Abstract
Cognitive impairment occurs frequently in Parkinson’s disease (PD) and the concept of Mild Cognitive Impairment in PD (PD-MCI) has recently emerged. Patients with mild impairment are at risk of developing dementia, and thus it is a topic of growing interest. Many previous studies have investigated the neural correlates of cognitive impairment, in particular executive dysfunction, in PD patients without dementia using neuroimaging techniques including structural MRI, functional MRI and PET imaging. These studies, which have provided a foundation for understanding which brain regions and neurotransmitter systems may be involved in executive dysfunction in PD, will be reviewed. Recent neuroimaging studies that have used specific criteria to classify patients as PD-MCI, in the hopes of gaining further insight into the underlying neural mechanisms will also be discussed. In particular, this review will cover key findings involving structural MRI investigating grey and white matter changes, functional MRI to examine changes in neural activation and PET imaging to investigate metabolic and neurochemical changes that have led to an improved understanding of pathology associated with executive dysfunction in PD without dementia and PD-MCI.
Parkinson’s disease (PD) has been typically described by the cardinal motor symptoms bradykinesia, rigidity and tremor (Gelb, Oliver, & Gilman, 1999). However, non-motor and neuropsychiatric symptoms such as autonomic dysfunction, fatigue, depression, anxiety or cognitive impairment occur frequently and are now well established as part of the disease (Chaudhuri, Healy, & Schapira, 2006; Park & Stacy, 2009). Cognitive impairment is known to be a common feature of PD existing in approximately 27% of all non-demented patients, although the prevalence may vary depending on the sample (Litvan et al., 2011). Dementia is also quite common in PD, with 80% of patients developing dementia over the course of their disease (Aarsland, Larsen, Lolk, & Andersen, 2003; Hely, Reid, Adena, Halliday, & Morris, 2008). Cognitive impairment and dementia in PD negatively affect quality of life (Schrag, 2004; Weintraub, Moberg, Duda, Katz, & Stern, 2004) and increase caregiver burden as well as nursing home placement (Aarsland, Larsen, Karlsen, Lim, & Tandberg, 1999; Aarsland, Larsen, Tandberg, & Laake, 2000; Müller, Kuhn, & Przuntek, 1997). However, the specific neural mechanisms underlying cognitive impairment and decline are still unclear, making it difficult to develop treatments for cognitive symptoms.
Cognitive impairment in PD not meeting the criteria for dementia (Emre et al., 2007) has been termed mild cognitive impairment (PD-MCI; Caviness et al., 2007), similar to the term used in the Alzheimer’s disease literature to refer to patients with memory impairment not meeting the criteria for Alzheimer’s disease (Petersen et al., 1999). However, PD-MCI can include not only memory deficits, but deficits in language, attention, executive function and visuospatial function as well (Caviness et al., 2007). These patients have an increased risk of developing dementia compared with cognitively normal patients (Janvin, Aarsland, & Larsen, 2005; Janvin, Larsen, Aarsland, & Hugdahl, 2006), and thus understanding the neurological basis of this condition is critical for the eventual development of treatment approaches. The specific criteria for impairment in PD-MCI have varied in the literature, however, it usually requires a score of 1–2 SD below the normative mean of healthy control scores on a given neuropsychological test. This lack of a cohesive definition of PD-MCI makes the interpretation of neuroimaging results difficult, as changes observed could be associated with varying severity of impairment depending on the study criteria used. As a result of differing methods of classification, there has been a recent proposal of consensus criteria for PD-MCI (Litvan et al., 2012).
Cognitive impairment in PD is heterogeneous with deficits varying from patient to patient, and it is not clear which of these patients will progress to dementia or how fast. Onset can be as early as the time of disease diagnosis, while others will become impaired at a later stage (Foltynie, Brayne, Robbins, & Barker, 2004; Williams-Gray, Foltynie, Brayne, Robbins, & Barker, 2007). Patients can present with a wide range of cognitive deficits on neuropsychological tests involving executive function, visuospatial function, attention, memory and language with some experiencing impairment in only one area of cognition, and others demonstrating multiple domain impairments (Caviness et al., 2007; Janvin, Aarsland, Larsen, & Hugdahl, 2003). This has led to subtype classifications including single domain and multi-domain, amnestic, or non-amnestic PD-MCI (Caviness et al., 2007; Janvin et al., 2006). There is evidence to suggest that deficits involving more posterior cortical regions are more predictive of future dementia than frontal-executive deficits (Williams-Gray et al., 2009), meaning not all types of PD-MCI patients may develop dementia. However, the implications of this heterogeneity are still not clear, and more research is needed to determine how these subtypes differ in pathology and course, and how this affects risk of dementia.
In particular, executive deficits have been most commonly reported in the literature (Dalrymple-Alford, Kalders, Jones, & Watson, 1994; Downes et al., 1989; Lewis, Cools, et al., 2003; Rogers et al., 1998; Taylor, Saint-Cyr, & Lang, 1986), and there have been many attempts to use neuroimaging techniques to examine brain changes associated with executive deficits in PD. Thus, the primary focus of this review will be brain changes associated with executive dysfunction in PD. Furthermore, as imaging studies classifying patients as PD-MCI according to specific criteria have recently emerged, this review will discuss findings related to cognition involving both PD patients without dementia and patients classified as PD-MCI. Firstly, studies using MRI techniques to uncover structural brain changes in grey and white matter in PD patients will be reviewed. Then, functional brain changes revealed with functional magnetic resonance imaging (fMRI) associated with executive dysfunction will be discussed. Finally, PET imaging studies demonstrating both brain metabolic changes, changes in neural activation and neurochemical changes associated with executive dysfunction will be reviewed.
Structural imaging of cognitive impairment in PD
Structural brain changes associated with cognitive impairment in PD have been of interest. In particular, voxel-based morphometry (VBM) has been a useful tool for assessing grey matter density throughout the brain. Pre-frontal and hippocampal atrophy was shown in early stage PD patients compared with healthy controls, and this atrophy was correlated with impaired performance on tests of vigilance and verbal memory, respectively, in one study examining grey matter changes with VBM (Brück, Kurki, Kaasinen, Vahlberg, & Rinne, 2004). Another study using VBM found significant grey matter loss in PD patients compared with normal controls in the limbic/paralimbic regions and pre-frontal cortex. They also found that impairment on a test of executive and visuospatial function correlated with grey matter loss in these patients in the dorsolateral pre-frontal cortex (DLPFC) and the para hippocampal gyrus (Nagano-Saito et al., 2005). Measures of executive dysfunction in PD patients have also been shown to correlate significantly with caudate nucleus, middle temporal gyri, left precuneus and cerebellar density compared with healthy controls (Camicioli et al., 2009), demonstrating the relationship between grey matter density and cognition in PD. Although evidence for structural changes in PD-MCI specifically is limited, one study investigating grey matter changes with VBM found atrophy in the left frontal lobe and both left and right temporal lobes of patients with PD-MCI compared with cognitively intact PD patients (Beyer, Janvin, Larsen, & Aarsland, 2007), indicating that atrophy in these regions may be associated with cognitive impairment. Another study investigating atrophy in PD-MCI found evidence of significant grey matter loss in the hippocampus compared with healthy controls, whereas patients with normal cognition showed no significant change (Weintraub et al., 2011). This was followed by a study showing that an Alzheimer’s disease pattern of atrophy involving the hippocampus and parietal-temporal cortex is able to predict global cognitive decline in non-demented PD patients with cognitive impairment (Weintraub et al., 2012). These findings implicate the hippocampus as an important region in worsening cognition over time.
However, significant grey matter atrophy has not always been found in PD patients with mild impairment compared with healthy controls (Apostolova et al., 2010; Hattori et al., 2012), and is far more pronounced and widespread in PD dementia (PDD) compared with non-demented PD patients without dementia and aged-matched controls (Apostolova et al., 2010; Burton, Mckeith, Burn, Williams, & Brien, 2004; Hattori et al., 2012; Nagano-Saito et al., 2005), suggesting brain atrophy may not be as prominent in the early stages of cognitive impairment. Catechol-O-Methyl Transferase (COMT) genotype may also have an effect on brain structure. One study found that in healthy subjects and PD patients, differences in COMT genotype resulted in different grey matter volumes, and these differences varied with age. Thus, COMT genotype, which is known to affect functional networks involved in executive processes in PD, may also have an impact on grey matter volume (Rowe et al., 2010).
White matter abnormalities have also been reported in PD-MCI with the use of diffusion tensor imaging. PD-MCI patients demonstrated significantly reduced fractional anisotropy (FA) values, representing a loss of white matter integrity, compared with cognitively normal PD patients and healthy controls. These reductions were strongly correlated with Mini Mental State Examination (MMSE) scores, measuring global cognitive function, bilaterally in the parietal regions (Hattori et al., 2012). This supports a previous finding that FA values were significantly reduced in the left parietal lobe of PD patients with executive impairments compared to those without, indicating a loss of white matter integrity (Matsui et al., 2007). Therefore, it seems likely that not only grey matter loss, but also white matter abnormalities in associative brain regions contribute to cognitive impairment in PD.
Functional magnetic resonance imaging of cognitive impairment in PD
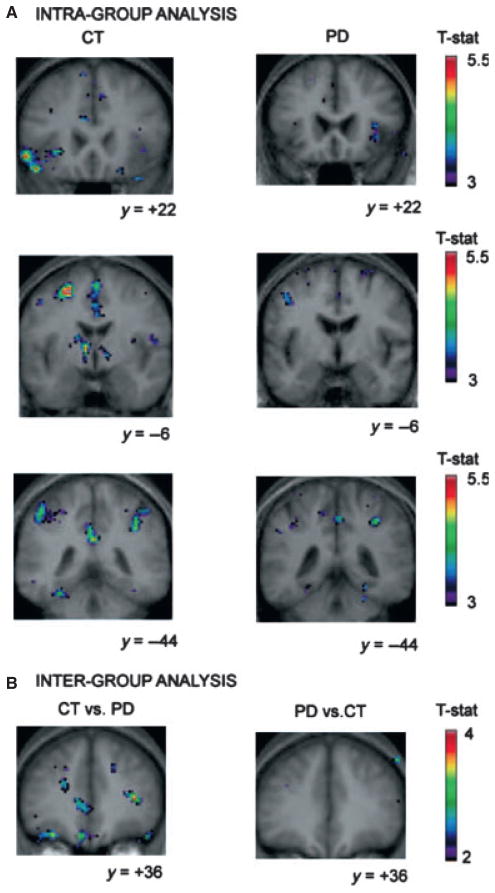
Changes in neural activation associated with active participation in cognitive tasks have been investigated in PD with functional magnetic resonance imaging (fMRI) to elucidate the brain networks involved and how they may be altered compared with healthy controls. The blood-oxygen-level-dependent (BOLD) signal obtained from fMRI can be used as an indirect measure of neural activity. Patients with PD show a different pattern of neural activation while performing cognitive tasks compared with normal controls. In one study, PD patients with cognitive impairment displayed reduced activation in fronto-striatal circuitry compared with cognitively normal PD patients and healthy controls while performing a working memory task (Lewis, Dove, Robbins, Barker, & Owen, 2003). This indicated abnormal cortico-striatal processing in PD, likely due to nigrostriatal dopamine depletion. However, dopamine medication state can also have an impact on the level of activity in cortical regions. PD patients undergoing fMRI scans showed increased cortical activation during a sensorimotor task in the ON state as compared to the OFF medication state, which correlated with improved motor performance (Mattay et al., 2002). However, during a working memory-task relying on the prefrontal cortex, increases in cortical activation were seen in the OFF versus ON dopamine medication state, which correlated with task errors. This suggests that the pathology of cognitive processes differ from the motor pathology in PD, as increases in cortical activation are not seen during motor tasks in the OFF state (Mattay et al., 2002). This led the authors to conclude that disruption of the mesocortical dopamine system could be affecting cognition. This is further supported by fMRI studies in PD patients demonstrating changes in activation compared with normal controls whereas performing a card sorting task involving set-shifting; an executive process that requires mental flexibility (Monchi, Petrides, Mejia-Constain, & Strafella, 2007; Monchi et al., 2004). Reductions in fronto-striatal activation were found only during behavioural shifting conditions requiring the caudate nucleus. Specifically, PD patients had less activation compared with controls when comparing retrieval with shift versus retrieval without shift conditions (Figure 1).
Figure 1.
Figure from Monchi et al. (2007). Images on the upper-left demonstrate brain activation in controls when comparing retrieval with shift versus retrieval without shift conditions in the left ventrolateral PFC, caudate nucleus, posterior cingulate and posterior parietal corticies. Images on the upper-right demonstrate that there is little or no activation in these regions in the PD group. The lower part of the figure demonstrates the increased activation in controls when comparing controls versus PD in the left DLPFC, orbitofrontal cortex and the right ventrolateral PFC, demonstrating the lack of recruitment of these brain regions in behavioural shifting in PD.
Conversely, increases in cortical activation were found when behaviour was simply maintained with the use of working memory, a process relying only on cortical regions and not the caudate (Monchi et al., 2004, 2007). This demonstrated that the level of activation in the brain was dependent on the striatal involvement in the task. In addition, these findings did not agree with the nigrostriatal model of dopamine depletion, which proposes that striatal dopamine loss results in reduced cortical activity. Therefore, these findings provided further evidence for mesocortical dopamine dysfunction affecting executive function in PD.
Genetic influences on cognition in PD have also been investigated with fMRI. One study found that PD patients with a Catechol-O-Methyl Transferase (COMT) genotype resulting in a low activity enzyme, and thus increased synaptic dopamine levels in the frontal cortex, display worse performance on the Tower of London (TOL) planning task which utilizes executive processes. Task performance was also associated with reduced BOLD signal in the fronto-parietal network (Williams-Gray, Hampshire, Robbins, Owen, & Barker, 2007), suggesting that too much cortical dopamine may impair cognitive function. Another investigation found that PD patients with this low activity COMT had more difficulty forming an attentional set during a set-shifting task, and that this was also associated with reduced activation in the fronto-parietal attention network (Williams-Gray, Hampshire, Barker, & Owen, 2008). Therefore, polymorphisms resulting in altered dopamine clearance in the cortex may also be a contributing factor to variability in cognitive performance in addition to disease related dopamine dysfunction.
PET imaging of cognitive impairment in PD
Metabolic changes
It is well established that PD patients show a distinct pattern of brain metabolic activity compared with healthy controls, as revealed by [18F] Fluorodeoxyglucose (FDG) PET. In non-demented PD patients, a network modelling approach was used with [18F] FDG PET to determine a covariance pattern that correlated with tests of memory and executive function in PD patients (Huang et al., 2007). A PD-related cognitive pattern (PDCP) was found characterized by reduced metabolism in frontal and parietal association areas and increases in cerebellar and dentate nuclei metabolism compared with controls, which were not altered by antiparkinsonian treatments (Huang et al., 2007). This pattern indicates that these regions may be disrupted in PD, resulting in cognitive deficits. Although this finding was not specific for patients with impairment, similar metabolic changes have been demonstrated in patients classified as PD-MCI. One study using [18F] FDG PET to examine glucose metabolism found metabolic reductions in the inferior parietal lobule and frontal lobe and increases in pons, cerebellum and dentate nuclei of multi-domain PD-MCI patients compared with cognitively normal PD patients and healthy controls (Huang et al., 2008). The single domain PD-MCI group showed similar changes, although less pronounced, occupying an intermediate stage between the PD group with normal cognition and multi-domain MCI patients (Huang et al., 2008). A second study that classified patients into PD-MCI subtypes found that single domain non-amnestic type PD-MCI had reduced glucose metabolism in the parieto-temporo-occipital regions, whereas the multi-domain type had more widespread parieto-occipital hypometabolism compared with normal controls (Lyoo, Jeong, Ryu, Rinne, & Lee, 2010). The multi-domain group also showed reduced metabolism in the lateral frontal, cingulate and parieto-temporo-occipital cortices compared with PD patients with normal cognition (Lyoo et al., 2010). These findings suggest that those patients with multiple types of cognitive difficulties have more widespread brain metabolic changes, than those patients with only one cognitive domain affected. PET imaging can also be a useful tool for evaluating metabolic brain changes over time. A longitudinal study evaluating glucose metabolism in non-demented PD patients at baseline found that those who converted to dementia several years later had significantly reduced glucose metabolism in the occipital and posterior cingulate cortices at baseline compared with controls, concluding that these metabolic reductions are indicative of future dementia (Bohnen et al., 2011). Therefore, cerebral glucose metabolism can reflect disruption of various networks involved in cognitive function in PD at baseline, and this may prove useful for identifying or differentiating specific cognitive phenotypes.
Changes in neural activation
Cerebral blood flow changes have been measured during the performance of executive tasks with the use of H215O PET as an indirect measure of neural activation. PD patients are thought to have abnormal basal ganglia outflow due to striatal dopamine depletion, affecting cortico-striatal processing. There is evidence to support this in the fMRI literature as outlined previously, and this has also been demonstrated with PET. PD patients show reduced activation as measured with H215O PET in the globus pallidus during the performance of the Tower of London planning task (TOL) relative to control conditions, whereas healthy controls show increased activation (Owen, Doyon, Dagher, Sadikot, & Evans, 1998). This can be interpreted as abnormal basal ganglia outflow to cortical regions. Abnormalities have also been detected in extrastriatal regions during this planning task, where L-Dopa medicated patients demonstrated reduced DLPFC blood flow relative to the OFF state during the task (Cools, Stefanova, Barker, Robbins, & Owen, 2002). This medication-induced reduction correlated with improved performance, supporting the hypothesis that DA medication restores functions in the dopamine depleted dorsolateral cortico-striatal circuitry (Cools, 2001, 2006). On the other hand, there is evidence to suggest that dopamine may actually ‘overdose’ the relatively preserved dopamine terminals in the ventral striatum, impairing performance on tasks requiring this circuitry such as reversal learning (Clatworthy et al., 2009; Cools, 2001; Cools, Altamirano, & D’Esposito, 2006; Swainson, 2000). These findings are in accordance with the post-mortem evidence that DA depletion is most severe in the putamen and dorsal caudate affecting motor function (Kish, Shannak, & Hornykiewicz, 1988). However, over time, in certain patients, dopamine loss may spread more ventro-medially to regions of the striatum involved in cognitive processing (Kish et al., 1988). Thus, cognitive performance may vary depending on the level of dopamine depletion, as well as medication state and the task being performed. In another H215O PET study, PD patients showed normal cortical activation, but reduced activation in the caudate nucleus compared with healthy controls while performing the TOL task (Dagher, Owen, Boecker, & Brooks, 2001). This demonstrated that PD patients could have normal levels of cortical activation despite abnormal processing in the striatum. Moreover, patients showed enhanced hippocampal activation compared with healthy controls, which could be representative of the recruitment of compensatory processes due to inadequate cortico-striatal function (Dagher et al., 2001).
Neurochemical changes
PET imaging studies investigating neurochemical changes associated with cognitive impairment in PD have also indicated the involvement of various neurotransmitter systems. In particular, the dopaminergic and cholinergic systems have been of primary interest. PD patients show many changes in the dopaminergic system related to cognitive function, many of which have been demonstrated using [18F] Fluorodopa (F-DOPA) PET, which provides a measure of dopamine synthesis capacity. A PET study using [18F] F-DOPA found that PD patients exhibited reduced Ki values, or dopamine synthesis in the putamen, right caudate and left ventral striatum compared with healthy controls, which expanded to include the anterior cingulate cortex in demented patients (Ito et al., 2002). This suggests that dopaminergic dysfunction in PD may progress from the nigrostriatal dopamine system to eventually affect the mesocortical system with worsening cognition. Another [18F] F-DOPA PET study found that PD patients right caudate [18F] F-DOPA uptake covaried significantly with Tower of London task scores and that left anterior putamen uptake covaried with scores on a verbal working memory task (Cheesman et al., 2005). Similarly, another report showed that reduced [18F] F-DOPA uptake in the right caudate nucleus was related to worse performance on the Stroop test, which requires response inhibition, relative to healthy controls and a similar trend was seen in the left caudate (Brück et al., 2001). Although primarily involved in motor function, putaminal dopamine synthesis has also been found to correlate with impaired performance on the Wisconsin Card Sorting task; a test of executive function, in PD patients (Cropley et al., 2008). Together, these findings demonstrate the relationship between nigrostriatal dopamine synthesis and cognitive performance, and strongly implicate a role for nigrostriatal dopamine dysfunction in executive impairment in PD. Some changes have also been seen with [18F] F-DOPA in the extrastriatal regions. In early PD patients, cortical uptake is increased compared with healthy controls, which could be representative of a compensatory response to diminished dopamine levels in PD (Brück, Aalto, Nurmi, Bergman, & Rinne, 2005). This activity was found to correlate positively with attention in the DLPFC and negatively with inhibition on the Stroop task in the anterior cingulate and medial pre-frontal cortex (Brück et al., 2005), suggesting that this increased dopaminergic activity could contribute to deficits of inhibitory control.
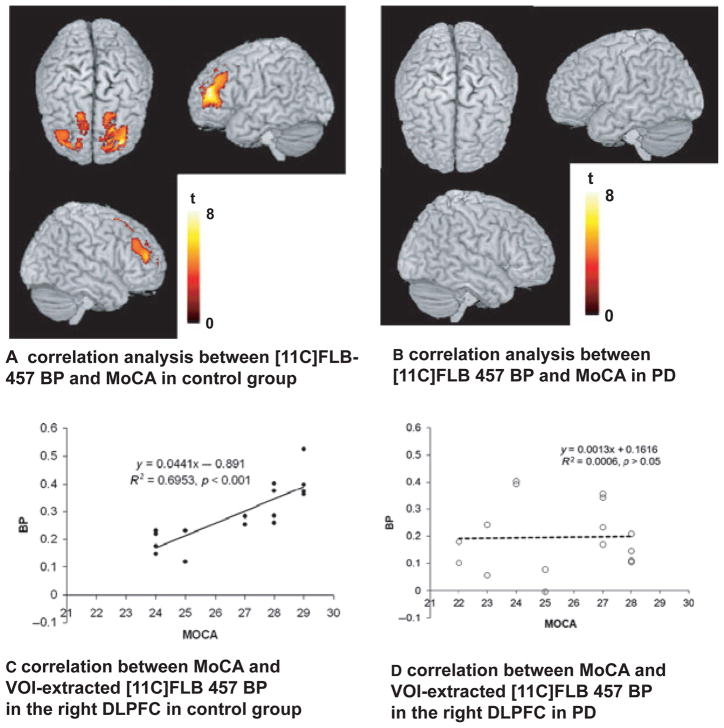
PET imaging in humans has demonstrated that dopamine is released during executive processes in the striatum (Monchi, Ko, & Strafella, 2006) as well as extrastriatal regions such as the anterior cingulate, frontal and temporal cortices (Aalto, Brück, Laine, Någren, & Rinne, 2005; Ko et al., 2009) with the use of radioligands that compete with endogenous dopamine for post-synpatic dopamine receptors. Striatal dopamine release as measured by [11C]-raclopride PET is significantly reduced in the dorsal caudate in PD patients compared with healthy controls during the performance of a spatial working memory task requiring executive function (Sawamoto et al., 2008). This implies that a lack of nigrostriatal dopamine release in the caudate, a region of the striatum involved in cognitive processes, may result in impairment. In addition, a recent PET study using the high-affinity extrastriatal D2/D3 receptor ligand [11C] FLB-457, found evidence of dopamine release in the right orbitofrontal cortex in healthy controls during the performance of an executive task, but no such release in PD patients (Ko et al., 2012). This further supports the notion that mesocortical DA disruption is associated with cognitive impairment in PD. There was also a correlation between D2 receptor availability and MoCA scores for both active and control scans in bilateral DLPFC and ACC in healthy controls, but no such correlation in PD patients (Figure 2), suggesting a potential disruption of the mesocortical dopamine system (Ko et al., 2012).
Figure 2.
Figure from Ko et al. (2012). The image on the left demonstrates the correlation between [11C] FLB BP and MoCA scores in the DLPFC of healthy controls. The graph below demonstrates extracted BP values from the significant cluster plotted against individual MoCA scores, confirming a positive correlation. On the right, the image demonstrates no significant correlation between [11C] FLB BP and MoCA in PD patients, indicating a disruption of normal DLPFC function.
It is well known that degeneration of the Nucleus basalis of Meynert, the main cholinergic nucleus of the brainstem, occurs in PD (Candy et al., 1983). Resulting changes in the cholinergic system of the brain have been shown to affect cognition in PD. Acetylcholinesterase activity reductions as measured by [11C] PMP PET were shown to be greater in PD than in Alzheimer’s disease relative to healthy controls, however, these reductions were more pronounced in PD with dementia than non-demented PD patients (Bohnen et al., 2003). Another study found that cortical acetylcholinesterase activity was reduced in non-demented PD patients and more so in demented PD patients compared with controls. These reductions were most robustly correlated with impairment on tests of attention and working memory, but also with tests of executive function such as the Stroop task and the Trail Making test. These reductions in acetylcholinesterase activity were independent of motor function or disease severity, indicating the significance of acetylcholine as a neurotransmitter involved in cognition (Bohnen et al., 2006). These findings also indicate that the function of acetylcholine extends to a range of cognitive processes, and that cortical acetylcholine depletion may accompany cognitive decline. A recent PET study assessing acetylcholinesterase activity reductions and striatal dopamine depletion found that both of these measures were independently associated with impairments in global cognitive function (Bohnen et al., 2012). Thus, it is likely that both brain acetylcholine depletion as well as nigrostriatal dopamine depletion contribute to cognitive changes seen in PD-MCI.
Imaging amyloid load with [11C]-PIB PET has been of interest in neurodegenerative disease. However, non-demented PD patients and even PD patients with dementia do not always show significant increases in amyloid load in the brain compared with healthy controls (Edison et al., 2008; Jokinen et al., 2010). Therefore, more work will be needed to determine whether or not amyloid load contributes to cognitive impairment in PD.
Although the majority of PET studies investigating cognition in PD have been focused on the dopaminergic and cholinergic systems, the involvement of other neurotransmitter systems such as the noradrenergic system and the serotonergic systems cannot be ruled out. There is degeneration of brainstem nuclei producing these neurotransmitters in PD patients (Halliday, Blumbergs, Cotton, Blessings, & Geffen, 1990), and reduced cortical levels of these transmitters and their metabolites (Scatton, Javoy-Agid, Rouquier, Dubois, & Agid,1983). PET studies investigating other symptoms of PD have shown receptor abnormalities in both noradrenergic and serotonergic systems in the brain (Boileau et al., 2008; Remy, Doder, Lees, Turjanski, & Brooks, 2005). In addition, there is some evidence that treatments targeting these systems may be effective (Marsh, Biglan, Gerstenhaber, & Williams, 2009).
Future directions
Neuroimaging has greatly advanced the field of cognitive impairment in Parkinson’s disease. Structural imaging studies have indicated that grey matter atrophy and integrity of white matter may be important factors in contributing to cognitive decline, especially with more severe impairment. However, as a result of conflicting evidence as to which brain regions undergo changes affecting cognition and at what disease stage this is most relevant, further study is needed to draw conclusions. The inconsistency in findings of grey matter atrophy in cognitively impaired PD patients could be a result of heterogeneous samples. For those studies gathering samples of non-demented PD patients, variability among patients in terms of disease stage and extent of cognitive impairment could contribute to differing results. For the studies looking specifically at PD-MCI, differing test methods and cut-offs for PD-MCI classification could cause discrepancies between findings. For example, in the study examining grey matter changes by Apostolova et al. (2010), the modified Petersen criteria were used, which requires a score of at least 1.5 SD below the mean on at least one neuropsychological test and a cognitive complaint, whereas a similar study by Weintraub et al. (2012) used a score of between 6 and 8 on the Dementia Rating Scale 2 as criteria for PD-MCI. Future studies will need to attempt to adhere to homogeneous criteria to minimize discrepancies between results, as well as distinguish PD patients with normal cognition from those with impairment to avoid heterogeneous samples.
Functional MRI and H215O PET has also revealed that Parkinson’s disease patients recruit different brain networks and show altered patterns of activation in cortico-striatal circuitry compared with controls. Namely, PD patients either show increased or decreased activation compared with healthy controls depending on the task they are performing and their current medication state. Cortical activation is also affected by cortical dopamine levels, which are in turn affected by genetic polymorphisms resulting in altered dopamine clearance. Thus, dopamine levels have an impact on both brain activation and performance on executive tasks. Future research should attempt to relate anatomical changes and neurochemical changes to neural activation in order to better understand how these pathologies affect cognitive function. PET imaging has provided valuable information regarding metabolic changes and the role of abnormal receptor and neurotransmitter function in PD. PD patients show a distinct pattern of brain metabolism compared with normal controls, characterized primarily by hypometabolism in frontal, parietal, temporal and occipital regions. The reason for differences between studies in anatomical location of hypometabolism may be due to the classification of PD patients. Studies looking at PD patients in general may have a sample with a very different cognitive profile than those studies that classify patients as PD-MCI according to criteria. Now that patients with mild cognitive impairment can be distinguished from those with normal cognition, it will be important to separate these groups in order to maximize the power to detect changes.
There is substantial evidence for striatal dopamine depletion as demonstrated by PET studies measuring dopamine uptake, synthesis and release as well as cortical dopamine abnormalities contributing to executive dysfunction in PD. However, it is still not known to what extent pathology in the individual nigrostriatal and mesocortical dopamine pathways affect cognition. Although the dopamine system in PD has been largely represented in the literature, recent evidence for cholinergic involvement in cognitive impairment has changed the view that dopamine dysfunction alone affects cognition. It is possible that relationships between cognitive function and both dopaminergic and cholinergic abnormalities have been found because they each have distinct roles in executive processing. However, there could also be a combined effect of pathology in these systems contributing to cognitive impairment in PD. Future study should look at the relative roles of these neurotransmitter systems in PD-MCI and also attempt to relate this to cognitive performance and risk of dementia for individual patients. More longitudinal imaging studies would be useful for determining how pathology in these systems progresses over time and how this relates to cognitive decline. Other neurotransmitter systems such as the noradrenergic and serotonergic systems, as well as amyloid deposition should also be considered to determine whether these are major factors in the development of cognitive impairment in PD.
Conclusions
Neuroimaging has provided evidence for a number of factors underlying executive dysfunction in PD. Structural MRI has revealed that grey matter atrophy and white matter abnormalities are associated with executive performance in PD and PD-MCI. Functional MRI and H215O PET have shown that PD patients have altered activation of brain networks, which is affected by dopamine levels and consequently affects performance on executive tasks. Genetic polymorphisms also have an impact on cortical dopamine and cognitive performance. PET imaging studies have demonstrated distinct patterns of resting metabolic changes in PD patients without dementia and PD-MCI related to cognitive function. Furthermore, PET imaging has provided evidence for neurochemical changes in the dopaminergic and cholinergic systems of the brain associated with executive impairment in PD patients without dementia and PD-MCI. More work will be needed to determine how structural, functional and neurochemical brain changes affect different aspects of cognition, and the resulting outcomes associated with these changes.
With the recent development of PD-MCI as a unique subset of patients, neuroimaging will continue to be a valuable tool. Imaging PD-MCI will enhance current knowledge, and hopefully lead to a more cohesive understanding of the disease mechanisms underlying cognitive impairment in PD.
Acknowledgments
This study was supported by Canadian Institutes of Health Research (MOP 110962). A.P.S. is also supported by Canada Research Chair program and E.J. Safra Foundation. L.C. is supported by a scholarship from Parkinson Society Canada.
References
- Aalto S, Bruck A, Laine M, Någren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: A positron emission tomography study using the high-affinity dopamine D2 receptor ligand [(11)C]FLB 457. Journal of Neuroscience. 2005;25:2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. International Journal of Geriatric Psychiatry. 1999;14:866–874. doi: 10.1002/(SICI)1099-1166(199910)14:10<866::AID-GPS38>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Larsen J, Lolk A, Andersen K. Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Archives of Neurology. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: A population-based, prospective study. Journal of the American Geriatrics Society. 2000;48:938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. 35400009126757.0100. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Beyer M, Green AE, Hwang KS, Morra JH, Chou YY, Thompson PM. Hippocampal, caudate, and ventricular changes in Parkinson’s disease with and without dementia. Movement Disorders. 2010;25:687–688. doi: 10.1002/mds.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. Journal of Neurology. 2007;78:254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, Dekosky ST. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. Journal of Neurology. 2006;253:242–247. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, Dekosky ST. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: An in vivo positron emission tomographic study. Archives of Neurology. 2003;60:1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Koeppe RA, Minoshima S, Giordani B, Albin RL, Frey KA, Kuhl DE. Cerebral glucose metabolic features of Parkinson disease and incident dementia: Longitudinal study. Journal of Nuclear Medicine. 2011;52:848–855. doi: 10.2967/jnumed.111.089946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller MLTM, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, Frey KA. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. Journal of Cerebral Blood Flow & Metabolism. 2012;32:1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Warsh JJ, Guttman M, Saint-Cyr JA, McCluskey T, Rusjan P, Kish SJ. Elevated serotonin transporter binding in depressed patients with Parkinson’s disease: A preliminary PET study with [11C]DASB. Movement Disorders. 2008;23:1776–1780. doi: 10.1002/mds.22212. [DOI] [PubMed] [Google Scholar]
- Brück A, Aalto S, Nurmi E, Bergman J, Rinne JO. Cortical 6-[(18)F]fluoro-L-dopa uptake and frontal cognitive functions in early Parkinson’s disease. Neurobiology of Aging. 2005;26:891–898. doi: 10.1016/j.neurobiolaging.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Brück A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:1467–1469. doi: 10.1136/jnnp.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück A, Portin R, Lindell A, Laihinen A, Bergman J, Haaparanta M, Rinne JO. Positron emission tomography shows that impaired frontal lobe functioning in Parkinson’s disease is related to dopaminergic hypofunction in the caudate nucleus. Neuroscience Letters. 2001;311:81–84. doi: 10.1016/S0304-3940(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Mckeith IG, Burn DJ, Williams ED, Brien JTO. Cerebral atrophy in Parkinson’s disease with and without dementia: A comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Gee M, Bouchard TP, Fisher NJ, Hanstock CC, Emery DJ, Martin WRW. Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in Parkinsonism. Parkinsonism & Related Disorders. 2009;15:187–195. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Candy JM, Perry RH, Perry EK, Irving D, Blessed G, Fairbairn AF, Tomlinson BE. Pathological changes in the nucleus of meynert in Alzheimer’s and Parkinson’s diseases. Journal of the Neurological Sciences. 1983;59:277–289. doi: 10.1016/0022-510X(83)90045-X. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, Adler CH. Defining mild cognitive impairment in Parkinson’s disease. Movement Disorders. 2007;22:1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AHV. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurology. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- Cheesman AL, Barker RA, Lewis SJG, Robbins TW, Owen AM, Brooks DJ. Lateralisation of striatal function: Evidence from [(18)F]-dopa PETin Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:1204–1210. doi: 10.1136/jnnp.2004.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJG, Brichard L, Hong YT, Izquierdo D, Clark L, Robbins TW. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. Journal of Neuroscience. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cerebral Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neuroscience and Biobehavioural Reviews. 2006;30:1–23. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Cools R, Altamirano L, D’Esposito M. Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: The role of the pre-frontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Bara-Jimenez W, Brown AK, Zhang XY, Sangare J, Innis RB. Pre- and post-synaptic dopamine imaging and its relation with frontostriatal cognitive function in Parkinson disease: PET studies with [(11)C]NNC 112 and [(18)F]FDOPA. Psychiatry Research. 2008;163:171–182. doi: 10.1016/j.pscychresns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. The role of the striatum and hippocampus in planning: A PET activation study in Parkinson’s disease. Brain. 2001;124:1020–1032. doi: 10.1093/brain/124.5.1020. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, Kalders AS, Jones RD, Watson RW. A central executive deficit in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1994;57:360–367. doi: 10.1136/jnnp.57.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Morris RG, Robbins TW. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson’s disease: Evidence for a specific attentional dysfunction. Neuropsychologia. 1989;27:1329–1343. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Brooks DJ. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [(11)C] PIB positron emission tomography. Journal of Neurology, Neurosurgery & Psychiatry. 2008;79:1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement Disorders. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Brayne CEG, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain. 2004;127(Pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- Gelb D, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Archives of Neurology. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. doi:10-1001/pubs.ArchNeurol.-ISSN-0003-9942-56-1-nsa7701. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Blumbergs PC, Cotton RG, Blessings WW, Geffen LB. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Research. 1990;510:104–107. doi: 10.1016/0006-8993(90)90733-R. [DOI] [PubMed] [Google Scholar]
- Hattori T, Orimo S, Aoki S, Ito K, Abe O, Amano A, Mizusawa H. Cognitive status correlates with white matter alteration in Parkinson’s disease. Human Brain Mapping. 2012;33:727–739. doi: 10.1002/hbm.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Movement Disorders. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70:1470–1477. doi: 10.1212/01.wnl.0000304050.05332.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson’s disease. NeuroImage. 2007;34:714–723. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Nagano-Saito A, Kato T, Arahata Y, Nakamura A, Kawasumi Y, Brooks DJ. Striatal and extrastriatal dysfunction in Parkinson’s disease with dementia: A 6-[(18)F]fluoro-l-dopa PET study. Brain. 2002;125:1358–1365. doi: 10.1093/brain/awf134. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson’s disease: A community-based, 4-year longitudinal study. Journal of Geriatric Psychiatry and Neurology. 2005;18:149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Aarsland D, Larsen JP, Hugdahl K. Neuropsychological profile of patients with Parkinson’s disease without dementia. Dementia and Geriatric Cognitive Disorders. 2003;15:126–131. doi: 10.1159/000068483. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: Progression to dementia. Movement Disorders. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- Jokinen P, Scheinin N, Aalto S, Någren K, Savisto N, Parkkola R, Rinne JO. [(11)C]PIB-, [(18)F]FDG-PET and MRI imaging in patients with Parkinson’s disease with and without dementia. Parkinsonism & Related Disorders. 2010;16:666–670. doi: 10.1016/j.parkreldis.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. New England Journal of Medicine. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Ko JH, Antonelli F, Monchi O, Ray N, Rusjan P, Houle S, Strafella AP. Prefrontal dopaminergic receptor abnormalities and executive functions in Parkinson’s disease. Human Brain Mapping. 2012 doi: 10.1002/hbm.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Ptito A, Monchi O, Cho SS, Van Eimeren T, Pellecchia G, Strafella AP. Increased dopamine release in the right anterior cingulate cortex during the performance of a sorting task: A [(11)C]FLB 457 PET study. NeuroImage. 2009;46:516–521. doi: 10.1016/j.neuroimage.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJG, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia. 2003;41:645–654. doi: 10.1016/S0028-3932(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. Journal of Neuroscience. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. Retrieved from http://ukpmc.ac.uk/abstract/MED/12867520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Weintraub D. MDS task force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Movement Disorders. 2011;26:1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement disorder society task force guidelines. Movement Disorders. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo CH, Jeong Y, Ryu YH, Rinne JO, Lee MS. Cerebral glucose metabolism of Parkinson’s disease patients with mild cognitive impairment. European Neurology. 2010;64:65–73. doi: 10.1159/000315036. [DOI] [PubMed] [Google Scholar]
- Marsh L, Biglan K, Gerstenhaber M, Williams JR. Atomoxetine for the treatment of executive dysfunction in Parkinson’s disease: A pilot open-label study. Movement Disorders. 2009;24:277–282. doi: 10.1002/mds.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Nishinaka K, Oda M, Niikawa H, Komatsu K, Kubori T, Udaka F. Wisconsin card sorting test in Parkinson’s disease: Diffusion tensor imaging. Acta Neurologica Scandinavica. 2007;116:108–112. doi: 10.1111/j.1600-0404.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, Weinberger DR. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Annals of Neurology. 2002;156–164 doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: A [(11)C] raclopride PET study. NeuroImage. 2006;33:907–912. doi: 10.1016/j.neuroimage.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson’s disease. Journal of Neuroscience. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain. 2007;130(Pt 1):233–244. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Kuhn W, Przuntek H. Non-motor symptoms of Parkinson disease. Significant impact on quality of life–using possible treatments. Fortschritte der Medizin. 1997;115:45–48. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19901251. [PubMed] [Google Scholar]
- Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, Ito K. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64:224–229. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson’s disease identified with PET: Implications for higher cortical functions. Brain. 1998;121:949–965. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- Park A, Stacy M. Non-motor symptoms in Parkinson’s disease. Journal of Neurology. 2009;256(Suppl 3):293–298. doi: 10.1007/s00415-009-5240-1. [DOI] [PubMed] [Google Scholar]
- Petersen R, Waring S, Ivnik R, Tangalos E, Kokmen E, Smith G. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. doi:10-1001/pubs.ArchNeurol.-ISSN-0003-9942-56-3-noc7845. [DOI] [PubMed] [Google Scholar]
- Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: Loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121:815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Williams-Gray CH, Bishop S, Fallon S, Barker RA, Owen AM. The val158met COMT polymorphism’s effect on atrophy in healthy aging and Parkinson’s disease. Neurobiology of Aging. 2010;31:1064–1068. doi: 10.1016/j.neurobiolaging.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131(Pt 5):1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Research. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schrag A. Psychiatric aspects of Parkinson’s disease–an update. Journal of Neurology. 2004;251:795–804. doi: 10.1007/s00415-004-0483-3. [DOI] [PubMed] [Google Scholar]
- Swainson R. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: Possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/S0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson’s disease. The cortical focus of neostriatal outflow. Brain. 1986;109(Pt 5):845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Dietz N, Duda JE, Wolk DA, Doshi J, Xie SX, Siderowf A. Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain. 2012;135:170–180. doi: 10.1093/brain/awr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Doshi J, Koka D, Davatzikos C, Siderowf AD, Duda JE, Clark CM. Neurodegeneration across stages of cognitive decline in Parkinson disease. Archives of Neurology. 2011;68:1562–1568. doi: 10.1001/archneurol.2011.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Moberg ÃPJ, Duda ÃJE, Katz ÃIR, Stern MB. Effect of psychatric and other nonmotor symptoms on disability in Parkinson’s disease. Journal of the American Geriatrics Society. 2004;784–788 doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Barker RA. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson’s disease is dependent on COMT val 158 met genotype. Brain. 2008;131(Pt 2):397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson’s disease. Journal of Neuroscience. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]