of cumulative cadmium intake causing Itai-itai disease. Toxicol Lett. 2005;159:192–201. doi: 10.1016/j.toxlet.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Engström B. Effects of chelating agents on oral uptake and renal deposition and excretion of cadmium. Environ Health Perspect. 1984;54:219–32. doi: 10.1289/ehp.54-1568190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Johnson JE, Singh PK, Jones MM, Yan H, Carter CE. In vivo studies of cadmium-induced apoptosis in testicular tissue of the rat and its modulation by a chelating agent. Toxicology. 1996;107:1–8. doi: 10.1016/0300-483x(95)03195-l. [DOI] [PubMed] [Google Scholar]
- Zhukalin M, Blanksma MK, Silva TD, et al. Characterization and in vitro cytotoxicity testing of ethanolamine-derived cadmium chelating agents. Biometals. 2007;20:61–72. doi: 10.1007/s10534-006-9015-1. [DOI] [PubMed] [Google Scholar]
- Flora SJ, Pachauri V. Chelation in metal intoxication. Int J Environ Res. 2010;7:2745–88. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Shinkai S. Novel cavity design using calix[n]arene skeletons: toward molecular recognition and metal binding. Chem Rev. 1997;97:1713–34. doi: 10.1021/cr960385x. [DOI] [PubMed] [Google Scholar]
- Iki N, Morohashi N, Narumi F, Miyano S. High complexation ability of thiacalixarene with transition metal ions. The effects of replacing methylene bridges of tetra(pt-butyl)calix[4]arenetetrol by epithio groups. Bull Chem Soc Jpn. 1998;71:1597–603. [Google Scholar]
- Iki N, Morohashi N, Kabuto C, Miyano S. Coordination of epithio groups of ptert-butylthiacalix[4]arene in a Zn2+ complex studied by X-ray crystallography. Chem Lett. 1999;28:219–20. [Google Scholar]
- Kumagai H, Hasegawa M, Miyanari S, et al. Facile synthesis of ptert-butylthiacalix[4]arene by the reaction of ptert-butylphenol with elemental sulfur in the presence of a base. Tetrahedron Lett. 1997;38:3971–2. [Google Scholar]
- Iki N, Fujimoto T, Miyano S. A new water-soluble host molecule derived from thiacalixarene. Chem Lett. 1998;27:625–6. [Google Scholar]
- Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–8. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Hazen-Martin DJ, Sens DA, Blackburn JG, Sens MA. Cadmium nephrotoxicity in human proximal tubule cell cultures. In Vitro Cell Dev Biol. 1989;25:784–90. doi: 10.1007/BF02623661. [DOI] [PubMed] [Google Scholar]
- Aydin HH, Celik HA, Deveci R, et al. Characterization of the cellular response during apoptosis induction in cadmium-treated Hep G2 human hepatoma cells. Biol Trace Elem Res. 2003;95:139–53. doi: 10.1385/BTER:95:2:139. [DOI] [PubMed] [Google Scholar]
- Gulisano M, Pacini S, Punzi T, et al. Cadmium modulates proliferation and differentiation of human neuroblasts. J Neurosci Res. 2009;87:228–37. doi: 10.1002/jnr.21830. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu X, Song X, Sun T. Remediation of cadmium-contaminated soil by extraction with para-sulphonato-thiacalix[4]arene, a novel supramolecular receptor. Environ Pollut. 2012;167:93–100. doi: 10.1016/j.envpol.2012.03.042. [DOI] [PubMed] [Google Scholar]
- Mestroni G, Alessio E, Sava G. International Patent. New salts of anionic complexes of Ru (III) as antimetastatic and antineoplastic agents PCT C07F 15/00, A61K 31/28, WO 98/00431.
- Rademaker-Lakhai JM, van den Bongard D, Pluim D, Beijnen JH, Schellens JH. A Phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin Cancer Res. 2004;10:3717–27. doi: 10.1158/1078-0432.CCR-03-0746. [DOI] [PubMed] [Google Scholar]
- Berger MR, Garzon FT, Keppler BK, Schmähl D. Efficacy of new ruthenium complexes against chemically induced autochthonous colorectal carcinoma in rats. Anticancer Res. 1989;9:761–5. [PubMed] [Google Scholar]
- Hartinger CG, Jakupec MA, Zorbas-Seifried S, et al. KP1019, a new redox-active anticancer agent preclinical development and results of a clinical phase I study in tumor patients. Chem Biodivers. 2008;5:2140–55. doi: 10.1002/cbdv.200890195. [DOI] [PubMed] [Google Scholar]
- Sutton BM, McGusty E, Walz DT, DiMartino MJ. Oral gold. Antiarthritic properties of alkylphosphinegold coordination complexes. J Med Chem. 1972;15:1095–8. doi: 10.1021/jm00281a001. [DOI] [PubMed] [Google Scholar]
- Mirabelli CK, Johnson RK, Hill DT, et al. Correlation of the in vitro cytotoxic and in vivo antitumor activities of gold(I) coordination complexes. J Med Chem. 1986;29:218–23. doi: 10.1021/jm00152a009. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Giovagnini L, Marzano C, et al. Gold dithiocarbamate derivatives as potential antineoplastic agents: design, spectroscopic properties, and in vitro antitumor activity. Inorg Chem. 2005;44:1867–81. doi: 10.1021/ic048260v. [DOI] [PubMed] [Google Scholar]
- Milacic V, Chen D, Ronconi L, Landis-Piwowar KR, Fregona D, Dou QP. A novel anticancer gold(III) dithiocarbamate compound inhibits the activity of a purified 20S proteasome and 26S proteasome in human breast cancer cell cultures and xenografts. Cancer Res. 2006;66:10478–86. doi: 10.1158/0008-5472.CAN-06-3017. [DOI] [PubMed] [Google Scholar]
- Milacic V, Chen D, Giovagnini L, Diez A, Fregona D, Dou QP. Pyrrolidine dithiocarbamate zinc(II) and -copper(II) complexes induce apoptosis in tumor cells by inhibiting the proteasomal activity. Toxicol Appl Pharmacol. 2008;231:24–33. doi: 10.1016/j.taap.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel KG, Gupta P, Harbach RH, Guida WC, Dou QP. Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells. Biochem Pharmacol. 2004;67:1139–51. doi: 10.1016/j.bcp.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Bjelogrlić S, Todorović T, Bacchi A, et al. Synthesis, structure and characterization of novel Cd(II) and Zn(II) complexes with the condensation product of 2-formylpyridine and selenosemicarbazide. Antiproliferative activity of the synthesized complexes and related selenosemicarbazone complexes. J Inorg Biochem. 2010;104:673–82. doi: 10.1016/j.jinorgbio.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Perret F, Coleman AW. Biochemistry of anionic calix[n]arenes. Chem Commun. 2011;47:7303–19. doi: 10.1039/c1cc11541c. [DOI] [PubMed] [Google Scholar]
- Da Silva E, Shahgaldian P, Coleman AW, et al. Haemolytic properties of some water-soluble para-sulphonato-calix-[n]-arenes. Int J Pharm. 2004;273:57–62. doi: 10.1016/j.ijpharm.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Paclet MH, Rousseau CF, Yannick C, Morel F, Coleman AW. An absence of non-specific immune response towards para-sulphonato-calix[n]arenes. J Inclusion Phenom Macrocyclic Chem. 2006;55:353–7. [Google Scholar]
- Coleman AW, Baggetto LG, Lazar AN, Michaud MH, Magnard S. International Patent. Calixarene derivatives as anticancer agent PCT A61P 35/00, A61K 31/6615, C07F 09/12, WO/2007/119027.
- Coleman AW, Jebors S, Cecillon S, et al. Toxicity and biodistribution of para-sulfonato-calix[4]arene in mice. New J Chem. 2008;32:780–2. [Google Scholar]
- Guo DS, Wang K, Liu Y. Selective binding behaviors of p-sulfonatocalixarenes in aqueous solution. J Incl Phenom Macrocycl Chem. 2008;62:1–21. [Google Scholar]
- Iwamoto KS, Mizuno T, Seyama T, Kyoizumi S. Mutant p53: epigenetic mutator of the T-cell receptor via induction of methylation. Mol Carcinog. 1999;25:113–21. doi: 10.1002/(sici)1098-2744(199906)25:2<113::aid-mc6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owen JJ. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989;337:181–4. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Neutralizing effect of TC4ATS-Na against cadmium toxicity.
Fig. S2. Western blot analysis of activated caspase-3 and cleaved PARP in epithelia-derived cell lines.
Fig. S3. TC4ATS-Cd elicits no adverse effects on tumor-bearing mice.
Fig. S4. Relative organ weights in tumor-bearing mice.
Table S1. In vivo toxicity tests of TC4ATS-Cd and CdCl2.