Abstract
This prospective study was to assess the efficacy of nonbismuth containing quadruple therapy as first-line H. pylori treatment and to determine the clinical factors influencing patient outcome. We enrolled 200 H. pylori-infected naïve patients. They were prescribed either a 7-day nonbismuth containing quadruple therapy group (EACM, esomeprazole 40 mg twice daily, amoxicillin 1 g twice daily, metronidazole 500 mg twice daily, and clarithromycin 500 mg twice daily) or a 7-day standard triple therapy group (EAC, esomeprazole 40 mg twice daily, amoxicillin 1 g twice daily, and clarithromycin 500 mg twice daily). Follow-up studies to assess treatment responses were carried out 8 weeks later. The eradication rates attained by EACM and EAC groups were 95.6% (95% confidence interval [CI] = 89.4%–98.3%) and 79.3% (95% CI = 70%–86.4%) in the per-protocol analysis (P < 0.001) and 88% (95% CI = 80.2%–93.0%) and 73% (95% I = 63.6%–80.3%) in the intention-to-treat analysis (P = 0.007). Clarithromycin resistance, metronidazole resistance, and dual clarithromycin and metronidazole resistances were the clinical factors influencing H. pylori eradication in EACM group. Clarithromycin resistance and dual clarithromycin and metronidazole resistances were the influential factor for EAC treatment. In conclusion, the results suggest that 7-day nonbismuth containing quadruple therapy could achieve a grade “A” report card for first-line H. pylori treatment.
1. Introduction
The Maastricht IV/Florence-Consensus Report recommended that the standard triple therapy should now be avoided in areas where clarithromycin resistance is high (>15–20%) [1]. The reported local primary resistance rate to clarithromycin in Taiwan ranged from 6 to 18% over time [2–7]. However, Chen et al. recently reported the primary resistance rates of metronidazole (52.8% versus 47.7%) and clarithromycin (13.9% versus 22.7%) in patients who lived in urban and rural areas of eastern Taiwan [8]. The bismuth containing quadruple therapy with 10-day duration could be the alternative first-line treatment, especially in areas with a high prevalence of clarithromycin and metronidazole resistance, because of its ability to overcome metronidazole resistance and achieve an eradication rate > 90% [9–11]. Other alternatives include sequential therapy, concomitant therapy, and hybrid therapy, which provide > or close to 90% eradication rates even in areas with high rates of clarithromycin and metronidazole resistance. However, there were inconsistent reports for the eradication rates of sequential therapy. For instance, Wu et al. [2] and Tsay et al. [12] both reported >90% intention-to-treat (ITT) and per-protocol (PP) eradication rates in Taiwan. In Korea, the reported PP eradication rate of sequential therapy was 85.7% without at least grade “B” report card in 2008 [13]. This year, there were two publications from Korea and China which reported a decrease in 10-day sequential therapy which was only 72.1% by ITT and 78.4% by PP analysis for the Korean report [14] and 72.1% by ITT and 76.5% by PP analysis for the Chinese report [15]. The bottom line is that the inconsistent results may imply that the efficacy of sequential therapy varies in different countries and may have declined over time. It might not be a perfect option in the first-line H. pylori eradication anymore, particularly in areas where clarithromycin resistance is high (>15–20%).
One of the other alternatives includes hybrid therapy, which could provide >95% eradication rate even in areas with high rates of clarithromycin and metronidazole resistance [16]. Hsu et al. reported near 100% PP eradication rates for 14-day hybrid therapy [16]. It has two phases: dual therapy with a PPI (standard dose, b.i.d.) and amoxicillin (1 g, b.i.d.) for 7 days, followed by a nonbismuth quadruple therapy consisting of PPI (standard dose, b.i.d.), amoxicillin (1 g, b.i.d.), clarithromycin (500 mg, b.i.d.), and metronidazole (500 mg, b.i.d.) for further 7 days. The benefit of the extended duration of amoxicillin administration is to further reduce the bacterial load to improve the eradication rate but then again it involves very complex regimens [16]. However, recently published reports from Korea and Iran reported eradication rates of 85.9% and 92.9% [17, 18]. It may need more studies to prove its efficacies in different countries and taking into account the local antibiotics resistance to metronidazole and clarithromycin. Besides, the complexity and lengthy duration of the prescription may be the disadvantage.
Concomitant therapy consists of a PPI (standard dose, b.i.d.) combined with clarithromycin (500 mg, b.i.d.), amoxicillin (1 g, b.i.d.), and metronidazole (500 mg, b.i.d.), prescribed all together at the same time which is more convenient than sequential therapy and hybrid therapy because of the shorter duration of treatment and less complex drug administration. As a matter of fact, the concomitant therapy was more convenient and achieves equally efficient eradication rates and is with consistent results over years. Studies with 10-day concomitant therapy achieved an efficacy of > or close to 90% for H. pylori eradication rates [2, 19–24]. The current study explored the influential role of 7-day nonbismuth containing quadruple therapy for first-line H. pylori eradication to determine which clinical factors influence patient outcome.
2. Materials and Methods
2.1. Patients
From August 2012 to March 2014, a total of 200 H. pylori-infected naïve patients were enrolled. All patients were at least 18 years of age and had received endoscope examinations that showed peptic ulcers or gastritis. They were randomly prescribed either a 7-day nonbismuth containing quadruple therapy group (EACM, esomeprazole 40 mg twice daily, amoxicillin 1 g twice daily, metronidazole 500 mg twice daily, and clarithromycin 500 mg twice daily for 7 days) or a 7-day standard triple therapy group (EAC, esomeprazole 40 mg twice daily, amoxicillin 1 g twice daily, and clarithromycin 500 mg twice daily, for 7 days) by their clinicians.
Criteria for exclusion included the following: (1) previous surgery of the stomach, such as partial gastrectomy; (2) use of antibiotics within the preceding 30 days; (3) regular use of a PPI or bismuth compounds (>3 times per week) in the 30 days before enrollment; (4) presence of serious medical condition(s) precluding participation or endoscopy with biopsy; (5) patients previously treated for H. pylori infection; (6) use of concomitant medication(s) known to interact with study medication (simvastatin was permitted); (7) presence of Zollinger-Ellison syndrome; (8) pregnancy or lactation; (9) allergy to any medication in the study; (10) contraindication(s) to the use of any of the study drugs; (11) participating in any clinical trial within the last 30 days; (12) unwillingness to abstain from alcoholic beverages; and (13) patients taking other medications including antipsychotics, and chronic nonsteroidal anti-inflammatory drugs were also excluded.
This study was approved by both the Institutional Review Board and the Ethics Committee of Chang Gung Memorial Hospital (IRB-101-0674A3). All patients provided their written informed consent before undergoing endoscopic interventions.
2.2. Outcomes and Follow-Up
The primary endpoint was the successful eradication of H. pylori. We also analyzed antibiotic susceptibility. The confirmation of H. pylori eradication failure was defined as positive results both for the rapid urease test and by histology after first-line eradication therapy. All registered patients were followed up at week 2 to assess drug compliance and adverse effects after they finished the medication regimens. Drug compliance was assessed via pill counts. Poor compliance was defined as failure to finish 80% of all medication due to adverse effects [25–28]. These patients underwent either an endoscopy or a urea breath test 8 weeks later. We also performed a back-up urea breath test on all participants to avoid any false-negative results.
2.3. Questionnaire
The questionnaire contained questions regarding personal history of smoking and alcohol drinking. The questionnaire was locally derived and not a validated or previously published quality of life questionnaire. Quality of life was not assessed. Smokers were defined as those who consumed more than 1 pack of cigarettes a week, and drinkers were those who drank more than 1 cup of alcoholic beverage per day. The adverse events evaluated included abdominal pain, diarrhea, constipation, dizziness, taste perversion, headache, anorexia, nausea, vomiting, and skin rash. Those who considered those symptoms disturbed their daily life were defined to have major adverse effects. Those who experienced these symptoms but did not consider them a disturbance to their daily life were defined to have minor adverse effects.
2.4. Culture and Antimicrobial Resistance
One antral gastric specimen and one corpus biopsy specimen were obtained for H. pylori isolation using previously described culture methods [29]. The biopsy specimens were cultured on plates containing Brucella chocolate agar with 7% sheep blood and incubated for 4-5 days under microaerobic conditions. The minimal inhibitory concentration (MIC) was determined by the agar dilution test. H. pylori strains with MIC values ≥0.5, ≥1, ≥1, ≥4, and ≥8 mg/L were considered to be the resistant breakpoints for amoxicillin, clarithromycin, levofloxacin, tetracycline, and metronidazole, respectively [2, 3, 16, 25].
2.5. Randomization
A computer-generated randomization list was used to generate a “random sequence.” We used a method combining blocking and stratified randomization to ensure a close balance of the numbers and patients' characteristics in each group. We set separate randomization within each of two groups of participants. We then set a block of every 10 participants. A computer-generated randomization list was drawn up by the statistician and given to the physician for randomization. Doctors determined patients' suitability to be enrolled in this study and allocated the next available number on entry into the trial. The code was revealed to the researchers once recruitment, data collection, and laboratory analyses were complete. An independent research assistant generated the computerized random number sequence. The sequence was concealed in an opaque envelope until the intervention was assigned. After the written informed consents were obtained from the participants, an independent research assistant assigned the therapies according to the treatment allocations kept in the envelopes. Each patient collected the medications on the same day from the pharmacy department in our hospital.
2.6. Statistical Analysis
The primary outcome variables were the eradication rate, presence of adverse events, and level of patient compliance. Using the SPSS program (Statistical Package for the Social Sciences version 18, Chicago, IL, USA), Chi-square tests with or without Yates' correction for continuity and Fisher's exact tests were used when appropriate to compare the major outcomes between groups. Eradication rates were analyzed by both ITT and per-protocol (PP) approaches. ITT analysis included all assigned patients who had taken at least one dose of the study medication. Patients whose infection status was unknown following treatment were considered treatment failures for the purposes of the ITT analysis. The PP analysis excluded patients with unknown H. pylori status following therapy and those with major protocol violations. A P value <0.05 was considered statistically significant. To determine the independent factors that affected treatment response, the clinical and bacterial parameters were analyzed by univariate and multivariate analyses.
3. Results
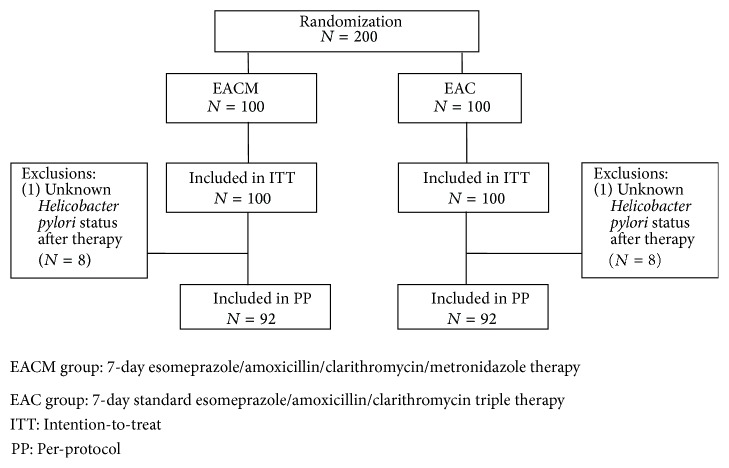
Figure 1 shows patient flowchart, according to the CONSORT statement advice. A total of 200 patients with positive H. pylori were recruited into the study and were randomly assigned to receive EACM and EAC therapy. The two treatment groups were matched with respect to baseline demographic data, clinical characteristics, and antibiotic resistance (Table 1). A total of 16 patients were excluded from the PP analysis (8 in each group), because of being lost to follow-up, resulting in 92 in the PP study for each of EACM and EAC groups. The eradication rates in the EAC and EACM groups are detailed in Table 2. They were 95.6% (95% confidence interval [CI] = 89.4–98.3%) and 79.3% (95% CI = 70%–86.7%), respectively, in the PP analysis (P < 0.001); 88% (95% CI = 80.2%–93%) and 73% (95% CI = 63.6%–80.3%), respectively, in the ITT analysis.
Figure 1.
Disposition of patients.
Table 1.
Demographic data and endoscopic appearances of the two patient groups.
| Characteristics | EACM (n = 92) | EAC (n = 92) | P value |
|---|---|---|---|
| Age (year) (mean ± SD) | 47.8 ± 11.6 | 52.8 ± 12.8 | 0.593 |
| Gender (male/female) | 45/47 | 46/46 | 0.883 |
| Smoking | 14 | 14 | 1.000 |
| Alcohol consumption | 20 | 14 | 0.254 |
| Previous history of peptic ulcer | 14 | 19 | 0.337 |
| Endoscopic findings | 0.964 | ||
| Gastritis | 32 | 34 | |
| Gastric ulcer | 22 | 22 | |
| Duodenal ulcer | 31 | 28 | |
| Gastric and duodenal ulcer | 7 | 8 |
EACM group: 7-day esomeprazole/amoxicillin/clarithromycin/metronidazole therapy; EAC group: 7-day standard esomeprazole/amoxicillin/clarithromycin/triple therapy.
Table 2.
Major outcomes of eradication therapy.
| Eradication rate | |||
|---|---|---|---|
| EACM (n = 92) | EAC (n = 92) | P value | |
| Intention-to-treat | 88% (88/100) | 73% (73/100) | 0.007 |
| Per-protocol | 95.6% (88/92) | 79.3% (73/92) | <0.001 |
| Adverse event | 30.4% (28/92) | 16.3% (15/92) | 0.024 |
| Compliance | 100% (92/92) | 100% (92/92) | 1.000 |
EACM group: 7-day esomeprazole/amoxicillin/clarithromycin/metronidazole therapy; EAC group: 7-day standard esomeprazole/amoxicillin/clarithromycin/triple therapy.
3.1. Adverse Events and Complications
The adverse event rates were 30.4% (28/92) in EACM group and 16.3% (15/92) in EAC group, P = 0.024 (Table 3). These adverse events included abdominal pain, constipation, diarrhea, dizziness, headache, and nausea/vomiting; there were more patients in the EACM group experiencing nausea sensation than the EAC group (30.4% versus 16.3%, P = 0.024). However, these were mild and did not markedly disturb the patients' daily activities and importantly both groups had good drug compliances (100% in all of them).
Table 3.
Adverse events during eradication therapies.
| Adverse event | EACM (n = 92) | EAC (n = 92) | P value |
|---|---|---|---|
| Abdominal pain | 9 (9.8%) | 6 (6.5%) | 0.419 |
| Constipation | 1 (1.1%) | 2 (2.2%) | 0.560 |
| Diarrhea | 11 (11.9%) | 6 (6.5%) | 0.203 |
| Dizziness | 7 (7.6%) | 4 (4.3%) | 0.315 |
| Headache | 6 (6.5%) | 2 (2.2%) | 0.148 |
| Nausea/vomiting | 10 (10.8%) | 2 (2.2%) | 0.017 |
| Skin rash | 0 (0%) | 0 (0%) | — |
| Taste perversion | 0 (0%) | 0 (0%) | — |
EACM group: 7-day esomeprazole/amoxicillin/clarithromycin/metronidazole therapy; EAC group: 7-day standard esomeprazole/amoxicillin/clarithromycin/triple therapy.
3.2. Antibiotic Resistance
Samples from 90 patients were cultured for H. pylori, and the positive culture rate was 75.6% (68/90). The results of the H. pylori eradication rate for different subgroups susceptible to amoxicillin, clarythromycin, and metronidazole for both EAC and EACM patients are foundin Table 4.
Table 4.
Univariate analysis of the clinical factors influencing the efficacy of H. pylori eradication.
| Principle parameter | EACM | EAC | |||||
|---|---|---|---|---|---|---|---|
| Case number | Eradication rate (%) | P value | Case number | Eradication rate (%) | P value | ||
| Age | <60 years | 75/78 | 96.1 | 0.578 | 52/64 | 81.2 | 0.496 |
| ≥60 years | 13/14 | 92.8 | 21/28 | 75.0 | |||
| Sex | Female | 44/47 | 93.6 | 0.328 | 33/46 | 71.1 | 0.071 |
| Male | 44/45 | 97.8 | 40/46 | 86.9 | |||
| Smoking | (−) | 74/78 | 94.9 | 0.386 | 61/78 | 78.2 | 0.523 |
| (+) | 14/14 | 100.0 | 12/14 | 85.7 | |||
| Alcohol consumption | (−) | 69/72 | 95.8 | 0.872 | 61/78 | 78.2 | 0.523 |
| (+) | 19/20 | 95.0 | 12/14 | 85.7 | |||
| Previous history of peptic ulcer | (−) | 74/78 | 94.9 | 0.386 | 57/73 | 78.1 | 0.557 |
| (+) | 14/14 | 100.0 | 16/19 | 84.2 | |||
| Compliance | Good | 92/92 | 100.0 | — | 92/92 | 100.0 | — |
| Poor | 0 | 0 | 0 | 0 | |||
|
| |||||||
| H. pylori culture (n = 68) | |||||||
| Amoxicillin | Susceptible | 31/34 | 91.2 | — | 15/33 | 45.4 | 0.367 |
| Resistance | 0 | — | 0/1 | 0 | |||
| Clarithromycin | Susceptible | 29/30 | 96.7 | 0.002 | 15/15 | 100 | <0.001 |
| Resistance | 2/4 | 50.0 | 0/19 | 0 | |||
| Metronidazole | Susceptible | 23/23 | 100 | 0.009 | 11/24 | 45.8 | 0.755 |
| Resistance | 8/11 | 72.7 | 4/10 | 40.0 | |||
| Dual resistance | Absent | 30/31 | 96.8 | <0.001 | 15/28 | 53.6 | <0.001 |
| Present | 1/3 | 33.3 | 0/6 | 0 | |||
EACM group: 7-day esomeprazole/amoxicillin/clarithromycin/metronidazole therapy; EAC group: 7-day standard esomeprazole/amoxicillin/clarithromycin triple therapy.
3.3. Factors Influencing the Efficacy of Anti-H. pylori Therapies
Univariate analysis for factors that influence the efficacy was shown in Table 4. Clarithromycin resistances (CLA-R) were significant factors in both the EACM group (P = 0.002) and the EAC group (P < 0.001). Metronidazole resistance (MET-R) is the risk factors for eradication failure in the EACM group (P = 0.009) but not the EAC group. However, dual resistances to both antibiotics were the risk factors for eradication failure in both EACM and EAC groups (P < 0.001).
4. Discussion
Our data confirm that the previously reported efficacy of using concomitant therapy achieves an efficacy of > or close to 90% for H. pylori eradication rates but we achieved a grade “A” PP result (95.6%) with 7-day regimen instead of 10-day one [2, 21–24]. These findings merit the recommendations of concomitant therapy as the optimal formula in addition to the advantage of being far more easy and convenient for patients to follow than the sequential and hybrid therapies.
Possible explanations for the discrepancies in the reports for both sequential and hybrid therapies as first-line H. pylori eradication regimens are different antibiotic resistances of H. pylori strains and different treatment durations. Besides the regional variations in eradication efficacies, sequential and hybrid therapy are much more complex in terms of medication requirements because the patients need to switch drugs during the treatment course. This might result in reducing patient compliance and physician preference to prescribe the regimen [16–18, 30]. Therefore, concomitant therapy might be a relatively optimal treatment option in terms of convenience and consistent efficacies among the three. Nevertheless, the inevitable problematic issue is that CLA-R and MET-R could be crucial in the efficacy of H. pylori eradication [31].
Currently, the decrease in the first-line H. pylori eradication rates in standard 7-day triple therapy is relevant to the progressively increasing CLA-R resistance rates in many parts of the world, particularly those areas with the CLA-R above 20% [30]. The current study showed eradication rates of 73% in ITT and 79.3% in the PP analysis in EAC group. Univariate and multivariate analyses of our data identified CLA-R as a factor that reduced the efficacy of concomitant therapy. None of our 19 patients with CLA-R assigned to EAC were eradicated and the same occurred to the other six patients with dual resistance (CLA-R and MET-R) as shown in Table 4. This was the same for EACM group. Two out of four CLA-R patients in the EACM group were eradicated (50%) while the eradication rates dropped to 33.3% when dual resistance was present. These findings implied that dual resistance could be a major factor affecting the outcome of both EACM and EAC patients. The bottom line is that the prevalence of dual resistance in our study was only 13.2% among the PP population (9/68) and the low number of patients makes the possibility of a type II error likely. For instance, the majority of dual resistances that had treatment failure in EAC arm were due to clarithromycin resistance as demonstrated by the fact that none of the 19 clarithromycin resistance strains were successfully eradicated while 4/10 of metronidazole resistance strains were still successfully eradicated under the EAC arm. Therefore, we should be careful during interpretation of these data. It would be overestimated by drawing conclusion that dual clarithromycin and metronidazole resistance influences the treatment outcome of EAC arm. The effect could be likely due to clarithromycin resistance alone.
When bismuth quadruple therapy is not available, nonbismuth containing quadruple therapy (concomitant therapy) is recommended in high prevalence of CLA-R areas by the Maastricht IV Consensus Report [1]. As mentioned earlier in this text, the reported local primary resistance rate to clarithromycin in Taiwan is increasing over time [2–8]. It was therefore suggested that standard seven-day triple therapy is not suitable for patients in Eastern Taiwan because the H. pylori eradication rates were only 57.5% by ITT analysis and 61.8% by PP analysis. This is the same in current study as we attained only an eradication rate of 79.3% by PP analysis and 73% by ITT analysis. This is the same in current study as we attained only an eradication rate of 79.3% by PP analysis and 73% by ITT analysis. Both these Taiwanese studies, including ours, suggested that the clinical practice for first-line eradication with standard triple therapy should be abandoned. However, for patients who encountered dual resistances, although 7-day EACM eradication rates are higher than EAC group (33.3% versus 0%, P = 0.134), it implies that in areas with high prevalence of CLA-R even concomitant therapy may not be suitable to eradicate patients with CLA-R and MET-R resistances. The impact of MET-R on the outcome of concomitant therapy is relatively less marked than CLA-R. Data on H. pylori eradication with concomitant therapy in isolated MET-R populations from previously published studies indicate that eradication rates are >85% [2, 23], which is similar to our own findings (72.7% in the EACM group versus 40% in the EAC group).
This study encounters several limitations. Firstly, the sample size is clearly insufficient for a treatment comparison. An adequate sample for reliably detecting clinically significant differences between the two arms would be much larger than the number of patients included in the study. Therefore this study should be considered to be a pilot study that reports preliminary results on the two first-line therapies rather than being treated as a comparative trial. Secondly, the small populations with antibiotic resistance data included in this study have impeded the evaluation of the effects of antibiotic resistance on eradication efficacy. Large sample sized, prospective randomized studies are mandatory to precisely interpret the association of antibiotic resistance to the efficacy of concomitant therapy in Taiwan. Up to now, standard triple therapy was still recommended by the Taiwanese National Health Insurance Administration as the first-line empiric regimen but the overall eradication rates have dropped to <80% as shown in current study. We believe that our results will help facilitate the selection of appropriate patients for concomitant therapy in Taiwan because the more significant rise in clarithromycin resistance is unavoidable for Taiwanese in the near future. It is therefore likely that eradication rates will continue to drop.
5. Conclusion
In conclusion, the results suggest that a 7-day nonbismuth containing quadruple therapy could achieve a grade “A” report card (>95% eradication rate) for first-line H. pylori treatment.
Acknowledgments
This work was funded by grants from the Research Foundation of Chang Gung Memorial Hospital (CMRPG8B0421), Taiwan. The authors would like to acknowledge Miss Ching-Yi Lin for her assistance in this study.
Disclosure
Wei-Chen Tai and Chih-Ming Liang are co-first authors.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Malfertheiner P., Megraud F., O'Morain C. A., et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 2.Wu D. C., Hsu P. I., Wu J. Y., et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H. pylori infection. Clinical Gastroenterology and Hepatology. 2010;8(1):36–41. doi: 10.1016/j.cgh.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou J.-M., Chen C.-C., Chen M.-J., et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. The Lancet. 2013;381(9862):205–213. doi: 10.1016/s0140-6736(12)61579-7. [DOI] [PubMed] [Google Scholar]
- 4.Poon S. K., Chang C. S., Su J., et al. Primary resistance to antibiotics and its clinical impact on the efficacy of Helicobacter pylori lansoprazole-based triple therapies. Alimentary Pharmacology and Therapeutics. 2002;16(2):291–296. doi: 10.1046/j.1365-2036.2002.01184.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang W. L., Sheu B. S., Cheng H. C., Yang Y. J., Yang H. B., Wu J. J. Resistance to metronidazole, clarithromycin and levofloxacin of Helicobacter pylori before and after clarithromycin-based therapy in Taiwan. Journal of Gastroenterology and Hepatology. 2009;24(7):1230–1235. doi: 10.1111/j.1440-1746.2009.05829.x. [DOI] [PubMed] [Google Scholar]
- 6.Hu C. T., Wu C. C., Lin C. Y., et al. Resistance rate to antibiotics of Helicobacter pylori isolates in eastern Taiwan. Journal of Gastroenterology and Hepatology. 2007;22:720–723. doi: 10.1111/j.1440-1746.2006.04743.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y. J., Yang J. C., Jeng Y. M., Chang M. H., Ni Y. H. Prevalence and rapid identification of clarithromycin-resistant Helicobacter pylori isolates in children. Pediatric Infectious Disease Journal. 2001;20(7):662–666. doi: 10.1097/00006454-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Chen M.-C., Lei W.-Y., Lin J.-S., et al. Levofloxacin-amoxicillin/clavulanate-rabeprazole versus a standard seven-day triple therapy for eradication of Helicobacter pylori infection. BioMed Research International. 2014;2014:7. doi: 10.1155/2014/158520.158520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo C. H., Kuo F. C., Hu H. M., et al. The optimal first-line therapy of Helicobacter pylori infection in year 2012. Gastroenterology Research and Practice. 2012;2012:8. doi: 10.1155/2012/168361.168361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Morain C., Borody T., Farley A., et al. Efficacy and safety of single-triple capsules of bismuth biskalcitrate, metronidazole and tetracycline, given with omeprazole, for the eradication of Helicobacter pylori: an international multicentre study. Alimentary Pharmacology and Therapeutics. 2003;17(3):415–420. doi: 10.1046/j.1365-2036.2003.01434.x. [DOI] [PubMed] [Google Scholar]
- 11.Laine L., Hunt R., EI-Zimaity H., Nguyen B., Osato M., Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. The American Journal of Gastroenterology. 2003;98(3):562–567. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsay F.-W., Tseng H. H., Hsu P.-I., et al. Sequential therapy achieves a higher eradication rate than standard triple therapy in Taiwan. Journal of Gastroenterology and Hepatology. 2012;27(3):498–503. doi: 10.1111/j.1440-1746.2011.06885.x. [DOI] [PubMed] [Google Scholar]
- 13.Choi W. H., Park D. I., Oh S. J., et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. The Korean Journal of Gastroenterology. 2008;51(5):280–284. [PubMed] [Google Scholar]
- 14.Lee J. W., Kim N., Kim J. M., et al. A comparison between 15-day sequential, 10-day sequential and proton pump inhibitor-based triple therapy for Helicobacter pylori infection in Korea. Scandinavian Journal of Gastroenterology. 2014;49(8):917–924. doi: 10.3109/00365521.2014.896409. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L., Zhang J., Chen M., et al. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: a randomized multicenter trial. The American Journal of Gastroenterology. 2014;109(4):535–541. doi: 10.1038/ajg.2014.26. [DOI] [PubMed] [Google Scholar]
- 16.Hsu P. I., Wu D. C., Wu J. Y., Graham D. Y. Modified sequential Helicobacter pylori herapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16(2):139–145. doi: 10.1111/j.1523-5378.2011.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh D. H., Lee D. H., Kang K. K., et al. The efficacy of hybrid therapy as first-line regimen for Helicobacter pylori infection compared with sequential therapy. Journal of Gastroenterology and Hepatology. 2014;29(6):1171–1176. doi: 10.1111/jgh.12518. [DOI] [PubMed] [Google Scholar]
- 18.Sardarian H., Fakheri H., Hosseini V., Taghvaei T., Maleki I., Mokhtare M. Comparison of hybrid and sequential therapies for helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013;18(2):129–134. doi: 10.1111/hel.12017. [DOI] [PubMed] [Google Scholar]
- 19.McNicholl A. G., Marin A. C., Molina-Infante J., et al. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut. 2014;63(2):244–249. doi: 10.1136/gutjnl-2013-304820. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y.-K., Wu M.-C., Wang S. S., et al. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. Journal of Digestive Diseases. 2012;13(4):232–238. doi: 10.1111/j.1751-2980.2012.00575.x. [DOI] [PubMed] [Google Scholar]
- 21.Molina-Infante J., Pazos-Pacheco C., Vinagre-Rodriguez G., et al. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resist ant strains. Helicobacter. 2012;17(4):269–276. doi: 10.1111/j.1523-5378.2012.00947.x. [DOI] [PubMed] [Google Scholar]
- 22.Georgopoulos S. D., Xirouchakis E., Martinez-Gonzalez B., et al. Clinical evaluation of a ten-day regimen with esomeprazole, metronidazole, amoxicillin, and clarithromycin for the eradication of Helicobacter pylori in a high clarithromycin resistance area. Helicobacter. 2013;18(6):459–467. doi: 10.1111/hel.12062. [DOI] [PubMed] [Google Scholar]
- 23.Georgopoulos S., Papastergiou V., Xirouchakis E., et al. Nonbismuth quadruple ‘concomitant’ therapy versus standard triple therapy, both of the duration of 10 days, for first-line H. Pylori eradication: a randomized trial. Journal of Clinical Gastroenterology. 2013;47(3):228–232. doi: 10.1097/mcg.0b013e31826015b0. [DOI] [PubMed] [Google Scholar]
- 24.Heo J., Jeon S. W., Jung J. T., et al. A randomised clinical trial of 10-day concomitant therapy and standard triple therapy for Helicobacter pylori eradication. Digestive and Liver Disease. 2014;46(11):980–984. doi: 10.1016/j.dld.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Chuah S. K., Hsu P. I., Chang K. C., et al. Randomized comparison of two non-bismuth-containing second-line rescue therapies for Helicobacter pylori . Helicobacter. 2012;17(3):216–223. doi: 10.1111/j.1523-5378.2012.00937.x. [DOI] [PubMed] [Google Scholar]
- 26.Tai W. C., Lee C. H., Chiou S. S., et al. The clinical and bacteriological factors for optimal levofloxacin-containing triple therapy in second-line Helicobacter pylori eradication. PLoS ONE. 2014;9(8) doi: 10.1371/journal.pone.0105822.e105822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang C.-M., Cheng J.-W., Kuo C.-M., et al. Levofloxacin-containing second-line anti-Helicobacter pylori eradication in Taiwanese real-world practice. Biomedical Journal. 2014;37(5):326–330. doi: 10.4103/2319-4170.125650. [DOI] [PubMed] [Google Scholar]
- 28.Chuah S. K., Tai W. C., Hsu P. I., et al. The efficacy of second-line anti-Helicobacter pylori therapy using an extended 14-day levofloxacin/amoxicillin/proton-pump inhibitor treatment—a Pilot Study. Helicobacter. 2012;17(5):374–381. doi: 10.1111/j.1523-5378.2012.00960.x. [DOI] [PubMed] [Google Scholar]
- 29.Hsu P. I., Hwang I. R., Cittelly D., et al. Clinical presentation in relation to diversity within the Helicobacter pylori cag pathogenicity island. The American Journal of Gastroenterology. 2002;97(9):2231–2238. doi: 10.1016/s0002-9270(02)04334-4. [DOI] [PubMed] [Google Scholar]
- 30.Hsu P. I., Wu D. C., Chen W. C., et al. Comparison of 7-day triple, 10-day sequential and 7-day concomitant therapies for Helicobacter pylori infection—a randomized controlled trial. Antimicrobial Agents and Chemotherapy. 2014;58(10):5936–5942. doi: 10.1128/aac.02922-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papastergiou V., Georgopoulos S. D., Karatapanis S. Treatment of Helicobacter pylori infection: meeting the challenge of antimicrobial resistance. World Journal of Gastroenterology. 2014;20(29):9898–9911. doi: 10.3748/wjg.v20.i29.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]