Abstract
Neural epidermal growth factor-like like (NELL) 1 and 2 constitute a family of multimeric and multimodular extracellular glycoproteins. Although the osteogenic effects of NELL1 and functions of NELL2 in neural development have been reported, their expression and functions in cancer are largely unknown. In this study, we examined expression of NELL1 and NELL2 in renal cell carcinoma (RCC) using clinical specimens and cell lines. We show that, whereas NELL1 and NELL2 proteins are strongly expressed in renal tubules in non-cancerous areas of RCC specimens, their expression is significantly downregulated in cancerous areas. Silencing of NELL1 and NELL2 mRNA expression was also detected in RCC cell lines. Analysis of NELL1/2 promoter methylation status indicated that the CpG islands in the NELL1 and NELL2 genes are hypermethylated in RCC cell lines. NELL1 and NELL2 bind to RCC cells, suggesting that these cells express a receptor for NELL1 and NELL2 that can transduce signals. Furthermore, we found that both NELL1 and NELL2 inhibit RCC cell migration, and NELL1 further inhibits RCC cell adhesion. These results suggest that silencing of NELL gene expression by promoter hypermethylation plays roles in RCC progression by affecting cancer cell behavior.
Keywords: Cell migration, methylation, NELL1, NELL2, renal cell carcinoma
Kidney cancers, including renal cell carcinoma (RCC) and urothelial carcinoma of the renal pelvis and ureter, account for approximately 3% of all cancer incidences. Although mortality rates of kidney cancers are stabilizing, the number of new cases is increasing in the USA.1 Kidney cancers are classified according to their histological type and origin. Cancers originating from kidney epithelia are called RCC. The most common subtype is clear cell RCC (CCRCC), which derives its histological appearance from intracellular accumulations of glycogen and lipid. The risk factors and molecular mechanisms of renal carcinogenesis have been extensively studied. Loss of heterozygosity or mutations of the von Hippel-Lindau (VHL) tumor suppressor gene and the mesenchymal–epithelial transition (c-MET) proto-oncogene, which are responsible for inherited tumor syndromes involving the kidney, have been found in sporadic RCC.2,3 Epigenetic inactivation by DNA methylation was shown to cause downregulation of the mRNA expression of RAS-associated domain family-1A (RASSF1A), a tumor suppressor gene, in RCC.4,5 However, the mechanisms of RCC development and progression remain unclear.
Neural epidermal growth factor-like (Nel) is a multimeric and multimodular extracellular glycoprotein with significant structural similarity to thrombospondin-1. Whereas Nel was originally identified in chickens,6 two related genes, Nel-like1 (NELL1) and Nel-like2 (NELL2), were subsequently described in mammals, including humans.7 Based on sequence similarities, mammalian NELL2 appears to be the ortholog of chicken Nel. Human NELL1 and NELL2 have approximately 55% homology at the amino acid level. The human NELL1 and NELL2 genes were mapped to chromosomes 11p15.1 and 12q12, respectively.
Previous studies showed that NELL1 plays important roles in osteogenic differentiation.8,9 NELL1 is overexpressed in patients with unilateral coronal synostosis,8 which is a common form of craniosynostosis. NELL1 also induces bone regeneration in calvarial defects10 and in a rat femoral distraction model.11
Nel-like2 is predominantly expressed in the nervous system and has been implicated in neural development.7,12–14 Recently, we showed that chicken Nel (NELL2) regulates retinal axon guidance15 and production of retinal ganglion cells during development.16
Expression of NELL1 and NELL2 genes has been studied in human lymphoma, in cancers of the central nervous system, prostate, and bladder, and in cancer cell lines.17–20 Furthermore, NELL1 and NELL2 expression was reported in adult and embryonic normal kidney.6,7,17 However, little is known about the expression and functions of NELL1 and NELL2 in RCC. Here we show that, whereas NELL1 and NELL2 are strongly expressed in non-cancerous renal tubules, their expression is downregulated in cancerous areas of CCRCC specimens and in RCC cell lines. In addition, the CpG islands in the NELL1 and NELL2 promoter regions are hypermethylated in RCC cells. In vitro, NELL1 suppresses the migration and adhesion of RCC cells, and NELL2 suppresses RCC cell migration. These results suggest that NELL protein expression is downregulated in RCC, presumably by promoter hypermethylation, and that lack of NELL expression may contribute to RCC progression by altered regulation of cancer cell behavior.
Materials and Methods
Clinical samples
Formalin-fixed paraffin-embedded CCRCC tissues were obtained from surgical pathology files (between 2010 and 2012) of the Pathology Section of Kanazawa University Hospital (Kanazawa, Japan). This project was approved by the research ethics committee on genetic analysis of Kanazawa University (approval no. 320).
Immunohistochemistry
Four-micron sections of CCRCC samples were analyzed by immunohistochemistry (IHC) using anti-NELL1 and anti-NELL2 antibodies (HPA051535, 1/100 and HPA035715, 1/300 dilutions; Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's protocol, except that antigen retrieval was carried out at 121°C for 15 min. The LSAB2 kit Universal (Dako, Glostrup, Denmark) was used for detection. NELL1 and NELL2 expression was scored for both cancerous and non-cancerous areas on a graded 0 (no expression) to +3 (very strong expression) scale. Immunohistochemistry scores were calculated by subtracting the scores of cancerous areas from those of adjacent non-cancerous areas. Therefore, positive IHC scores indicated downregulation of NELL expression in cancer cells, whereas negative scores indicated upregulation.
Cell lines
Renal cell carcinoma (OS-RC-2, TUHR14TKB, and VMRC-RCW) and HEK293T cell lines were obtained from the Riken Cell Bank (Tsukuba, Japan). Renal cell carcinoma and HEK293T cells were cultured in RPMI and DMEM, respectively, containing 10% FBS and penicillin/streptomycin.
Quantitative RT-PCR, methylation-specific PCR, and bisulfide sequencing PCR
Quantitative RT-PCR (qPCR) was carried out with first-strand cDNAs of the cell lines and SYBR PremixEx TaqII (Takara Bio, Otsu, Japan) using the Viia7 system (Life Technologies, Carlsbad, CA, USA). Unmethylated cytosines in the genomic DNAs from the cell lines were converted to uracil by bisulfide reactions using the EZ DNA Methylation-Gold Kit (ZYMO Research, Irvine, CA, USA). Methylation-specific PCR (MSP) was carried out using the TaKaRa EpiScope MSP Kit. Bisulfide sequencing PCR (BSP) was carried out using the bisulfide-treated genomic DNAs and EpiTaq HS (Takara). The PCR products were inserted into the pGEM-T easy vector (Promega KK, Tokyo, Japan) and transformed into DH5α, and plasmids isolated from 10 colonies of each sample were sequenced. The primer sequences and PCR conditions used are shown in Table1.
Table 1.
Primer sequences and PCR conditions
| Gene | PCR | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) | Annealing temperature | PCR cycles |
|---|---|---|---|---|---|
| NELL1 | Quantitative RT-PCR | ATGGCCAGGTGTGGACCTTG | TCTGGTGTCACATTCTGGGC | 60 | 40 |
| NELL2 | Quantitative RT-PCR | CCACTTAAGTCGGCTCTTGC | ATGGTCTGGTCCTTGCACTC | 60 | 40 |
| GAPDH | Quantitative RT-PCR | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCC | 60 | 40 |
| NELL1 | MSP-Methyl. | GATTTCGATTTCGGTGTTTC | CTACGTTCGTCTAAAAACCCG | 60 | 35 |
| NELL2 | MSP-Methyl. | CGTTTTATTTGGTAGGGGGC | TTCCCTACCGAAAACGAAA | 60 | 35 |
| NELL1 | MSP-Unmethyl. | TGGGATTTTGATTTTGGTGTTTT | ACTACATTCATCTAAAAACCCAAAC | 55 | 40 |
| NELL2 | MSP-Unmethyl. | TTATGTTTTATTTGGTAGGGGGT | AATTCCCTACCAAAAACAAAAC | 63 | 35 |
| NELL1 | Bisulfite sequencing | TTATAGGGGTTTAATAGGAGAGAGG | CCTAACTTAAAACCR*CCAAACTTACTAA | 55 | 35 |
| NELL2 | Bisulfite sequencing | TGTTTTTTGTTGTATGTTGGTTT | AAAAAAAAAAACCTCCCCAAAC | 55 | 35 |
R(A+G). Methyl., methylated; MSP, methylation-specific PCR; Unmethyl., unmethylated.
5-Azacytidine treatment of RCC cell lines
The OS-RC-2, VMRC-RCW, and TUHR14TKB cells were plated in 100-mm plates on day 0. The cells were treated with 5 μM 5-azacytidine (Sigma-Aldrich) for 5 days. Total RNA extraction, cDNA synthesis and qPCR were carried out as described above.
Luciferase assay
NELL1 and NELL2 putative promoter regions were ligated into pCpGL-basic21 (kindly provided by Prof. Michael Rehli, University Hospital Regensburg, Regensburg, Germany). NELL1 and NELL2 transcriptional factors, RUNX222 and E2F123 were kindly provided by Prof. Yoshiaki Ito (National University of Singapore, Singapore) (pEF-BOS-RUNX2)24 and Prof. Kristian Helin (University of Copenhagen, BRIC, Copenhagen, Denmark, E2F1/pCMV-HA,25 Addgene plasmid #24225). RUNX2 was amplified from pEF-BOS-RUNX2 and inserted into pCMV-HA-N (Takara Bio). After incubation with/without methyltransferases, NELL1/pCpGL and NELL2/pCpGL were transfected with their transcriptional factors and pGL 4.74 into HEK293T cells. The luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega KK).
Preparation of alkaline phosphatase fusion proteins
NELL1 protein fused to an alkaline phosphatase (AP) tag (NELL1-AP), NELL2-AP, and AP proteins were prepared and purified as previously described.26
Cell migration assay
The cell migration assay was carried out basically as previously described.27 The lower side of the Transwell membrane inserts (product #3422; Corning, Corning, NY, USA) was coated with 40 nM or 400 nM AP, NELL1-AP, or NELL2-AP protein at 4°C overnight, blocked with 20 mg/mL BSA in PBS for 3 h at 4°C, and then washed with RPMI-1640. OS-RC-2 or VMRC-RCW cells (1 × 105 cells) were dissociated with 1 mM EDTA and were seeded inside the Transwell. The cells were then allowed to migrate to the lower side of the membrane in RPMI-1640 with 1% FBS at 37°C overnight, fixed with 4% paraformaldehyde, and then stained with hematoxylin. The upper side of the membrane was wiped with a cotton swab, and the cells that had migrated to the lower side of the membrane were quantified. For each Transwell membrane, at least three high-power fields were examined under a microscope. Each experiment was repeated three times.
Cell adhesion assay
Ninety-six-well cell culture plates were coated with AP, NELL1-AP, or NELL2-AP protein (400 nM each) at 4°C overnight. After blocking with 10 mg/mL BSA, the wells were washed twice with PBS, and OS-RC-2 or VMRC-RCW cells were then plated at a density of 2.5 × 104 cells/well. The cells were cultured at 37°C for 3 h in RPMI-1640 with 2% FBS, washed twice with PBS, and then fixed with 4% paraformaldehyde. Cell numbers in three high-power fields per well were counted using an inverted microscope. Experiments were repeated under the same conditions at least three times.
Statistical analysis
For statistical analysis, anova (for IHC scores with more than three parameters) and Student's t-test (for IHC scores with two parameters, and cell migration and adhesion assays) were used. Supplementary information is available in Document S1.
Results
NELL1 and NELL2 protein expression is downregulated in CCRCC
We first examined and compared NELL1 and NELL2 protein expression in cancerous regions and adjacent non-cancerous areas in tissue samples of 64 CCRCC patients. Both NELL1 and NELL2 proteins were abundantly expressed in renal tubules in non-cancerous regions (Fig.1). At higher magnification (Fig.1e,g), NELL1 and NELL2 proteins were observed to be localized in granular cytoplasmic patterns, consistent with the fact that NELL1 and NELL2 are secreted glycoproteins and thus processed in the secretory pathway. In contrast, NELL1 and NELL2 expression was significantly lower in cancerous areas (IHC scores, 1.88 (NELL1) and 2.17 (NELL2); expression grade of cancerous area/non-cancerous area, 1.05/2.09 (NELL1, P < 0.01) and 0.72/2.93 (NELL2, P < 0.01); Fig.1h). No significant correlations were observed between the degree of NELL downregulation and clinicopathological parameters, including sex or age of patients, or vascular involvement or grade of tumors (Table2).
Fig 1.
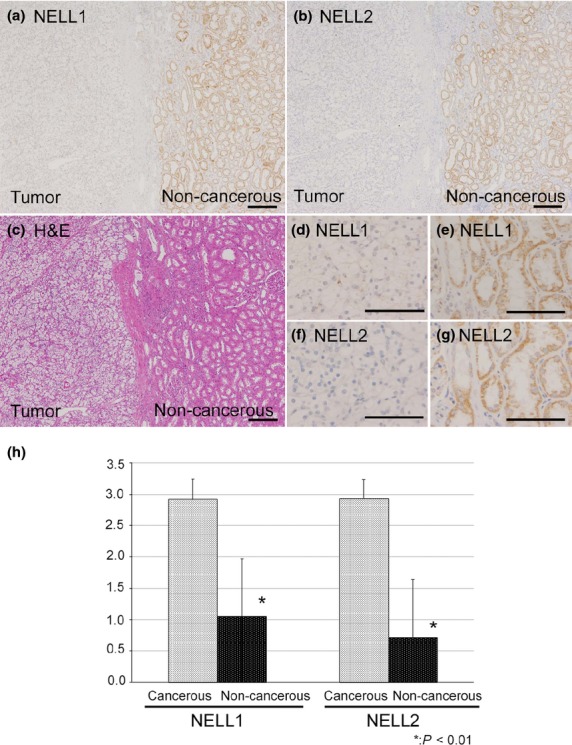
Protein expression of neural epidermal growth factor-like like 1 (NELL1) and NELL2 in clear cell renal cell carcinoma (CCRCC) tissues. (a, b, d–g) Formalin-fixed paraffin-embedded sections of CCRCC tissues were immunostained with anti-NELL1 (a, d, e) or anti-NELL2 (b, f, g) antibody. Images (d, e) and (f, g) are higher magnification views of (a) and (b), respectively. Both NELL1 and NELL2 were detected in the cytoplasm of renal tubule cells in non-cancerous (a, b, right side [non-cancerous]), but not in cancerous areas (a, b, left side [tumor]). (c) H&E stain. Scale bar = 200 μm (a–c), 100 μm (d–g). (h) Average NELL1 and NELL2 expression grades in the cancerous and non-cancerous areas.
Table 2.
Correlation between neural epidermal growth factor-like like 1 (NELL1) and NELL2 subtraction scores (immunohistochemistry [IHC] score) and clinicopathological parameters
| No. of cases | Average of NELL1 IHC scores | P-value | Average of NELL2 IHC scores | P-value | |
|---|---|---|---|---|---|
| Overall | 64 | 1.88 | 2.17 | ||
| Sex | |||||
| Male | 43 | 1.84 | 0.71† | 2.16 | 0.93† |
| Female | 21 | 1.95 | 2.19 | ||
| Age, years | |||||
| <60 | 26 | 1.65 | 0.19† | 1.88 | 0.10† |
| ≥60 | 38 | 2.03 | 2.37 | ||
| Grade | |||||
| G1 | 20 | 2.10 | 0.18‡ | 2.30 | 0.46‡ |
| G2 | 43 | 1.74 | 2.09 | ||
| G3 | 1 | 3.00 | 3.00 | ||
| Vascular involvement | |||||
| v0 | 42 | 1.86 | 0.86† | 2.19 | 0.86† |
| v1 | 22 | 1.90 | 2.14 | ||
| pT | |||||
| pT1 | 47 | 1.83 | 0.85§ | 2.11 | 0.69§ |
| pT2 | 4 | 2.00 | 2.50 | ||
| pT3 | 13 | 2.00 | 2.31 | ||
| pT4 | 0 | ||||
Student's t-test was carried out. ‡More than three parameters, however only two parameters had more than three samples. Student's t-test was carried out between G1 and G2. §anova was carried out.
Downregulation of NELL1 and NELL2 expression in RCC cell lines
We next examined and compared NELL1 and NELL2 mRNA and protein expression in RCC cell lines with that in the HEK293T cell line derived from human embryonic kidney using qRT-PCR and Western blot. We found by RT-PCR that NELL1 and NELL2 mRNA expression levels in the cancer cell lines were 10–1000 times lower than those in HEK293T cells (Fig.2). Consistent with this finding, the Western blot analysis showed that the protein expression of NELL1 and NELL2 was decreased in the RCC cells compared to that observed in HEK293 cells (Fig. S1). The combined results indicate that NELL1 and NELL2 expression is downregulated in RCC at both the mRNA and protein levels.
Fig 2.

mRNA expression of NELL1 and NELL2 in renal cell carcinoma cell lines. NELL1 (a) and NELL2 (b) mRNA expression levels in renal cell carcinoma cell lines OS-RC-2, VMRC-RCW, and TUHR14TKB and control HEK293T cells were analyzed using quantitative RT-PCR. Expression levels are quantified as NELL1 or NELL2/GAPDH and plotted as means ± SD.
Promoter hypermethylation of NELL1 and NELL2 genes in RCC
Aberrant DNA hypermethylation of promoter CpG islands with associated gene silencing plays crucial roles in tumor progression. Because hypermethylation of the NELL1 5′-UTR has been reported in esophageal adenocarcinoma and colorectal cancer,28,29 we speculated that the downregulation of the NELL1 and NELL2 genes observed in RCC may be caused by promoter hypermethylation in those genes. To test this hypothesis, the methylation status of putative promoter CpG islands of NELL1 and NELL2 genes in RCC cell lines was examined using MSP. NELL1 gene promoter hypermethylation was detected in VMRC-RCW and TUHR14TKB cells. NELL2 promoter hypermethylation was detected in VMRC-RCW cells, and weak hypermethylation was observed in OS-RC-2 cells. No significant methylation was detected in the NELL1 or NELL2 promoter regions in control HEK293 cells (Fig.3).
Fig 3.

Methylation-specific PCR analysis of the NELL CpG islands in renal cell carcinoma cells. Genomic DNA was analyzed by PCR using specific primers for methylated (Methyl) or unmethylated (Unmethyl) CpG islands in NELL1 or NELL2 putative promoter regions (NELL1 methylated, NELL1 unmethylated, NELL2 methylated, and NELL2 unmethylated).
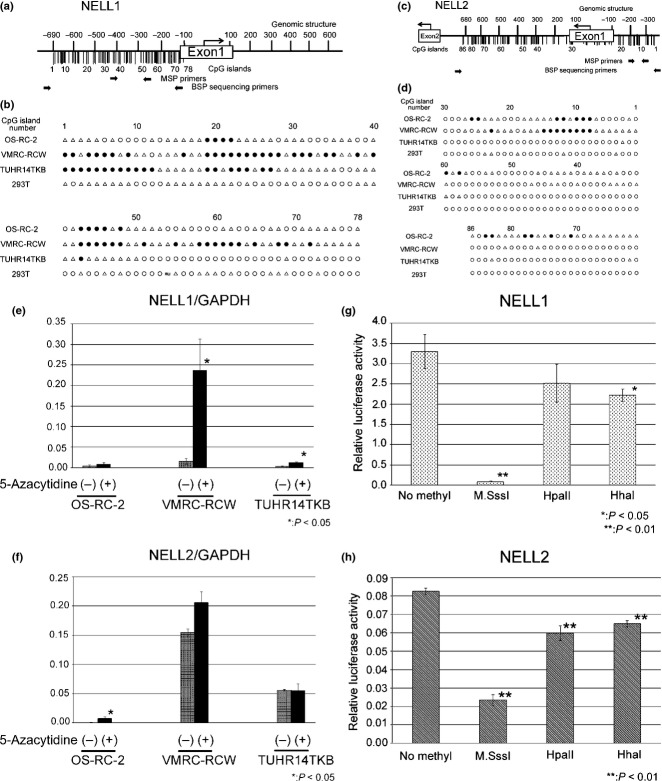
To further investigate promoter methylation status, we carried out BSP of the CpG islands that included the putative promoter regions of NELL1 (−690 to −100) and NELL2 (−320 to +678) in RCC cell lines (Fig.4). We numbered the CpG islands in these regions in the 5′ to 3′ direction (NELL1, Nos. 1–78; NELL2, Nos. 1–86). The analyses of the NELL1 promoter detected CpG island hypermethylation throughout the analyzed region in VMRC-RCW cells, high methylation of CpG islands around No. 20 and No. 45 in OS-RC2 cells, and frequent methylation of CpG islands from No. 1 to No. 30 in TUHR14TKB cells (Fig.4b). The analyses of the NELL2 promoter region detected methylation of CpG islands around No. 10 in both OS-RC2 and VMRC-RCW cells (Fig.4d). The analyses of the NELL2 promoter region showed methylation of CpG islands around No. 10 in both the OS-RC2 and VMRC-RCW cells and around No. 80 in the OS-RC-2 cells (Fig.4d). No significant CpG island hypermethylation was detected at the NELL1/2 promoter regions in the HEK293T cells.
Fig 4.
Hypermethylation of CpG islands in the putative promoter region of the NELL1 and NELL2 genes. (a, c) CpG islands (vertical bars) in the putative promoter regions of NELL1 (a) and NELL2 (c) genes are numbered in the 5′ to 3′ direction (NELL1, Nos. 1–78; NELL2, Nos. 1–86). Positions of primer sequences for methylation-specific PCR (MSP) and bisulfide sequencing PCR (BSP) are also shown. (b, d) Methylation status of CpG islands. ●, Methylated CpG islands (methylation detected in more than 8 of 10 clones); ○, unmethylated islands (methylation not detected in any of 10 clones); Δ, incompletely methylated CpG islands (methylation detected in one to seven clones). *This nucleotide in HEK293T differed from the registered sequence in the database. (e, f) NELL1 and NELL2 mRNA expression levels in OS-RC-2, VMRC-RCW and TUHR14TKB cells with/without 5-azacytidine treatment. The NELL1, NELL2 and GAPDH mRNA expression levels were measured using quantitative PCR. NELL1 and NELL2 mRNA expression levels were normalized by that of GAPDH and plotted as the mean ± SD. (g, h) Effects of promoter methylation on NELL1 and NELL2 expression. Firefly luciferase reporter constructs containing putative promoter regions of NELL1 and NELL2 genes (NELL1/pCpGL and NELL2/pCpGL) were treated with M.SssI, HpaII, or HhaI methyltransferases and then transfected into HEK293T cells with RUNX2/pCMVHA (for NELL1/pCpGL) or E2F1/pCMVHA (for NELL2/pCpGL) and the Renilla luciferase expression vector pGL 4.74. The ratio of the firefly/Renilla luciferase activity was plotted as the mean ± SD.
If the NELL1/2 downregulation observed in RCC cells is caused by promoter hypermethylation, the expression would be recovered by CpG demethylation. In order to test this hypothesis, we treated RCC cell lines with 5 μM 5-azacytidine and examined the effects on the NELL1/2 mRNA expression. As shown in Figure4(e), the NELL1 expression was elevated in VMRC-RCW cells. The NELL1 expression in TUHR14TKB cells was also significantly increased after 5-azacytidine treatment. The NELL2 expression in OS-RC-2 cells was elevated by 5-azacytidine treatment (Fig.4f). A slight increase in NELL2 expression was observed in VMRC-RCW cells, although the difference was not statistically significant (P = 0.071). Whereas the increases of NELL1 and NELL2 expression by 5-azacytidine were statistically significant in most of the above RCC cell lines, their expression levels after treatment were still lower than those in HKE293T (Figs2,4e,f), suggesting that promoter methylation partially contributes to the silencing of NELL expression.
In order to confirm the effects of CpG methylation on NELL1/2 expression, we carried out a luciferase assay using the putative promoter regions of NELL1 and NELL2. Consequently, reporter constructs containing unmethylated NELL1 and NELL2 promoter regions showed high luciferase activity. In contrast, the luciferase activity was significantly reduced by treatment with M.SssI, which methylates all cytosine residues within the dinucleotide recognition sequence 5′-CpG-3′. Treatment with HpaII, which methylates the second cytosine residue in 5′-CCGG-3′, or HhaI, which methylates the first cytosine residue in 5′-GCGC-3′, resulted in partial reduction of the luciferase activity (Fig.4g,h).
These data indicate that the CpG island of NELL1 and NELL2 promoter regions is hypermethylated in RCC, and suggest that downregulation of NELL1 and NELL2 expression in RCC was caused, at least in part, by promoter hypermethylation.
NELL1 and NELL2 bind to RCC cells
The above data on NELL1 and NELL2 expression may suggest that downregulation of NELL1 and NELL2 gene expression is involved in the development of RCC. If that is the case, it is likely that NELL1 and NELL2 proteins exert their functions by binding to a cell surface receptor and transducing intracellular signals. We therefore examined whether NELL proteins can bind to RCC cell lines using NELL1-AP or NELL2-AP as a probe (Fig. S2a). We incubated RCC and HEK293T cells with NELL1-AP or NELL2-AP, and binding was detected using the AP enzyme reaction.30 Significant binding activity was detected in all cell lines tested, including HEK293T cells (Fig. S2b). These results indicate that NELL1 and NELL2 can bind to RCC cells, and suggest that NELL1 and NELL2 can be recognized through a specific receptor expressed on those cells.
Effects of NELL proteins on RCC cell behavior
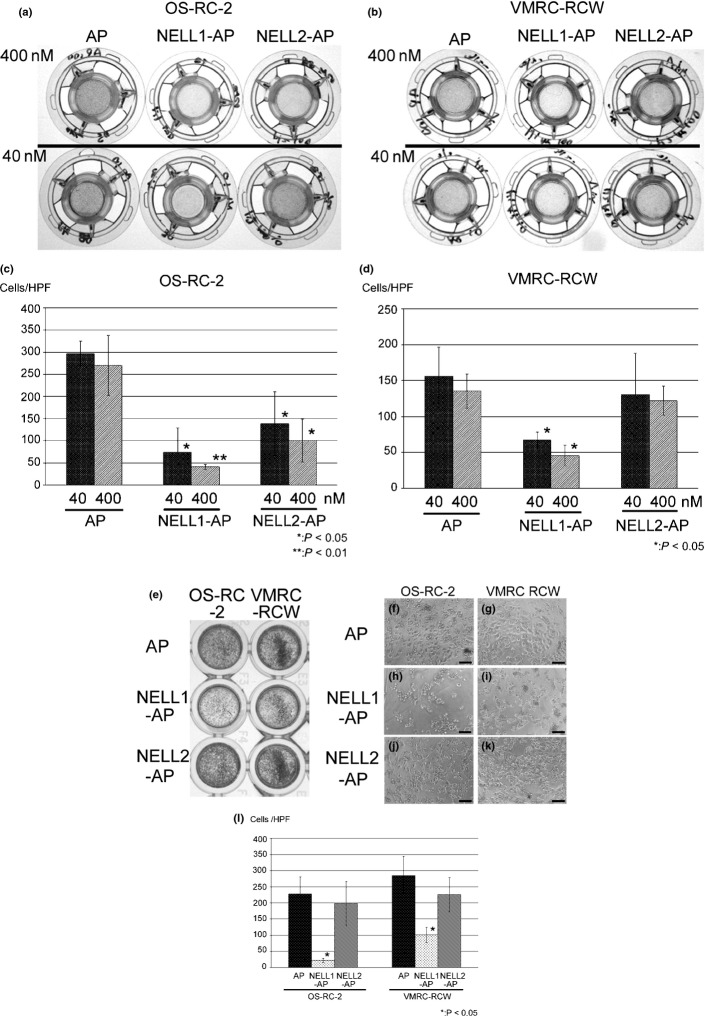
We previously showed that chicken Nel (NELL2) can act as an inhibitory axon guidance molecule and regulate morphology of the growth cone.15 As many axon guidance molecules also regulate behavior of the cell body, we examined whether NELL proteins can affect the migration and adhesion of RCC cells. We first tested the effects of NELL1 and NELL2 on cell migration by using a transfilter assay system. NELL1-AP significantly inhibited the migration of OS-RC-2 and VMRC-RCW cells in a dose-dependent manner. NELL2-AP also significantly inhibited the migration of OS-RC-2, but not that of VMRC-RCW cells (Fig.5a–d).
Fig 5.
Effect of neural epidermal growth factor-like like 1 (NELL1) and NELL2 on renal cell carcinoma cell migration and adhesion. (a–d) Transfilter cell migration assay. The bottom side of the Transwell filter was coated with 40 nM or 400 nM alkaline phosphatase (AP), NELL1-AP or NELL2-AP. OS-RC-2 (a) or VMRC-RCW (b) cells were placed inside the Transwell chamber and allowed to migrate to the bottom side of the filter overnight. The cells that migrated to the bottom side of the filter were stained with hematoxylin and photographed. (c, d) Quantification of cell migration. The numbers of cells that migrated to the bottom side of the filter were plotted as mean ± SD. (e–l) Cell adhesion assay. OS-RC-2 (left three wells of e, and f, h, j) and VMRC-RCW (right three wells of e, and g, i, k) cells were seeded on AP (e, f, g), NELL1-AP (e, h, i), or NELL2-AP (e, j, k) coated dishes (400 nM each). Low (e) and high (f–k) power views after cell adhesion assay. Adherent cells were stained with hematoxylin. Adherent cell numbers were quantified and plotted as means ± SD (l). HPF, high-power field.
We next examined whether NELL1 or NELL2 proteins affect cell adhesion using NELL-AP protein-coated dishes. Significant numbers of OS-RC-2 and VMRC-RCW cells adhered to the AP-coated control dish. In contrast, the number of adherent cells on the NELL1-AP substratum was dramatically decreased. NELL2-AP did not affect adhesion of the RCC cells (Fig.5e–l). The combined results indicate that NELL1 and NELL2 can regulate RCC cell behavior.
Discussion
In the present study, we analyzed the expression, genomic DNA methylation, and functions of NELL1 and NELL2 in RCC. Immunohistochemical studies of clinical samples showed that, whereas NELL1 and NELL2 are strongly expressed in non-cancerous renal tubules, their expression is significantly downregulated in CCRCC areas (Fig.1). Consistent with the results of clinical samples, NELL1 and NELL2 mRNA and protein expression was also reduced in RCC cell lines compared with that in HEK293T cells, which are derived from non-cancerous kidney (Figs2,S1).
NELL1 and NELL2 expression has been previously reported in other cancers using clinical samples and cell lines. For example, NELL1 gene loss was observed in more than 40% of Hodgkin's lymphoma patients.20 NELL2 mRNA expression is upregulated in benign prostate hyperplasia and prostate cancer.18 NELL2 is also considered as a candidate biomarker for bladder cancer.19 Among nervous system tumors, NELL1 and NELL2 are predominantly expressed in neuroblastoma cell lines and are also expressed in medulloblastoma, central neurocytoma, and some astrocytic tumors.17 A recent study showed that posterior fossa ependymoma is composed of two molecularly distinct groups, and that NELL2 is the most significant marker for the group that represents less invasive and less metastatic tumors with better prognosis.31 These results suggest that NELL may be involved in cancer development.
Our studies of DNA methylation status showed that the CpG islands in the NELL1 and NELL2 promoters are hypermethylated in RCC cells, whereas no significant NELL1/2 promoter hypermethylation was detected in control HEK293 cells. The degree of promoter hypermethylation and effect of 5-azacytidine treatment varied between RCC cell lines. In the RCC cell lines in which promoter hypermethylation was particularly prominent (NELL1 in VMRC-RCW and TUHR14TKB cells, NELL2 in OS-RC-2 cells), the NELL mRNA expression was significantly recovered by 5-azacytidine treatment (Fig.4e,f). In contrast, recovery of NELL2 expression was not statistically significant in VMRC-RCW and TUHR14TKB cells. Interestingly, CpG islands around No. 80 in the NELL2 promoter were methylated only in OS-RC-2, but not in VMRC-RCW or TUHR14TKB cells (Fig.4d). In addition, NELL2 expression levels without treatment were much lower in OS-RC-2 cells than those in VMRC-RCW or TUHR14TKB cells. These results suggest that CpG methylation around No. 80 may be important for NELL2 downregulation, although it is plausible that other factors are also involved in NELL2 downregulation in OS-RC-2 cells, because the NELL2 expression level restored by 5-azacytidine treatment was still lower than those in untreated VMRC-RCW, TUHR14TKB, and HEK293T cells.
DNA methylation of gene promoters is an important mechanism of transcriptional downregulation of gene expression. In cancer cells, many tumor suppressor genes have been shown to be silenced by promoter hypermethylation, including p16/CDKN2/MTS1,32 retinoblastoma gene,33 and EphA7.34 In RCC, transcriptional silencing by promoter hypermethylation was reported for several tumor-suppressor genes, including VHL35 and RASSF1A,36 and differentially expressed in adenocarcinoma of the lung (DAL-1/4.1B).37 DNA methylation profiling of 10 tumor suppressor genes in 100 kidney cancers showed hypermethylation of promoter DNA in 93% of kidney tumor cases, approximately two-thirds of which have two or more hypermethylated genes.38
Promoter hypermethylation and silencing of the NELL1 gene were detected in approximately 44% of colorectal carcinomas.29 In esophageal cancer, methylation levels of the NELL1 gene are significantly higher in Barrett's metaplasia, dysplasia in Barrett's esophagus, and esophageal adenocarcinoma than those in normal esophagus, and inversely correlate with patient survival rates. These findings indicate that promoter hypermethylation of NELL1 is a common event that occurs at early stages of Barrett's-associated esophageal cancer progression and is a potential biomarker of poor prognosis in early stage esophageal adenocarcinoma.28
In vitro assays of cell behavior in the present study indicated that both NELL1 and NELL2 inhibit RCC cell migration and that NELL1 further inhibits RCC cell adhesion. As regulation of cell migration and adhesion plays crucial roles in cancer invasion and metastasis, these results raise the possibility that loss of NELL expression in RCC is involved in cancer progression. Although no significant correlation was found between NELL expression levels and the size or extent of the primary tumor (pT stages), our data show that hypermethylation and downregulation of the NELL genes already occurs at early stages of RCC progression. These findings are consistent with esophageal cancer data,28 and suggest that decreased NELL expression plays roles in early oncogenesis.
Our results showing that NELL1 and NELL2 bind to RCC cells suggest that these molecules regulate the behavior of cancer cells by binding to a cell surface receptor and transducing intracellular signals. We also previously reported that chicken Nel (NELL2) can bind to and regulate the behavior of retinal axons.15 Although no receptors have been identified for NELL2, recent studies have shown that integrins (β139 and α3β140) act as cell surface receptors for NELL1. Binding of NELL1 to these integrins promotes adhesion of several different types of cells, including bone marrow stromal cells, multipotential mesenchymal cells, chondroprogenitor cells, pre-osteoblasts, and human osteocarcinoma cells.39,40 As it is likely that NELL1 protein interacts with a variety of receptors through its different domains, the opposite effects of NELL1 on cell adhesion may be mediated by different NELL1 receptors that interact with different domains of NELL1. Further studies, especially regarding identification of additional receptors and signaling pathways, are required to fully understand NELL functions in cancer cell behavior regulation.
Acknowledgments
We thank Professors Michael Rehli, Yoshiaki Ito, and Kristian Helin for gifting plasmids, Dr. Alasdair MacKenzie (University of Aberdeen) for helpful discussion, and Mr. Takashi Mizukami, Ms. Ryoko Tokuda, and Ms. Sanae Funaoka (Kanazawa University) for technical assistance.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Neural epidermal growth factor-like like 1 (NELL1) and NELL2 protein expression in renal cell carcinoma cell lines.
Fig. S2. Binding activity of neural epidermal growth factor-like like protein fused to an alkaline phosphatase tag (NELL-AP) to renal cell carcinoma cells.
Doc. S1. Supplementary materials and methods.
References
- Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; . (eds). Available from URL: http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]
- Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Junker K, Nakaigawa N, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–50. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- Dreijerink K, Braga E, Kuzmin I, et al. The candidate tumor suppressor gene, RASSF1A, from human chromosome 3p21.3 is involved in kidney tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:7504–9. doi: 10.1073/pnas.131216298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Dammann R, Pfeifer GP. Hypermethylation of the CpG island of the RASSF1A gene in ovarian and renal cell carcinomas. Int J Cancer. 2001;94:212–7. doi: 10.1002/ijc.1466. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S, Noji S, Koyama E, et al. New gene, NEL, encoding A M(R) 93-K protein with EGF-like repeats is strongly expressed in neural tissues of early-stage chick-embryos. Dev Dyn. 1995;203:212–22. doi: 10.1002/aja.1002030209. [DOI] [PubMed] [Google Scholar]
- Watanabe TK, Katagiri T, Suzuki M, et al. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. 1996;38:273–6. doi: 10.1006/geno.1996.0628. [DOI] [PubMed] [Google Scholar]
- Ting K, Vastardis H, Mulliken JB, et al. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14:80–9. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Kuroda S, Carpenter D, et al. Craniosynostosis in transgenic mice overexpressing NELL-1. J Clin Invest. 2002;110:861–70. doi: 10.1172/JCI15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaloo T, Cowan CM, Chou YF, et al. Nell-1-induced bone regeneration in calvarial defects. Am J Pathol. 2006;169:903–15. doi: 10.2353/ajpath.2006.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Peng JA, Yuan M, et al. NELL1 promotes high-quality bone regeneration in rat femoral distraction osteogenesis model. Bone. 2011;48:485–95. doi: 10.1016/j.bone.2010.10.166. [DOI] [PubMed] [Google Scholar]
- Kim H, Ha CM, Choi J, et al. Ontogeny and the possible function of a novel epidermal growth factor-like repeat domain-containing protein, NELL2, in the rat brain. J Neurochem. 2002;83:1389–400. doi: 10.1046/j.1471-4159.2002.01245.x. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Oyasu M, Kawakami M, et al. Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun. 1999;265:79–86. doi: 10.1006/bbrc.1999.1638. [DOI] [PubMed] [Google Scholar]
- Oyasu M, Kuroda S, Nakashita M, Fujimiya M, Kikkawa U, Saito N. Immunocytochemical localization of a neuron-specific thrombospondin-1-like protein, NELL2: light and electron microscopic studies in the rat brain. Mol Brain Res. 2000;76:151–60. doi: 10.1016/s0169-328x(99)00342-3. [DOI] [PubMed] [Google Scholar]
- Jiang YL, Obama H, Kuan SL, et al. In vitro guidance of retinal axons by a tectal lamina-specific glycoprotein Nel. Mol Cell Neurosci. 2009;41:113–9. doi: 10.1016/j.mcn.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto C, Kuan SL, Findlay AS, et al. Nel positively regulates the genesis of retinal ganglion cells by promoting their differentiation and survival during development. Mol Biol Cell. 2014;25:234–44. doi: 10.1091/mbc.E13-08-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Matsuhashi S, Tabuchi K, et al. Brain specific human genes, NELL1 and NELL2, are predominantly expressed in neuroblastoma and other embryonal neuroepithelial tumors. Neurol Med Chir. 2001;41:582–8. doi: 10.2176/nmc.41.582. [DOI] [PubMed] [Google Scholar]
- Shah US, Getzenberg RH. Fingerprinting the diseased prostate: associations between BPH and prostate cancer. J Cell Biochem. 2004;91:161–9. doi: 10.1002/jcb.10739. [DOI] [PubMed] [Google Scholar]
- Osman I, Bajorin DF, Sun TT, et al. Novel blood biomarkers of human urinary bladder cancer. Clin Cancer Res. 2006;12:3374–80. doi: 10.1158/1078-0432.CCR-05-2081. [DOI] [PubMed] [Google Scholar]
- Slovak ML, Bedell V, Hsu YH, et al. molecular karyotypes of hodgkin and reed-sternberg cells at disease onset reveal distinct copy number alterations in chemosensitive versus refractory hodgkin lymphoma. Clin Cancer Res. 2011;17:3443–54. doi: 10.1158/1078-0432.CCR-10-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M, Rehli M. Functional analysis of promoter CpG methylation using a CpG-free luciferase reporter vector. Epigenetics. 2006;1:127–30. doi: 10.4161/epi.1.3.3327. [DOI] [PubMed] [Google Scholar]
- Truong T, Zhang XL, Pathmanathan D, Soo C, Ting K. Craniosynostosis-associated gene NELL-1 is regulated by Runx2. J Bone Miner Res. 2007;22:7–18. doi: 10.1359/jbmr.061012. [DOI] [PubMed] [Google Scholar]
- Kim DH, Roh YG, Lee HH, et al. The E2F1 oncogene transcriptionally regulates NELL2 in cancer cells. DNA Cell Biol. 2013;32:517–23. doi: 10.1089/dna.2013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang YW, Yasui N, Ito K, et al. A RUNX2/PEBP2alpha A/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc Natl Acad Sci U S A. 2000;97:10549–54. doi: 10.1073/pnas.180309597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Petersen BO, Holm K, Bartek J, Helin K. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol Cell Biol. 1996;16:1047–57. doi: 10.1128/mcb.16.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Nakamoto C, Obama H, Durward E, Nakamoto M. Structure-function analysis of Nel, a thrombospondin-1-like glycoprotein involved in neural development and functions. J Biol Chem. 2012;287:3282–91. doi: 10.1074/jbc.M111.281485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka H, Obama H, Kelly ML, Matsui T, Nakamoto M. Biphasic functions of the kinase-defective Ephb6 receptor in cell adhesion and migration. J Biol Chem. 2005;280:29355–63. doi: 10.1074/jbc.M500010200. [DOI] [PubMed] [Google Scholar]
- Jin Z, Mori Y, Yang J, et al. Hypermethylation of the nel-like 1 gene is a common and early event and is associated with poor prognosis in early-stage esophageal adenocarcinoma. Oncogene. 2007;26:6332–40. doi: 10.1038/sj.onc.1210461. [DOI] [PubMed] [Google Scholar]
- Mori Y, Cai K, Cheng YL, et al. A genome-wide search identifies epigenetic silencing of somatostatin, tachykinin-1, and 5 other genes in colon cancer. Gastroenterology. 2006;131:797–808. doi: 10.1053/j.gastro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Cheng HJ, Feldheim DA, Hattori M, Lu Q, Vanderhaeghen P. Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods Enzymol. 2000;327:19–35. doi: 10.1016/s0076-6879(00)27264-9. [DOI] [PubMed] [Google Scholar]
- Witt H, Mack SC, Ryzhova M, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20:143–57. doi: 10.1016/j.ccr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo A, Herman JG, Mao L, et al. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995;1:686–92. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Greger V, Passarge E, Höpping W, Messmer E, Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. 1989;83:155–8. doi: 10.1007/BF00286709. [DOI] [PubMed] [Google Scholar]
- Wang JD, Kataoka H, Suzuki M, et al. Downregulation of EphA7 by hypermethylation in colorectal cancer. Oncogene. 2005;24:5637–47. doi: 10.1038/sj.onc.1208720. [DOI] [PubMed] [Google Scholar]
- Herman JG, Latif F, Weng Y, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994;91:9700–4. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey C, Martinez A, Zatyka M, et al. Epigenetic inactivation of the RASSF1A 3p21.3 tumor suppressor gene in both clear cell and papillary renal cell carcinoma. Cancer Res. 2001;61:7277–81. [PubMed] [Google Scholar]
- Yamada D, Kikuchi S, Williams YN, et al. Promoter hypermethylation of the potential tumor suppressor DAL-1/4.1B gene in renal clear cell carcinoma. Int J Cancer. 2006;118:916–23. doi: 10.1002/ijc.21450. [DOI] [PubMed] [Google Scholar]
- Dulaimi E, Ibanez de Caceres I, Uzzo RG, et al. Promoter hypermethylation profile of kidney cancer. Clin Cancer Res. 2004;10:3972–9. doi: 10.1158/1078-0432.CCR-04-0175. [DOI] [PubMed] [Google Scholar]
- Shen J, James AW, Chung J, et al. NELL-1 promotes cell adhesion and differentiation via integrin ss 1. J Cell Biochem. 2012;113:3620–8. doi: 10.1002/jcb.24253. [DOI] [PubMed] [Google Scholar]
- Hasebe A, Nakamura Y, Tashima H, et al. The C-terminal region of NELL1 mediates osteoblastic cell adhesion through integrin alpha 3 beta 1. FEBS Lett. 2012;586:2500–6. doi: 10.1016/j.febslet.2012.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Neural epidermal growth factor-like like 1 (NELL1) and NELL2 protein expression in renal cell carcinoma cell lines.
Fig. S2. Binding activity of neural epidermal growth factor-like like protein fused to an alkaline phosphatase tag (NELL-AP) to renal cell carcinoma cells.
Doc. S1. Supplementary materials and methods.