Abstract
Impulsivity is a complex behaviour composed of different domains encompassing behavioural disinhibition, risky decision-making and delay discounting abnormalities. To investigate regional brain correlates between levels of individual impulsivity and grey matter volume, we performed voxel-based morphometric correlation analysis in 34 young, healthy subjects using impulsivity scores measured with Barratt Impulsivity Scale-11 and computerized Kirby’s delay discounting task. The VBM analysis showed that impulsivity appears to be reliant on a network of cortical (medial prefrontal cortex and dorsolateral prefrontal cortex) and subcortical (ventral striatum) structures emphasizing the importance of brain networks associated with reward related decision-making in daily life as morphological biomarkers for impulsivity in a normal healthy population. While our results in healthy volunteers may not directly extend to pathological conditions, they provide an insight into the mechanisms of impulsive behaviour in patients with abnormalities in prefrontal/frontal-striatal connections, such as in drug abuse, pathological gambling, ADHD and Parkinson’s disease.
Keywords: Decision making, Impulsivity, Medial prefrontal cortex, Ventral striatum, Magnetic resonance imaging, Voxel based morphometry
Introduction
Impulsive actions represents one of the major features of psychiatric conditions and chemical and behavioral addiction disorders (Alessi and Petry 2003; Barkley et al. 2001; Kirby et al. 1999; Kaladjian et al. 2011). Impulsivity is a complex behaviour composed of different domains encompassing behavioural dis-inhibition, risky decision-making and delay discounting abnormalities (Dom et al. 2006; Reynolds et al. 2006; Swann et al. 2002). Brain areas like the dorsolateral prefrontal cortex (DLPFC), the medial prefrontal cortex (MePFC) including the orbitofrontal cortex (OFC) and the anterior cingulate cortex (ACC) and the ventral striatum (VST) have all been reported to be critically involved in impulsive behaviours (Stuss et al. 1992; Rolls 2004; Bechara and Van Der Linden 2005). Patients with damage to those prefrontal regions show increased impulsivity on inhibition tasks (Bechara et al. 1999) and exhibit greater impulsivity and aggressive behavior compared to patients with other frontal cortex lesions (Grafman et al. 1996). Various neuropsychological frameworks have been used to explore the individual behavioral differences of impulsivity. Roughly there are two different approaches to evaluate impulsivity level: subjective self-reported measures of personality that rely on an individual’s self-perception of behaviour and objective behavioural tasks that estimate the performance level (Dom et al., 2006). Both laboratory and self-report personality measures of impulsivity appear to be related to risk of psychopathology (Swann et al. 2002). How these two approaches can be combined to identify a common or overlapping neural network characterizing level of impulsivity is poorly investigated.
A few studies have investigated the relationship between structural changes in grey matter (GM) and impulsivity/self-control impairments (Koprivová et al. 2009; McAlonan et al. 2007). Previous observations using self-reported measurement reported an inverse correlation between impulsive behaviour and GM volume in the lateral OFC (Lui et al. 2009; Matsuo et al. 2009). Boes et al. (2009) showed that GM volume in the right MePFC predicted impulse control in adolescents. Studies using behavioural tasks such as the delay discounting paradigm have also demonstrated a relationship between impulsivity level and regional GM volume (Bjork et al. 2009; Schwartz et al. 2010) as well as white matter volume (Yu 2012). All together these reports demonstrated that structural changes at the cortical and subcortical level may be responsible for different degrees of impulsive behavior.
In this study, we investigated the relationship between individual impulsivity level measured, combining a self-reported method [i.e. Barratt Impulsivity Scale (BIS)] and objective behavioural test [i.e. computerized Kirby’s delay discounting task (DDT)], and GM volume using a voxel-based morphometry (VBM) method. This method allows an unbiased assessment of regional variations in brain structures using structural magnetic resonance images (MRI), and when combined with regression analysis permits us to identify potential relationships between behaviour and local brain morphology as well as to determine interregional connectivity among remote brain areas (Worsley et al. 2005; Lerch et al. 2006). Our hypothesis was that impulsivity level would be correlated with the brain GM volume in those regions, i.e. the DLPFC, MePFC (including ACC and OFC) and VST, known to be associated with decision making processes and possibly reflecting a potential predisposing factor to impulsivity-related variations.
Materials and Methods
Subjects
Thirty four right-handed young healthy subjects (23.4 age ± 4.3 years; age range: 18–35 years; 11 females) were enrolled in this study. All participants were recruited through a local advertisement. Prescreening was conducted by telephone before the subjects underwent a semi-structured interview during the first visit. Exclusion criteria included history of psychiatric and/or neurological disorders including epilepsy, any previous exposure to stimulant drugs, head injury, and migraine. To confirm handedness and emotional status, the Edinburgh Handedness Inventory and Beck Depression Inventory (BDI) were administered. Applicants who showed a laterality index less than 40 (left-handedness) for Edinburgh Handedness Inventory and depression score higher than ten were excluded. Brain MRI was performed within 1 week after the behavioural task performance for each subject. Written informed consent was obtained in all cases before study enrolment and the study protocol was approved by the Ethical Committee of the Centre for Addiction and Mental Health Research, University of Toronto.
Behavioural Measurement
Barratt Impulsivity Scale
A self-report trait measure of impulsivity was collected using the Barratt Impulsivity Scale-11 (BIS). The BIS is a self-report questionnaire containing 30 questions, on a 4-point Likert scale reflecting frequency of occurrence. Scoring yields a total and three subscale scores: attentional (rapid shifts and impatience with complexity), motor (impetuous action) and non-planning (lack of future orientation) impulsiveness (Patton et al. 1995). Higher scores demonstrate higher impulsivity.
Kirby’s Delay Discounting Task
As a laboratory measure of impulsivity, computerized Kirby’s delay discounting task (DDT) was used (Kirby et al. 1999). The task was composed of 27 trials; in each trial the amounts of monetary reward for immediate and delay options were decided by the fixed k value and the delay time was based on the hyperbolic function of delay discount, V = A/(1 + kD), where V is the value of the delayed outcome, A is the delayed reward, D is the length of the delay, and k expresses the steepness of the discount function (de Wit et al. 2002; Mitchell 1999; Richards et al. 1999). Based on this function, higher k-values are associated with a preference for immediate but small rewards and lower k-values indicate a preference for delayed but large rewards. Thus, low k-values are an index of minor impulsivity. Subjects were instructed that they had to make preference judgments about hypothetical rewards shown on a computer screen. All reward choices were made by pressing either the ← or → key with the subject’s dominant hand (right hand for all subjects). Choice options were presented on the screen until response selection; the inter-stimulation interval was 2 s. The trial order was fixed across the subjects based on Kirby’s inventory (Kirby et al. 1999).
The individual discounting values (k) were obtained from all subjects. The detailed method for computing individual discounting values (k) has been described elsewhere (Kirby et al. 1999; Kirby and Petry 2004). The k-values were estimated as the geometric mean between the lowest implied indifference k-value in which subjects chose the delayed option, and the highest implied indifference k-value in which subjects chose the immediate option. For all analyses the distributions of k-values were approximately normalized using the natural-log transformation [ln(k)].
Structural MRI
MRI scans were obtained using a 1.5T high-resolution MRI scanner (GE Signa EXCITE). T1-weighted 3D gradient echo imaging (FSPGR with repletion time = 11.9 ms, echo time = 5 ms, flip angle = 40°, field of view = 24 cm, slice thickness = 1 mm, NEX = 1, matrix size = 256 × 192) was performed to obtain 176 images covering the entire brain.
Voxel-Based Morphometry (VBM) Analysis
Images were processed using a VBM protocol implemented in VBM8 tool-box (http://dbm.neuro.uni-jena.de/vbm/) of the SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) with default parameter incorporating the DARTEL toolbox. Images were bias-corrected, tissue classified, and registered using linear (12-parameter affine) and non-linear transformations, within a unified model (Ashburner and Friston 2005). Final modified GM images were smoothed with an isotropic Gaussian kernel of 8 mm full-width at half maximum. To exclude from the statistical analysis pixels assigned by the segmentation to GM with low probability values and pixels with a low inter-subject anatomical overlay after normalization, the mean image of normalized GM from all subjects was used to create a GM mask, whose threshold was set at a value of 0.30 (pixels with computed GM fraction values >30 % were selected) and then used as an explicit mask for the statistical analysis.
In SPM8 voxel-wise multiple regression analysis of GM images and impulsivity scores with age and total brain volume as nuisance variables was performed with a P- threshold <0.001 uncorrected and an extent threshold of 100 continuous voxels. Small volume correction (SVC) for regional multiple comparisons was performed on regions of interest (ROI) associated with impulsivity level (i.e. DLPFC, MePFC and VST) by using a sphere with 10 mm-radius centered in the peak coordinates. SVC results are reported as significant at P < 0.05 with family-wise error correction on the voxel-level. Bonferroni correction was applied for multiple tests of correlation for BIS subscales.
All the analyses involving behavioural data were performed with SPSS software (SPSS Inc., Chicago), and the significance level was set at P < 0.05. For the visualization of the t-score statistics (SPM{t} map), the significant voxels were projected onto the ICBM152 brain mash image thus allowing anatomic identification using BrainNet viewer (http://www.nitrc.org/projects/bnv/) with a P-threshold <0.001, uncorrected. The MNI coordinate of the local maximum of each cluster was converted into Talairach coordinates (Talairach and Tornoux 1988) and listed in Tables 2 and 3 along with T-value and cluster size.
Table 2.
Regions showing the GM volumetric correlation with BIS total and sub-scales
| Region | BA | Coordinatesa
|
T-score | SVC | Cluster Sizeb | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | Corrected P value | ||||
| Positive correlation with BIS total score | |||||||
| Lt anterior cingulate gyrus | BA 24 | −9 | 37 | −2 | 5.29 | 0.001 | 3820 |
| BA 32 | −6 | 32 | 23 | 5.17 | 0.001 | ||
| Lt medial frontal gyrus | BA 10 | −3 | 43 | −7 | 5.22 | 0.001 | |
| Lt middle frontal gyrus (DLPFC) | BA 46 | −41 | 43 | 9 | 4.30 | 0.007 | 635 |
| Positive correlation with non-planning impulsivity score | |||||||
| Lt anterior cingulate gyrus | BA 32 | −2 | 46 | −2 | 5.87 | 0.0001 | 3561 |
| Rt middle cingulate gyrus | BA 24 | 5 | −2 | 36 | 5.60 | 0.0001 | |
| Lt middle cingulate gyrus | BA 32 | −3 | 15 | 35 | 5.17 | 0.0001 | |
| Lt middle frontal gyrus (DLPFC) | BA 47 | −41 | 31 | 2 | 4.75 | 0.003 | 306 |
| Rt orbitofrontal gyrus | BA 11 | 5 | 22 | −20 | 4.37 | 0.008 | 131 |
| Positive correlation with attentional impulsivity score | |||||||
| Lt medial frontal gyrus | BA 10/11 | −3 | 37 | −14 | 5.06 | 0.001 | 672 |
| Rt medial frontal gyrus | BA 11 | 8 | 45 | −17 | 4.03 | 0.01 | |
| Lt middle frontal gyrus (DLPFC) | BA 10/46 | −41 | 47 | 15 | 4.90 | 0.002 | 611 |
L left, R right, DLPFC dorsolateral prefrontal cortex, BA Brodmann’s area
Talairach coordinate (mm), SVC small volume correction (radius = 10 mm from statistical peak) was applied using a reporting criterion of P < 0.05 with family wise error corrected for multiple comparison
No. of voxels
Table 3.
Regions showing the correlation between GM volume and ln(k)
| Region | BA | Coordinatesa
|
T-score | SVC | Cluster sizeb | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | Corrected P value | ||||
| Positive correlation | |||||||
| Rt medial frontal gyrus | BA 11 | 11 | 46 | −17 | 5.32 | 0.001 | 238 |
| Rt orbitofrontal gyrus | BA 11 | 3 | 56 | −20 | 3.81 | 0.005 | |
| Lt medial frontal gyrus | BA 9 | −12 | 38 | 18 | 5.07 | 0.001 | 609 |
| Lt anterior cingulate gyrus | BA 24 | −8 | 31 | 2 | 4.51 | 0.004 | |
| Rt anterior cingulate gyrus | BA 32 | 17 | 15 | 33 | 4.50 | 0.004 | 265 |
| Lt middle cingulate gyrus | BA 24 | −3 | −11 | 36 | 4.03 | 0.01 | 102 |
| Negative correlation | |||||||
| Lt ventral putamen | −29 | 11 | −8 | 4.48 | 0.005 | 226 | |
| Rt ventral putamen | 29 | 17 | −5 | 4.33 | 0.006 | 120 | |
L left, R right, BA Brodmann’s area
Talairach coordinate (mm), SVC small volume correction (radius = 10 mm from statistical peak) was applied using a reporting criterion of P < 0.05 with family wise error corrected for multiple comparison
No. of voxels
Results
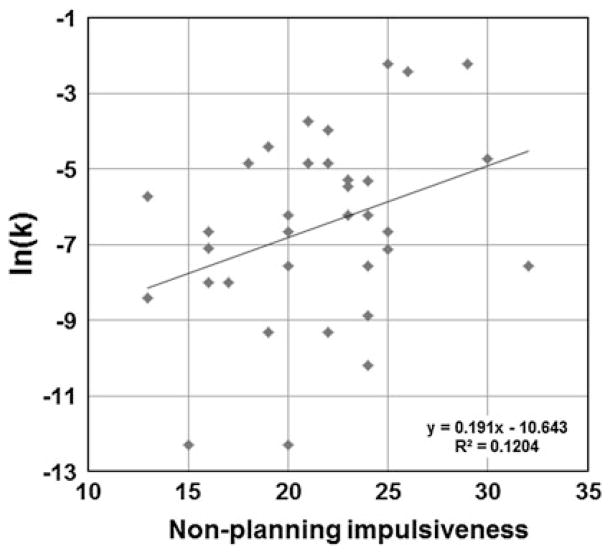
The mean (±SD) and range for BIS and log transformed k-value [ln(k)] are shown in Table 1. The mean BIS total score for all subjects was 58.7 (SD = 10.0, range 36–78), which is representative of the normal range for the general population (Spinella 2007). The mean ln(k) of DDT was −6.5 (SD: 2.5, range was −12.3 to −2.2). BIS total score did not correlate with ln(k) (Pearson’s correlation coefficient r = 0.28, P = 0.11). Within the sub-scores, the non-planning impulsivity scale of BIS showed significant positive correlation with ln(k) (Pearson’s correlation coefficient r = 0.35, P < 0.05) (Fig. 1) and a negative correlation with age (Pearson’s correlation coefficient r = −0.044, P < 0.05), suggesting that lack of careful thinking and planning was associated with higher delay discounting tendency and younger age. No significant sex dependent difference was found for either the total or subscales of BIS or ln(k).
Table 1.
Results of impulsivity level using self-reported (BIS) and behavioural task (Kirby’s DDT)
| Mean (±SD) | Range | |
|---|---|---|
| BIS total | 58.7 (10.7) | 36–78 |
| Non-planning | 21.5 (4.5) | 13–32 |
| Attention | 15.3 (3.6) | 14–21 |
| Motor | 21.9 (4.5) | 14–31 |
| Kirby’s ln(k)* | −6.5 (2.5) | −12.3 to −2.2 |
Log transformed k-value
Fig. 1.
Positive correlation between Barratt impulsivity scale (BIS) non-planning impulsivity score and delay discounting task (DDT) ln(k) (Pearson’s correlation coefficient r = 0.35, P < 0.05)
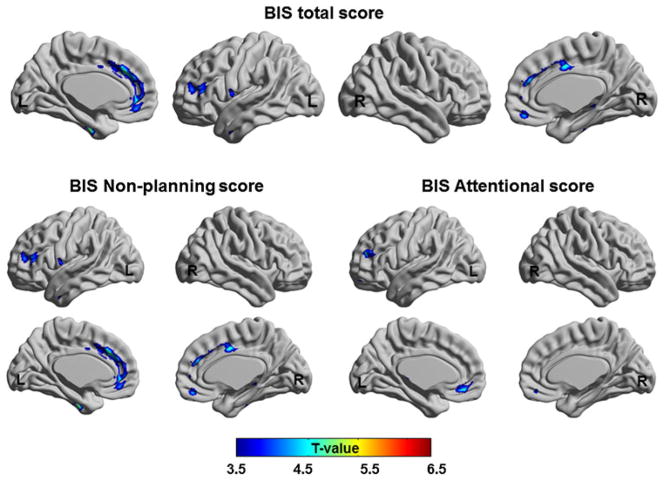
The BIS total score showed a correlation in the ROIs defined in our a priori hypothesis, i.e. MePFC, including the ACC (BA 24/32) and medial OFC (BA10), and DLPFC (BA 46) (Table 2; Fig. 2). Additionally, in the whole brain VBM analysis, positive correlations with level of GM volume were found in middle/inferior temporal (x = −50, y = 0, z = −29, T = 4.01, BA 20/21) and parahippocampal gyri (x = 35, y = −41, z = −3, T = 4.64, BA 19/35), implying that individuals who showed higher GM volume had higher impulsive tendency.
Fig. 2.
Brain regions show the positive correlation of GM volume with BIS total score (upper figure) and BIS sub-scores (lower figure)
When correlation analysis was performed for each score of the BIS sub-scales, the GM volume in ROIs correlated with the non-planning impulsivity scale of BIS at the level of the left pre/subgenual ACC (BA 32) and DLPFC (BA 47), bilateral middle ACC (BA 24/32), and right medial OFC (BA 11), In addition, a positive correlation was observed in the superior temporal gyrus (x = −48, y = 14, z = −6, T = 5.95, BA 38) and the right parahippocampal gyrus (x = 38, y = −48, z = 1, T = 6.76, BA 19) in whole brain level analysis. The left DLPFC (BA 10/46) and bilateral MePFC GM (BA 10/11) volumes were significantly correlated with the attention related impulsivity scale (Table 2; Fig. 2). There were no significant correlations between regional GM volumes and the motor impulsivity scale of BIS.
The ln(k) of the DDT showed some commonalities with the BIS, i.e. positive volumetric correlation in the MePFC including the ACC (BA 24/32) and medial OFC (BA 11) (Table 3; Fig. 3). In addition, GM volume showed a significant negative correlation at the level of the bilateral ventral putamen implying that individuals with lower GM volume in VST had higher impulsive tendency (Table 3; Fig. 3).
Fig. 3.
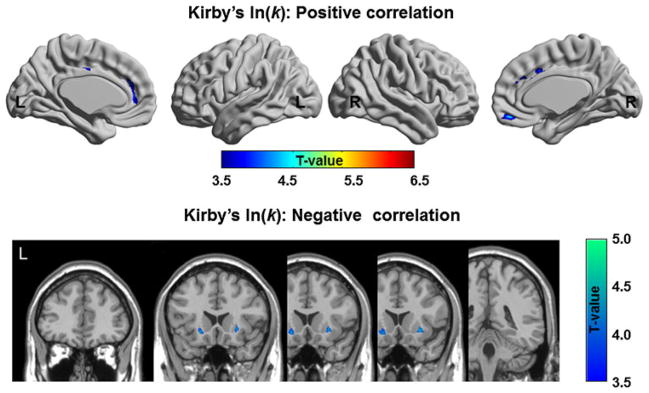
Brain regions show the positive and negative correlation of GM volume with ln(k)
Discussion
Using VBM analysis, we observed a relationship between levels of impulsivity and GM volume in several brain structures associated with decision making and the reward network (i.e. DLPFC, MePFC and VST). In particular, while the non-planning impulsivity score presented a widespread correlation within the MePFC including the dorsal ACC and medial OFC, the correlation of attention related impulsivity subscore was more limited to the medial OFC area. Whereas the origin of these differences can be debatable, we could speculate that the larger correlation area observed in the non-planning impulsivity score may be related to the specific and stronger contribution of these regions in the MePFC in reward-based decision-making and learning (Bush et al. 2002) associated with this task.
Our observation seems to complement previous reports (Matsuo et al. 2009) where instead at the level of the lateral part of the OFC a negative correlation was observed between GM and BIS measured impulsivity. This is not surprising given that it is well known that the medial and lateral OFC have distinct neuro-anatomical connections (Ferry et al. 2000; Kondo et al. 2003, 2005) and functional roles (Noonan et al. 2010; Mar et al. 2011; Bechara 2005). Experimental studies in animals seem to support this different function with a dissociation between the effects of medial and lateral OFC lesions in value guided decision making in macaque monkeys (Noonan et al. 2010). Thus, it seems reasonable to think that the positive relationship between GM volume and impulsivity in the MePFC/medial OFC may be the result of the engagement of reflective system (Bechara 2005) during decision making in subjects with higher impulsivity. Previous studies showed GM volume correlation in the OFC with motor impulsivity (Matsuo et al. 2009). The lack of a similar correlation in our study may be due to the functional differences described above between the lateral and medial OFC and the fact that the latter region may be less sensitive to motor-related impulsivity.
The DLPFC as well was associated with a positive relationship between GM volume and impulsivity level measured with BIS. This correlation seems particularly strong in relation to non-planning and attention/cognitive impulsivity sub-scores but not with motor impulsivity. So far, certain anatomical studies seemed to endorse this association between DLPFC and impulsivity level (Bjork et al. 2009), while others do not (Boes et al. 2009; Matsuo et al. 2009). In support of the former, there are also fMRI (Monterosso et al. 2007; McClure et al. 2004) and neurophysiological experiments conducted either with repetitive transcranial magnetic stimulation (rTMS) or transcranial direct current stimulation (Fecteau et al. 2007; Koch et al. 2005; van’t Wout et al. 2005). By applying rTMS to the right DLPFC in young healthy subjects, we were able to temporarily affect impulsivity level as measured by the DDT (Cho et al. 2010). In particular, rTMS of the right DLPFC induced healthy subjects to trade immediate rewards for delayed larger rewards. We proposed that these results were due to a removal of inhibitory control exerted by this prefrontal area (Aron et al. 2004; Conway and Fthenaki 2003) or by altering time perception (Koch et al. 2003).
Functional interactions between the DLPFC and MePFC have been documented both during behavioral tasks (MacDonald et al. 2000; Cole and Schneider 2007) and brain stimulation (Ohnishi et al. 2004; Knoch et al. 2006; Cho and Strafella 2009; Mayberg et al. 2005). It is thought that these prefrontal areas are part of multiple nodes in a large network of cortical and subcortical areas involved in choice of reward that varies over time (Hariri et al. 2006; Kable and Glimcher 2007; McClure et al. 2004; Xu et al. 2009). A recent study also showed the importance of context-dependent communication between the DLPFC and MePFC during normative decision making that related to monetary reward (Baumgartner et al. 2011).
The impulsivity level measured by DDT showed some commonalities with the BIS in the correlation analysis with GM in several brain areas such as the ACC and medial OFC. The ACC is known to be engaged in socio-cognitive processing, such as the selection of consequential versus inconsequential choices (Turk et al. 2004). Interestingly, because most BIS items are focused on the behavioural pattern in a social context and the DD paradigm also mimics economic decision making in real life, the positive correlation of BIS and DD levels [ln(k)] with ACC GM volume may indicate that the social context inherent in the task and questionnaire items may contribute to decision making. Other neuroimaging studies showed a similar correlation in the ACC. For example, in healthy subjects, activation in the ACC was positively correlated with impulsivity level during a task requiring response inhibition suggesting a significant regulatory role for this region (Brown et al. 2006). The pregenual ACC covaried with the subgenual part of the ACC (BA 25), a target area of the mesocortical dopamine system originating in the ventral tegmental area (Williams and Goldman-Rakic 1998) and also connected with the amygdala (Freedman et al. 2000). Even if it is unclear at the moment if these changes in GM volume, observed in ours and related studies, are part of a compensatory mechanism or primary phenomenon, our observations seem to suggest that the ACC provides significant regulatory input in modulating the level of impulsivity.
Reduced GM volume in the bilateral ventral putamen was associated with higher impulsivity levels measured by DDT in this study. This observation may not be surprising given that, contrary to DDT, BIS measures impulsive personality traits rather than sensitivity to reward (Moeller et al. 2001). This subcortical area is critical for processing reward: activation of this area occurs in response to rewarding stimuli (O’Doherty 2004) regardless of gender, age or race, and valence (positive vs. negative) (Hariri et al. 2006). Striatal dopamine is also implicated in impulsive decision-making behaviours (de Wit et al. 2002). Recent fMRI studies showed that the VST may encode the amount of potential financial gain (Kable and Glimcher 2007) and reward delays in a DDT (Xu et al. 2009). Specifically, the ventral putamen has been suggested to be implicated in encoding the valence of events regardless of the outcome (Mattfeld et al. 2011).
Related to impulsive control, Bechara (2005) provided an interesting conceptual framework with two separate but interacting neural systems that control decision making, i.e. a hyperactive impulsive system (i.e. amygdala-VST) and a deficient reflective/executive system (i.e. PFC), both responsible for impulsive choice and behavior. However, how the balance of these two systems is controlled in the normal functioning healthy brain is still not clear. In our study, we identified a morphological correlation with impulsivity score at the level of the parahippocampal gyrus. This region is functionally and anatomically interconnected with the MePFC (Carmichael and Price 1996) and generally associated with emotional regulation, experience of loss or punishment and spatial memory (Gilbert et al. 2010; Elliott et al. 2000; Ploner et al. 2000; Moscovitch et al. 2005). Thus, in our case, it may be possible that the parahippocampal gyrus may contribute to the emotional salience associated with the decision making process. This is also consistent with previous studies demonstrating the involvement of this region in impulsive behaviour (Völlm et al. 2007; Soloff et al. 2008; Carmona et al. 2005; Cilia et al. 2008).
In summary, our voxel-based VBM analysis showed that impulsivity appears to be reliant on a network of cortical and subcortical structures, emphasizing the importance of brain networks associated with reward related decision making in daily life as morphological biomarkers for impulsivity in a normal healthy population. While our results in healthy volunteers may not directly extend to other pathological impulsive conditions, they provide an insight into the etiology of development of impulsive behaviour in groups of patients with abnormalities in fronto-frontal/fronto-striatal connections (Cilia et al. 2011; Konrad and Eickhoff 2010), such as in drug abuse, pathological gambling, ADHD and Parkinson’s disease.
Acknowledgments
This work was funded by the Canadian Institutes of Health Research to APS (MOP-110962). APS is supported by the Edmond J. Safra Philanthropic Foundation and the Canada Research Chair Program.
References
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Processes. 2003;64(3):345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) J Abnorm Child Psychol. 2001;29(6):541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nat Neurosci. 2011;14(11):1468–1474. doi: 10.1038/nn.2933. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18(6):734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW. Delay discounting correlates with proportional lateral frontal cortex volumes. Biol Psychiatry. 2009;65(8):710–713. doi: 10.1016/j.biopsych.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Soc Cogn Affect Neurosci. 2009;4(1):1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6(2):239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371(2):179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Carmona S, Vilarroya O, Bielsa A, Trèmols V, Soliva JC, Rovira M, Tomàs J, Raheb C, Gispert JD, Batlle S, Bulbena A. Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett. 2005;389(2):88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE. 2009;4(8):e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Ko JH, Pellecchia G, Van Eimeren T, Cilia R, Strafella AP. Continuous theta burst stimulation of right dorsolateral prefrontal cortex induces changes in impulsivity level. Brain Stimul. 2010;3(3):170–176. doi: 10.1016/j.brs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilia R, Siri C, Marotta G, Isaias IU, DeGaspari D, Canesi M, Pezzoli G, Antonini A. Functional abnormalities underlying pathological gambling in Parkinson disease. Arch Neurol. 2008;65(12):1604–1611. doi: 10.1001/archneur.65.12.1604. [DOI] [PubMed] [Google Scholar]
- Cilia R, Cho SS, van Eimeren T, Marotta G, Siri C, Ko JH, Pellecchia G, Pezzoli G, Antonini A, Strafella AP. Pathological gambling in patients with Parkinson’s disease is associated with frontostriatal disconnection: a path modeling analysis. Mov Disord. 2011;26(2):225–233. doi: 10.1002/mds.23480. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Conway MA, Fthenaki A. Disruption of inhibitory control of memory following lesions to the frontal and temporal lobes. Cortex. 2003;39(4–5):667–686. doi: 10.1016/s0010-9452(08)70859-1. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27(5):813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, van den Brink W, Sabbe B. Behavioural aspects of impulsivity in alcoholics with and without a cluster-B personality disorder. Alcohol Alcoholism. 2006;41(4):412–420. doi: 10.1093/alcalc/agl030. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20(16):6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Theoret H, Boggio PS, Fregni F. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J Neurosci. 2007;27(23):6212–6218. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425(3):447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421(2):172–188. [PubMed] [Google Scholar]
- Gilbert AM, Prasad K, Goradia D, Nutche J, Keshavan M, Frank E. Grey matter volume reductions in the emotion network of patients with depression and coronary artery disease. Psychiatry Res. 2010;181(1):9–14. doi: 10.1016/j.pscychresns.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam head injury study. Neurology. 1996;46(5):1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26(51):13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Anton JL, Mazzola-Pomietto P. Impulsivity and neural correlates of response inhibition in schizophrenia. Psychol Med. 2011;41(2):291–299. doi: 10.1017/S0033291710000796. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99(4):461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128(1):78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Knoch D, Treyer V, Regard M, Muri RM, Buck A, Weber B. Lateralized and frequency-dependent effects of prefrontal rTMS on regional cerebral blood flow. Neuroimage. 2006;31(2):641–648. doi: 10.1016/j.neuroimage.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Caltagirone C. Underestimation of time perception after repetitive transcranial magnetic stimulation. Neurology. 2003;60(11):1844–1846. doi: 10.1212/wnl.60.11.1844. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Carlesimo GA, Turriziani P, Caltagirone C. rTMS evidence of different delay and decision processes in a fronto-parietal neuronal network activated during spatial working memory. Neuroimage. 2005;24(1):34–39. doi: 10.1016/j.neuroimage.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003;465(4):499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2005;493(4):479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31(6):904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivová J, Horácek J, Tintera J, Prasko J, Raszka M, Ibrahim I, Höschl C. Medial frontal and dorsal cortical morphometric abnormalities are related to obsessive-compulsive disorder. Neurosci Lett. 2009;464(1):62–66. doi: 10.1016/j.neulet.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31(3):993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, Zhang T, Li X, Li D, Zou L, Tang H, Zhou XJ, Mechelli A, Collier DA, Sweeney JA, Li T, Gong Q. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166(2):196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31(17):6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30(4):1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattfeld AT, Gluck MA, Stark CE. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learn Mem. 2011;18(11):703–711. doi: 10.1101/lm.022889.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Chua SE, Murphy DGM, Suckling J, Tai K-S, Yip LKC, Leung P, Ho TP. Mapping brain structure in attention deficit-hyperactivity disorder: a voxel-based MRI study of regional grey and white matter volume. Psychiatry Res. 2007;154(2):171–180. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146(4):455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158(11):1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat. 2005;207(1):35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TE, Sallet J, Buckley MJ, Rushworth MF. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci USA. 2010;107(47):20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Imabayashi E, Okabe S, Takano H, Arai N, Ugawa Y. rCBF changes elicited by rTMS over DLPFC in humans. Suppl Clin Neurophysiol. 2004;57:715–720. doi: 10.1016/s1567-424x(09)70412-x. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud-Péchoux S, Baulac M, Clémenceau S, Samson S, Pierrot-Deseilligny C. Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb Cortex. 2000;10(12):1211–1216. doi: 10.1093/cercor/10.12.1211. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Indiv Differ. 2006;40(2):305–315. [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71(2):121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50(4):1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff P, Nutche J, Goradia D, Diwadkar V. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Res. 2008;164(3):223–236. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella M. Normative data and a short form of the Barratt Impulsiveness Scale. Int J Neurosci. 2007;117(3):359–368. doi: 10.1080/00207450600588881. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gow CA, Hetherington CR. “No longer Gage”: frontal lobe dysfunction and emotional changes. J Consult Clin Psychol. 1992;60(3):349–359. doi: 10.1037//0022-006x.60.3.349. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51(12):988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tornoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Georg Thieme; Stuttgart: 1988. [Google Scholar]
- Turk DJ, Banfield JF, Walling BR, Heatherton TF, Grafton ST, Handy TC, Gazzaniga MS, Macrae CN. From facial cue to dinner for two: the neural substrates of personal choice. Neuroimage. 2004;22(3):1281–1290. doi: 10.1016/j.neuroimage.2004.02.037. [DOI] [PubMed] [Google Scholar]
- van’t Wout M, Kahn RS, Sanfey AG, Aleman A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex affects strategic decision-making. Neuro Report. 2005;16(16):1849–1852. doi: 10.1097/01.wnr.0000183907.08149.14. [DOI] [PubMed] [Google Scholar]
- Völlm B, Richardson P, McKie S, Elliott R, Dolan M, Deakin B. Neuronal correlates of reward and loss in Cluster B personality disorders: a functional magnetic resonance imaging study. Psychiatry Res. 2007;156(2):151–167. doi: 10.1016/j.pscychresns.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8(4):321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Chen JI, Lerch J, Evans AC. Comparing functional connectivity via thresholding correlations and singular value decomposition. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):913–920. doi: 10.1098/rstb.2005.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Liang ZY, Wang K, Li S, Jiang T. Neural mechanism of intertemporal choice: from discounting future gains to future losses. Brain Res. 2009;1261:65–74. doi: 10.1016/j.brainres.2008.12.061. [DOI] [PubMed] [Google Scholar]
- Yu R. Regional white matter volumes correlate with delay discounting. PLoS ONE. 2012;7(2):e32595. doi: 10.1371/journal.pone.0032595. [DOI] [PMC free article] [PubMed] [Google Scholar]