Abstract
Purpose
Recently we reported on day blindness in sheep caused by a mutation in the CNGA3 gene, thus making affected sheep a naturally occurring large animal model for therapeutic intervention in CNGA3 achromatopsia patients. The purpose of this study was to characterize flicker cone function in normal and day blind sheep, with the aim of generating a normative data base for ongoing gene therapy studies.
Methods
Electoretinographic (ERG) cone responses were evoked with full-field conditions in 10 normal, 6 heterozygous carriers and 36 day blind sheep. Following light adaptation (10 min, 30 cd/m2), responses were recorded at four increasing light intensities (1, 2.5, 5 and 10 cd s/m2). At each of these intensities, a single photopic flash response followed by 8 cone flicker responses (10–80 Hz) was recorded. Results were used to generate a normative data base for the three groups. Differences between day blind and normal control animals were tested in two age-matched groups (n = 10 per group).
Results
The normal sheep cone ERG wave is bipartite in nature, with critical flicker fusion frequency (CFF) >80 Hz. In all four flash intensities, the single photopic flash a-wave and b-wave amplitudes were significantly lower (p < 0.005), and implicit times significantly delayed (p < 0.0001), in day blind animals. In all four flash intensities, CFF values were significantly lower (p < 0.0001) in day blind sheep.
Conclusions
Cone function is severely depressed in day blind sheep. Our results will provide a normative data base for ongoing gene therapy studies.
Keywords: Critical flicker fusion frequency (CFF), Electroretinography (ERG), Photopic response
Introduction
Congenital achromatopsia is a hereditary vision disorder with a prevalence of approximately 1:30,000 [1]. Cone photoreceptor function is primarily affected, and in cases of complete achromatopsia, only rod vision is present. This leads to severe impairment of visual acuity and loss of color vision. Affected patients are legally blind from birth and also suffer from hemeralopia, nystagmus and severe photophobia [1].
Achromatopsia is recessively inherited, and in most cases caused by mutations in the cone cell cyclic nucleotide-gated (CNG) ion channel subunit A3 (a subunit) [2, 3] or B3 (b subunit) [4–6]. Recently, mutations within two additional genes, GNAT2 (which encodes the α subunit of cone transducin) and PDE6C, were also shown to cause achromatopsia in rare cases [7, 8]. All these genes encode crucial components of cone-specific phototransduction. In previous studies, mainly in North American and European populations, the most frequent mutations were found in the CNGB3 gene, explaining up to 50 % of all achromatopsia cases [6]. Mutations in CNGA3 account for approximately 20 % of cases [9], though in Israel, mutations in CNGA3 gene are by far the most prevalent cause of disease, accounting for over 70 % of cases [10].
In 2010, we reported on a novel, congenital day blindness in sheep that is inherited as an autosomal recessive disease in the Improved Awassi breed in Israel [11]. Interestingly, we determined that the day blindness is caused by a C → T substitution leading to the appearance of a stop codon at reside 236 of the ovine CNGA3 gene [12], thus making affected sheep a naturally occurring large animal model for human CNGA3 achromatopsia.
The eye in general and the retina in particular are ideal candidates for gene therapy, being readily accessible for intervention as well as for functional and structural assessment. In recent years, a number of research teams have shown the efficacy and safety of gene therapy using adeno-associated Virus vectors in restoring or preserving retinal function in a wide variety of retinal degenerative animal models [13, 14], including mouse models of CNGA3 achromatopsia [15, 16]. Moreover, as demonstrated in the case of leber congenital amaurosis, positive results in a naturally occurring large animal model, in that case RPE65 mutant dogs [17–19], can serve as a basis for clinical trials of gene therapy in human patients [20]. Therefore, we have recently started gene therapy trials in our sheep model, with positive preliminary results that may pave the way to gene therapy of CNGA3 achromatopsia patients [21].
The aim of the present work is to study flicker cone function in normal and day blind sheep in order to establish a normative data base that will be used as reference values in our ongoing gene therapy trials in this animal model of CNGA3 achromatopsia.
Methods
Animals
Ten (homozygous normal) control unaffected sheep, six heterozygous carriers of the CNGA3 mutation and 36 (homozygous affected) day blind sheep were studied (Table 1). Status of each animal was validated by genotyping [12] and visual function was confirmed by evaluating the menace response and by behavioral maze testing. Fundii images of all animals were captured using a fundus camera (Smartscope VET2, Acrivet, Hennigsdorf, Germany), and a representative image from each group is shown in Fig. 1. No gross differences were noted between fundii of animals from the three groups [22]. Additional phenotypic data, including behavioral and histopathological findings, were described in our previous manuscript [11] and will be detailed in another manuscript that is currently being prepared. Animals were maintained at the experimental flock of the Volcani Center at Bet Dagan, Israel. The day blind sheep originated from the Ein Harod Improved Awassi dairy flock and were part of an experimental group established to serve as a naturally occurring, large animal model for studying achromatopsia [11, 12]. Experimental protocols were approved by the Volcani Center Animal Care and Use Committee (authorization no. 40712IL) and were conducted in accordance with guidelines of the Association for Research in Vision and Ophthalmology.
Table 1.
Study animals
| Group | Gender | Age at ERG recording (months)
|
|
|---|---|---|---|
| Female, male | Mean, SD | Median, range | |
| Normal controls | 8, 2 | 30.2, 16.1 | 31.5, 4–52 |
| Day blind sheep | |||
| Subgroup age-matched to controls | 6, 4 | 29.6, 16.4 | 28, 4–46 |
| Entire group | 24, 12 | 16.79, 15.08 | 9, 4–56 |
| Carriers | 6, 0 | 5.5, 0.55 | 5.5, 5–6 |
A total of 10 normal, 36 day blind sheep and 6 heterozygous carriers were evaluated electroretinographically. A sub group of 10 day blind sheep (second row), age-matched to the normal controls, was used for statistical analysis of the effect of day blindness on cone function
Fig. 1.
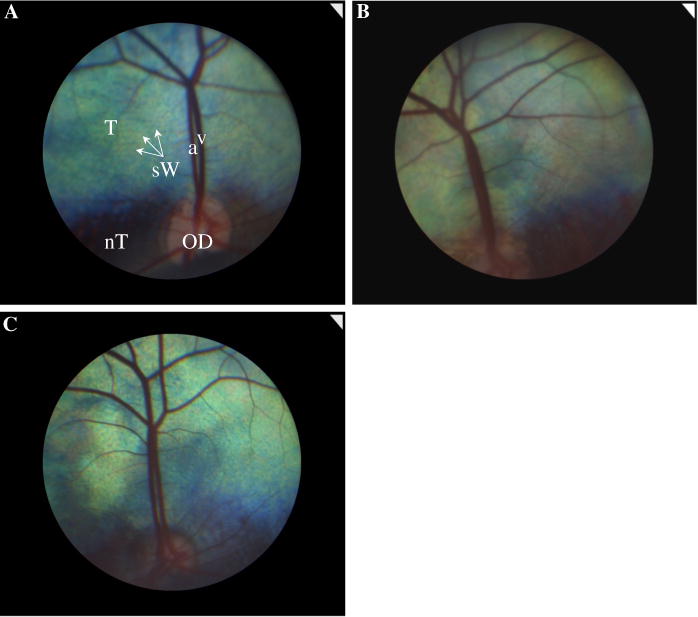
Representative fundus images of the left eyes in a normal (a), carrier (b) and day blind (c) sheep. OD optic disk, T tapetum, nT nontapetum, v retinal vein, a retinal artery, sW stars of Winslow (the black dots seen throughout the tapetal area)
Electroretinography (ERG)
Pupils were dilated with 0.5 % tropicamide (Mydramide, Fischer Pharmaceutical Labs, Israel) and 10 % phenylephrine hydrochloride (Efrin-10, Fischer Pharmaceutical Labs, Israel) solutions at least 20 min before recording. Animals were premedicated with an intramuscular injection of acepromazine (0.1 mg/kg; 10 mg/ml compounded preparation, Vetmarket Pharmacy, Israel) and pethidine (3 mg/kg; Dolestine, Teva Pharmaceutical Industries, Israel). Anesthesia was induced with an intravenous injection of propofol (4 mg/kg; Propofol-lipuro, B. Braun Medical Supplies, Philippines) and diazepam (0.15 mg/kg; Assival, Teva Pharmaceutical Industries, Israel). Animals were intubated and anesthesia was maintained with 3 % isoflurane (Forane, Abbott Laboratories, England) in 100 % oxygen (5–6 l/min). During anesthesia, animals were ventilated (2 % isoflurane and 1.5–2 l/min oxygen), hydrated with intravenous 0.9 % saline infusion and constantly monitored by a veterinary surgeon.
Animals were positioned in lateral recumbency, eyelids were retracted with Barraquer eyelid retractor and globes were center positioned using 1–2 subconjunctival stay sutures. To improve conduction, the recorded eyes were kept moist with a drop of 1.4 % hydroxymethylcellulose (Celluspan, Fischer Pharmaceutical Labs, Tel-Aviv, Israel). Signals were recorded using a Jet contact lens electrode (ERG-Jet, Fabrinal SA, La Chaux-de-Fonds, Switzerland). Subcutaneous needles (CareFusion, Wisconsin) served as reference and ground electrodes, and were placed at the ipsilateral lateral canthus and the pinna of the ear, respectively (Fig. 2). Both eyes were recorded in random order. After recording the first eye, the entire protocol, including light adaptation, was repeated in the second eye. The complete procedure lasted approximately 38 min, including 10 min of light adaptation and 9 min of recording time per eye. Impedance was kept under 5 KΩ.
Fig. 2.
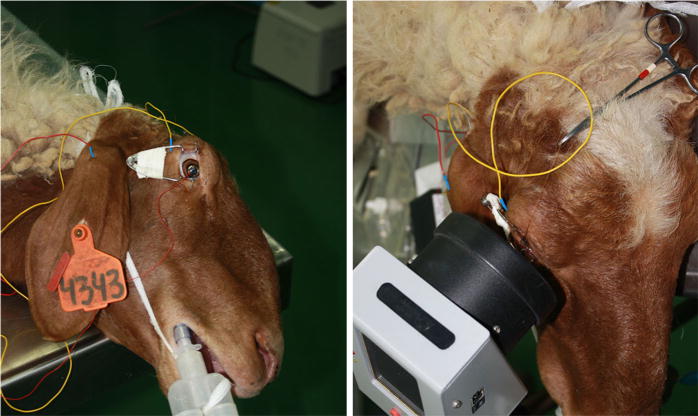
A sheep prepared for an ERG recording using an HMsERG mini Ganzfeld. Subcutaneous needles were placed at the lateral canthus and the pinna of the ear and served as reference and ground electrodes, respectively. Distance between cornea and mini Ganzfeld was approximately 2 cm
All recordings were conducted using a Handheld Multi-species Electroretinography system (HMsERG, Ocuscience, Nevada) with a bandpass of 0.3–300 Hz. Background adaptation light and stimuli were delivered using a handheld mini Ganzfeld (Fig. 2). Cone function was evaluated using a custom-written protocol. Following 10 min of light adaptation (30 cd/m2), responses to 4 increasing light intensities (1, 2.5, 5 and 10 cd s/m2) were recorded. At each of the four light intensities, 32 flashes, presented at 1 Hz, were averaged to generate the single photopic flash response; this was followed by 8 cone flicker responses (flashes presented at 10–80 Hz, with 128 responses averaged at each frequency) (Fig. 3). Recordings were conducted in a fluorescent-lit, indoor experimental facility at the Volcani Center with an ambient light intensity of 0.06 cd/m2 (LI-189 photometer, LI-COR, Lincoln, USA).
Fig. 3.
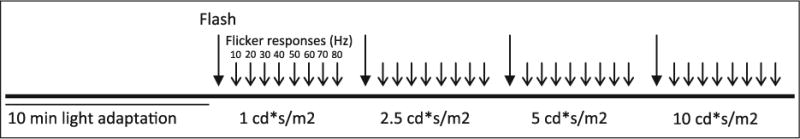
Photopic ERG recording protocol. Following 10 min of light adaptation (30 cd/m2), responses were recorded at four increasing intensities (1, 2.5, 5 and 10 cd s/m2). At each of the four light intensities, 32 flashes, presented at 1 Hz, were averaged to generate the single photopic flash response (Flash); this was followed by eight cone flicker responses (flashes presented at 10–80 Hz, with 128 responses averaged at each frequency). After recording the first eye, the entire protocol, including light adaptation, was repeated in the second eye. The recording time, including light adaptation, was approximately 19 min per eye
Data analysis
A- and b-wave amplitudes of single photopic flash response were measured from baseline to the first trough, and from that trough to the next positive peak, respectively. The kinetics of the responses were measured in terms of implicit times (IT), which is the corresponding time interval between the stimulus onset to the trough or to the positive peak. Amplitudes of the flicker response were measured in the same manner. The critical flicker fusion frequency (CFF), or the highest frequency at any light intensity that an animal can resolve flicker, was determined for each intensity [23]. A- and b-wave amplitudes of left and right eye responses to all four intensities were compared using either the Wilcoxon signed rank test (for the normal and carrier groups) or the paired Student t test (for the day blind group). As no significant differences (p > 0.05) were found between eyes in any test, except for the b-wave amplitude at 1 cd s/m2 of control group, responses of the left and right eyes were averaged for each sheep [24]. This data were used to generate a normative data base for day blind (n = 36), carrier (n = 6) and normal control (n = 10) groups (Table 1).
To test for differences in cone function between day blind and normal control sheep, we selected a subgroup of 10 day blind sheep that were age-matched to the ten normal control animals (Table 1). Responses of the left and right eyes were averaged for each sheep [24]. Log-transformed and crude data of the photopic single flash ERG response parameters were subjected to analysis of variance (ANOVA) using the General Linear Model procedure of the Jump In® computer package (SAS Inst. Inc., Cary, NC). The statistical model included the effects of study group (day blind vs. control), age (young < 1 year vs. mature > 1 year) and gender. Repeated measures ANOVA model was applied to assess the difference between control (n = 10) and age-matched day blind sheep (n = 10) for all flicker response amplitudes at the highest intensity of 10 cd s/m2. The Mann–Whitney nonparametric test was used to test for differences between the ten (homozygous normal) control sheep and the six heterozygous carriers. The effects of age (in months) and gender on the response parameters within each of the three groups were analyzed using ANOVA. Results with a p value <0.05 were considered statistically significant.
Results
Day blind versus normal sheep
Photopic single flash ERG
Representative single flash photopic signals of a day blind and a control animal in response to the highest intensity level (10 cd s/m2) are shown in Fig. 4. The b-wave contour is bipartite in nature.
Fig. 4.
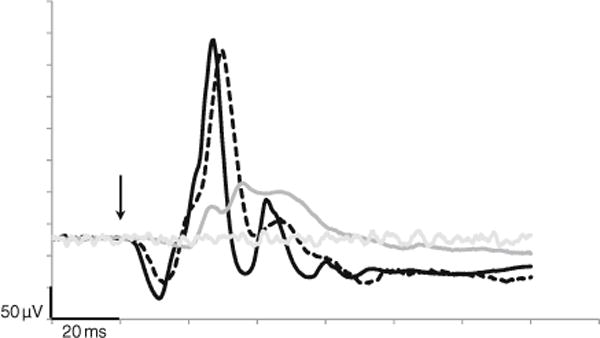
Representative photopic, single flash tracings of a normal (black), carrier (dashed) and a day blind (gray) sheep in response to the highest flash intensity (10 cd s/m2). Background noise is presented in light gray and flash onset indicated by the arrow
Mean ± SE photopic single flash parameters of the entire (n = 36) day blind study group is presented in Table 2. Mean ± SE parameters of the control (n = 10) and carrier (n = 6) sheep are presented in Fig. 5, alongside results of a subgroup of 10 select day blind sheep, age-matched to the control group, that were used for statistical comparison as described in “Methods”. Statistical analysis demonstrated significant differences between normal control and age-matched day blind sheep in all photopic single flash parameters that were evaluated (n = 10 in each group). At all four intensities, a- and b-wave amplitudes of day blind sheep were significantly lower than those of control animals (p < 0.005), and a- and b-wave IT were significantly delayed (p < 0.0001) in day blind animals (Fig. 5). Effects of age and gender were not significant.
Table 2.
Mean ± SE photopic single flash amplitude (uV) and implicit time (msec) and CFF values of the entire (n = 36) day blind study group
| A-wave
|
B-wave
|
CFF | |||
|---|---|---|---|---|---|
| Amplitude | Implicit time | Amplitude | Implicit time | ||
| 1 cd s/m2 | 4.12 ± 0.38 | 17.41 ± 0.46 | 37.33 ± 2.53 | 40.41 ± 0.6 | 39.07 ± 1.65 |
| 2.5 cd s/m2 | 7.28 ± 0.48 | 18.11 ± 0.25 | 64.63 ± 5.21 | 38.17 ± 0.48 | 34.4 ± 1.35 |
| 5 cd s/m2 | 11.46 ± 0.8 | 17.8 ± 0.25 | 76.79 ± 6.51 | 36.2 ± 0.47 | 32 ± 1.06 |
| 10 cd s/m2 | 17.59 ± 1.16 | 18.24 ± 0.22 | 80.55 ± 7.05 | 35.72 ± 0.43 | 29.46 ± 1.7 |
Fig. 5.
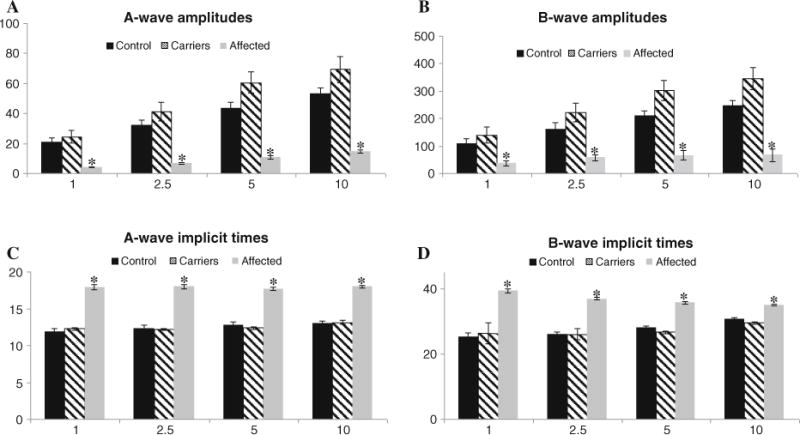
Mean ± SE a- and b-wave amplitudes (a, b) and implicit times (c, d) of the single flash photopic responses in 10 control (black), 6 carriers (striped) and age-matched day blind sheep (gray). Significant differences between control and day blind were found in all single flash ERG parameters (n = 10 in each group, p < 0.0005) and are denoted by asterisk
Photopic flicker response
Representative flicker tracings at eight frequencies (10–80 Hz), using the highest flash intensity (10 cd s/m2), are shown in Fig. 6. Mean ± SE CFF of the entire (n = 36) day blind study group is presented in Table 2. Mean ± SE CFF of the control (n = 10) and carrier (n = 6) sheep are presented in Fig. 7, alongside results of a subgroup of 10 select day blind sheep, age-matched to the control group, that were used for statistical comparison as described in “Methods”. At all four intensities, the CFF values were significantly lower in day blind sheep in all light intensities (p < 0.0001, Fig. 7a). Furthermore, at all four intensities, flicker responses of normal animals were still detectable at 80 Hz, implying that CFF of normal sheep is higher than 80 Hz.
Fig. 6.
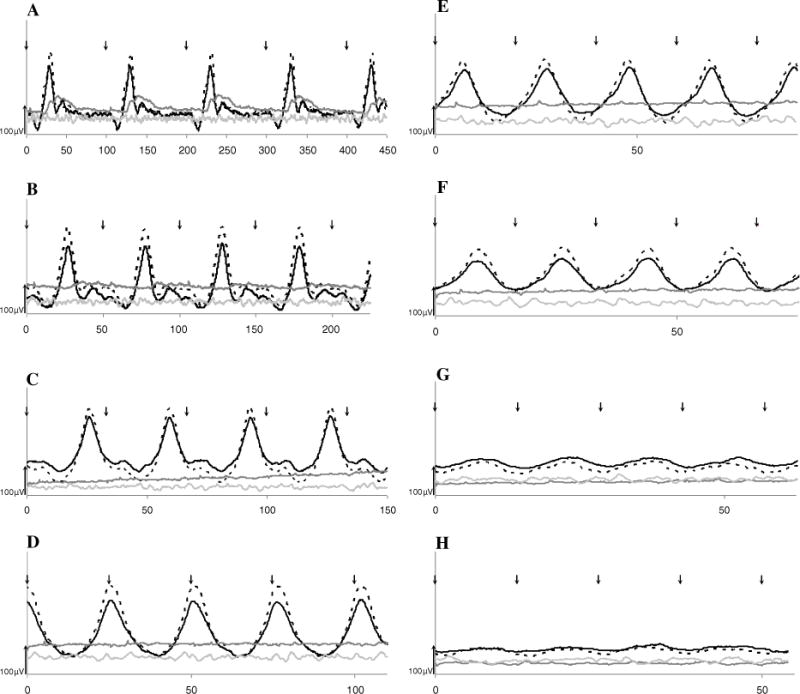
Representative cone flicker (10–80 Hz, a–h) tracings of a control (black), carrier (dashed) and day blind (gray) sheep at the highest intensity (10 cd s/m2). Background noise recording is presented in light gray and flash onset indicated by arrows. Note that the x-axis scale differs in each panel. In the day blind sheep, CFF (the highest flicker frequency at which flicker responses are still detectable) is 20 Hz (b), while the CFF of the control sheep is obviously above 80 Hz, as flicker responses are still detectable at this frequency (Fig. 6h)
Fig. 7.
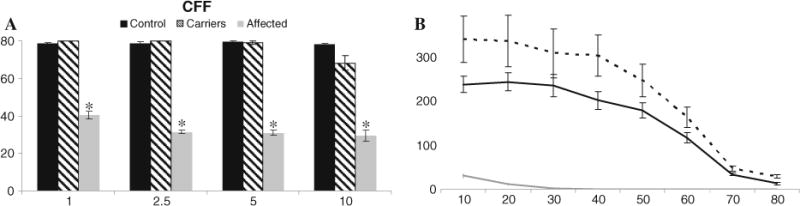
Flicker responses in 10 control (black), 6 carriers (striped) and 10 age-matched day blind sheep (gray). a. Mean ± SE CFF values. In all four intensities, flicker responses of normal sheep were still detectable at 80 Hz (implying that in normal sheep CFF > 80 Hz), and CFF values were significantly higher than in day blind sheep (p < 0.0001), as denoted by asterisk. b. Mean ± SE amplitude of the flicker responses of day blind (gray), carriers (dashed) and normal (black) sheep as a function of the stimulating frequency. Responses to the 10 cd s/m2 flicker are plotted. Flicker responses of control sheep were significantly higher than those of day blind sheep at all eight frequencies (p < 0.001)
Flicker mean ± SE amplitude responses as a function of the stimulus frequency (at 10 cd s/m2) are shown in Fig. 7b. Flicker responses of control sheep were significantly higher than those of day blind seep at all eight frequencies (p < 0.001).
Effect of age and gender
A significant, age-dependent decline of the photopic single flash a-wave amplitude was seen at the three highest intensities (p ≤ 0.05) of the day blind group (n = 36). At the two intermediate intensities, a-wave amplitude was significantly higher in females (p ≤ 0.05). None of the other photopic single flash or flicker response parameters of day blind sheep were affected by age or gender.
Effect of carrier status
A Mann–Whitney nonparametric test was used to test for potential differences between the six heterozygous carriers and ten normal controls (Table 1). No significant differences were found between the groups in 17 of the 20 parameters evaluated (data not shown). In the three parameters where statistical differences were found (a-wave amplitude at 5 cd s/m2 and b-wave amplitudes at 5 and 10 cd s/m2), carriers in fact had significantly higher amplitudes than normal controls, indicating that their function was not impaired by their heterozygous genotype.
Discussion
In this study, we describe cone function in the ovine CNGA3 achromatopsia model and compare it with control sheep. In our first report of the disease [11] we studied photopic responses of only six day blind and six control animals to just one stimulus intensity, and flicker responses to just one frequency were recorded. That preliminary study aimed to demonstrate cone dysfunction in affected animals, but did not constitute an exhaustive investigation of cone function in normal and day blind sheep. In the current study, we performed a comprehensive test of cone function using four light intensities, and a range of flicker frequencies, in a large sample of 36 affected sheep, six carriers and 10 control sheep. Our objective was to establish a normative data base of flicker responses in day blind and normal sheep that will be used as reference values of experimental and control animals in our ongoing CNGA3 gene therapy study. Additional phenotypic data, including behavioral tests and histopathology, will be presented in a future manuscript.
There are several reports of ERG recordings in achromatopsia patients. In a recently published study, most of the CNGA3 mutated patients had nonrecordable photopic and 30 Hz flicker responses. Photopic ERG responses of CNGB3 patients were more variable and ranged from nonrecordable to barely detectable responses [25]. Fahim et al. suggest that this difference demonstrates that the beta subunit loss is better tolerated than the alpha subunit loss [25]. A difference in the role of the two subunits was also proposed by other investigators of achromatopsia patients [9, 26] and of CNGB3−/−, CNG3−/− and CNGA3−/− mouse models [27–29]. Genead et al. [30] report that single flash, light-adapted responses of achromatopsia patients were nondetectable in 17 % of subjects, whereas 83 % of subjects had showed markedly reduced b-wave responses, with an average amplitude of 34.9 μV (lower normal limit was 132.8 μV). All patients had nondetectable 32-Hz flicker responses. However, no differences in function were noted between CNGB3 and CNGA3 patients [30]. Significant attenuation, or absence, of photopic ERG responses in achromatopsia patients were also shown in other studies [31, 32].
There have been several previous studies of cone function in sheep [33–36]. However, in most of these studies, critical parameters such as duration of light adaptation or stimulus intensity are not reported thus precluding comparison to our results. Nevertheless, the b-wave bipartite nature that we noted (Fig. 4) was also described by Smith et al. [35] and demonstrated by Graydon et al. [34], though we cannot discount the possibility that this may have been a spike artifact induced by the use of a 1 Hz stimulus. In addition, Smith et al. [34] also noted the very high CFF values of the sheep photopic ERG. They were still able to distinguish flicker responses at a maximal stimulus frequency of 60 fps (i.e., 120 Hz), while we were able to discern flicker responses at 80 Hz (Figs. 6, 7). While neither we nor Smith et al. [34] were able to determine the actual CFF value in sheep, it seems to be much higher than that of humans or rodents [37].
Similar to what has been reported in achromatopsia patients [25, 30–32], our results demonstrate significantly reduced photopic single flash a- and b-wave amplitudes, and increased implicit times, at several light intensities (Figs. 4, 5). The nondetectable responses to 30 or 32 Hz flicker stimuli that were described in human patients [25, 30] were also observed in our study, as the CFF values of day blind sheep are 30–40 Hz (depending on intensity, Fig. 7a). The implication of this is that even though normal sheep have extremely high CFF values, day blind sheep have CFF values remarkably similar to those of human achromatopsia patients. In addition, we found that a-wave amplitudes of the photopic single flash response decline with age at intensities ≥2.5 cd s/m2. This finding may indicate progression of the disease with age and warrants further investigation.
Although Khan et al. [32] showed that rod responses are impaired in some CNGB3 achromatopsia patients, most ERG studies in achromatopsia patients demonstrate normal, or only slightly reduced, rod responses [30, 31]. Similarly, in our preliminary study, we did not find differences in scotopic responses of affected and control sheep [11]. Since extended dark adaptation of sheep is not as simple as in commonly used laboratory animals and would likely require lengthy anesthesia, we decided to perform only photopic recordings, an approach that has also been used in the most recent ERG study of achromatopsia patients [25].
This study reinforces the reliability of the CNGA3 mutated sheep as a suitable model for human achromatopsia and provides a strong foundation for future studies using this model in gene therapy studies.
Acknowledgments
This study was supported (in part) by Grant No. 3-00000-8290 from the Chief Scientist Office of the Ministry of Health, Israel, and by Grant No. 2011445 from the U.S.-Israel Binational Science Foundation, as well as unrestricted awards from The Joseph Alexander Foundation, Yedidut 1 Research Grant and Macula Vision Research Foundation. The authors thank Tali Bdolah-Abram for her help with statistical analysis of the data.
Footnotes
Conflict of interest None.
Contributor Information
Raaya Ezra-Elia, Koret School of Veterinary Medicine, Hebrew University of Jerusalem, PO Box 12, 7610001 Rehovot, Israel.
Eyal Banin, Department of Ophthalmology, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
Hen Honig, Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel.
Alexander Rosov, Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel.
Alexey Obolensky, Department of Ophthalmology, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
Edward Averbukh, Department of Ophthalmology, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
William W. Hauswirth, Department of Ophthalmology, University of Florida, Gainesville, FL, USA
Elisha Gootwine, Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel.
Ron Ofri, Email: ron.ofri@mail.huji.ac.il, Koret School of Veterinary Medicine, Hebrew University of Jerusalem, PO Box 12, 7610001 Rehovot, Israel.
References
- 1.Simunovic MP, Moore AT. The cone dystrophies. Eye. 1998;12(Pt 3b):553–565. doi: 10.1038/eye.1998.145. [DOI] [PubMed] [Google Scholar]
- 2.Kohl S, Marx T, Giddings I, Jägle H, Jacobson SG, Apfelstedt-Sylla E, Zrenner E, Sharpe LT, Wissinger B. Total colourblindness is caused by mutations in the gene encoding the alpha-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet. 1998;19(3):257–259. doi: 10.1038/935. [DOI] [PubMed] [Google Scholar]
- 3.Wissinger B, Gamer D, Jägle H, Giorda R, Marx T, Mayer S, Tippmann S, Broghammer M, Jurklies B, Rosenberg T, Jacobson SG, Sener EC, Tatlipinar S, Hoyng CB, Castellan C, Bitoun P, Andreasson S, Rudolph G, Kellner U, Lorenz B, Wolff G, Verellen-Dumoulin C, Schwartz M, Cremers FP, Apfelstedt-Sylla E, Zrenner E, Salati R, Sharpe LT, Kohl S. CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet. 2001;69(4):722–737. doi: 10.1086/323613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winick JD, Blundell ML, Galke BL, Salam AA, Leal SM, Karayiorgou M. Homozygosity mapping of the Achromatopsia locus in the Pingelapese. Am J Hum Genet. 1999;64(6):1679–1685. doi: 10.1086/302423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bright SR, Brown TE, Varnum MD. Disease-associated mutations in CNGB3 produce gain of function alterations in cone cyclic nucleotide-gated channels. Mol Vis. 2005;11:1141–1150. [PubMed] [Google Scholar]
- 6.Kohl S, Varsanyi B, Antunes GA, Baumann B, Hoyng CB, Jägle H, Rosenberg T, Kellner U, Lorenz B, Salati R, Jurklies B, Farkas A, Andreasson S, Weleber RG, Jacobson SG, Rudolph G, Castellan C, Dollfus H, Legius E, Anastasi M, Bitoun P, Lev D, Sieving PA, Munier FL, Zrenner E, Sharpe LT, Cremers FP, Wissinger B. CNGB3 mutations account for 50 % of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005;13(3):302–308. doi: 10.1038/sj.ejhg.5201269. [DOI] [PubMed] [Google Scholar]
- 7.Kohl S, Baumann B, Rosenberg T, Kellner U, Lorenz B, Vadalà M, Jacobson SG, Wissinger B. Mutations in the cone photoreceptor G-protein alpha-subunit gene GNAT2 in patients with achromatopsia. Am J Hum Genet. 2002;71(2):422–425. doi: 10.1086/341835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiadens AA, den Hollander AI, Roosing S, Nabuurs SB, Zekveld-Vroon RC, Collin RW, De Baere E, Koenekoop RK, van Schooneveld MJ, Strom TM, van Lith-Verhoeven JJ, Lotery AJ, van Moll-Ramirez N, Leroy BP, van den Born LI, Hoyng CB, Cremers FP, Klaver CC. Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am J Hum Genet. 2009;85(2):240–247. doi: 10.1016/j.ajhg.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiadens AA, Slingerland NW, Roosing S, van Schooneveld MJ, van Lith-Verhoeven JJ, van Moll-Ramirez N, van den Born LI, Hoyng CB, Cremers FP, Klaver CC. Genetic etiology and clinical consequences of complete and incomplete achromatopsia. Ophthalmology. 2009;116(10):1984.e1981–1989.e1981. doi: 10.1016/j.ophtha.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 10.Zelinger L, Greenberg A, Kohl S, Banin E, Sharon D. An ancient autosomal haplotype bearing a rare achromatopsia-causing founder mutation is shared among Arab Muslims and Oriental Jews. Hum Genet. 2010;128(3):261–267. doi: 10.1007/s00439-010-0846-z. [DOI] [PubMed] [Google Scholar]
- 11.Shamir MH, Ofri R, Bor A, Brenner O, Reicher S, Obolensky A, Averbukh E, Banin E, Gootwine E. A novel day blindness in sheep: epidemiological, behavioural, electrophysiological and histopathological studies. Vet J. 2010;185(2):130–137. doi: 10.1016/j.tvjl.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Reicher S, Seroussi E, Gootwine E. A mutation in gene CNGA3 is associated with day blindness in sheep. Genomics. 2010;95(2):101–104. doi: 10.1016/j.ygeno.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Al-Saikhan FI. The gene therapy revolution in ophthalmology. Saudi J Ophthalmol. 2013;27(2):107–111. doi: 10.1016/j.sjopt.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahel JA, Roska B. Gene therapy for blindness. Annu Rev Neurosci. 2013;36:467–488. doi: 10.1146/annurev-neuro-062012-170304. [DOI] [PubMed] [Google Scholar]
- 15.Pang JJ, Deng WT, Dai X, Lei B, Everhart D, Umino Y, Li J, Zhang K, Mao S, Boye SL, Liu L, Chiodo VA, Liu X, Shi W, Tao Y, Chang B, Hauswirth WW. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS ONE. 2012;7(4):e35250. doi: 10.1371/journal.pone.0035250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalakis S, Mühlfriedel R, Tanimoto N, Krishnamoorthy V, Koch S, Fischer MD, Becirovic E, Bai L, Huber G, Beck SC, Fahl E, Büning H, Paquet-Durand F, Zong X, Gollisch T, Biel M, Seeliger MW. Restoration of cone vision in the CNGA3−/− mouse model of congenital complete lack of cone photoreceptor function. Mol Ther. 2010;18(12):2057–2063. doi: 10.1038/mt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 18.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, Hauswirth WW, Jacobson SG. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12(6):1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narfström K, Vaegan, Katz M, Bragadottir R, Rakoczy EP, Seeliger M. Assessment of structure and function over a 3-year period after gene transfer in RPE65−/− dogs. Doc Ophthalmol. 2005;111(1):39–48. doi: 10.1007/s10633-005-3159-0. [DOI] [PubMed] [Google Scholar]
- 20.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular sub-retinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Averbukh E, Ofri R, Gootwine E, Ezra-Elia R, Honig HH, Rosov A, Yamin E, Obolensky A, Hauswirth WW, Banin E. Recovery of visual function following gene therapy in a large animal model of CNGA3 achromatopsia. Paper presented at the ARVO 2013; Seattle. 2013. [Google Scholar]
- 22.Galán A, Martín-Suárez EM, Granados MM, Gallardo JM, Molleda JM. Comparative fluorescein angiography of the normal sheep and goat ocular fundi. Vet Ophthalmol. 2006;9(1):7–15. doi: 10.1111/j.1463-5224.2005.00425.x. [DOI] [PubMed] [Google Scholar]
- 23.Lisney TJ, Ekesten B, Tauson R, Håstad O, Odeen A. Using electroretinograms to assess flicker fusion frequency in domestic hens Gallus gallus domesticus. Vision Res. 2012;62:125–133. doi: 10.1016/j.visres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Labelle AL, Hamor RE, Narfström K, Breaux CB. Electroretinography in the western gray kangaroo (Macr-opus fuliginosus) Vet Ophthalmol. 2010;13(Suppl):41–46. doi: 10.1111/j.1463-5224.2010.00810.x. [DOI] [PubMed] [Google Scholar]
- 25.Fahim AT, Khan NW, Zahid S, Schachar IH, Branham K, Kohl S, Wissinger B, Elner VM, Heckenlively JR, Jayasundera T. Diagnostic fundus autofluorescence patterns in achromatopsia. Am J Ophthalmol. 2013;156(6):1211.e1212–1219.e1212. doi: 10.1016/j.ajo.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Nishiguchi KM, Sandberg MA, Gorji N, Berson EL, Dryja TP. Cone cGMP-gated channel mutations and clinical findings in patients with achromatopsia, macular degeneration, and other hereditary cone diseases. Hum Mutat. 2005;25(3):248–258. doi: 10.1002/humu.20142. [DOI] [PubMed] [Google Scholar]
- 27.Ding XQ, Harry CS, Umino Y, Matveev AV, Fliesler SJ, Barlow RB. Impaired cone function and cone degeneration resulting from CNGB3 deficiency: down-regulation of CNGA3 biosynthesis as a potential mechanism. Hum Mol Genet. 2009;18(24):4770–4780. doi: 10.1093/hmg/ddp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biel M, Seeliger M, Pfeifer A, Kohler K, Gerstner A, Ludwig A, Jaissle G, Fauser S, Zrenner E, Hofmann F. Selective loss of cone function in mice lacking the cyclic nucleotide-gated channel CNG3. Proc Natl Acad Sci USA. 1999;96(13):7553–7557. doi: 10.1073/pnas.96.13.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanimoto N, Sothilingam V, Gloeckner G, Bryda EC, Humphries P, Biel M, Seeliger MW. Auditory event-related signals in mouse ERG recordings. Doc Ophthalmol. 2014;128(1):25–32. doi: 10.1007/s10633-013-9417-7. [DOI] [PubMed] [Google Scholar]
- 30.Genead MA, Fishman GA, Rha J, Dubis AM, Bonci DM, Dubra A, Stone EM, Neitz M, Carroll J. Photoreceptor structure and function in patients with congenital achromatopsia. Invest Ophthalmol Vis Sci. 2011;52(10):7298–7308. doi: 10.1167/iovs.11-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiadens AA, Roosing S, Collin RW, van Moll-Ramirez N, van Lith-Verhoeven JJ, van Schooneveld MJ, den Hollander AI, van den Born LI, Hoyng CB, Cremers FP, Klaver CC. Comprehensive analysis of the achromatopsia genes CNGA3 and CNGB3 in progressive cone dystrophy. Ophthalmology. 2010;117(4):825.e821–830.e821. doi: 10.1016/j.ophtha.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Khan NW, Wissinger B, Kohl S, Sieving PA. CNGB3 achromatopsia with progressive loss of residual cone function and impaired rod-mediated function. Invest Ophthalmol Vis Sci. 2007;48(8):3864–3871. doi: 10.1167/iovs.06-1521. [DOI] [PubMed] [Google Scholar]
- 33.Knave B, Moller A, Persson HE. A component analysis of the electroretinogram. Vision Res. 1972;12(10):1669–1684. doi: 10.1016/0042-6989(72)90038-7. [DOI] [PubMed] [Google Scholar]
- 34.Smith EL, Witzel DA, Pitts DG. The waveform and scotopic CFF of the sheep electroretinogram. Vision Res. 1976;16(11):1241–1245. doi: 10.1016/0042-6989(76)90048-1. [DOI] [PubMed] [Google Scholar]
- 35.Graydon RJ, Jolly RD. Ceroid-lipofuscinosis (Batten’s disease). Sequential electrophysiologic and pathologic changes in the retina of the ovine model. Invest Ophthalmol Vis Sci. 1984;25(3):294–301. [PubMed] [Google Scholar]
- 36.Regnier A, Andreoletti O, Albaric O, Gruson DC, Schelcher F, Toutain PL. Clinical, electroretinographic and histomorphometric evaluation of the retina in sheep with natural scrapie. BMC Vet Res. 2011;7:25. doi: 10.1186/1746-6148-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin GR, Kraft TW. Flicker assessment of rod and cone function in a model of retinal degeneration. Doc Ophthalmol. 2007;115(3):165–172. doi: 10.1007/s10633-007-9066-9. [DOI] [PubMed] [Google Scholar]


