Abstract
Background
The Chinese sturgeon (Acipenser sinensis) is endangered through anthropogenic activities including over-fishing, damming, shipping, and pollution. Controlled reproduction has been adopted and successfully conducted for conservation. However, little information is available on the reproductive regulation of the species. In this study, we conducted de novo transcriptome assembly of the gonad tissue to create a comprehensive dataset for A. sinensis.
Results
The Illumina sequencing platform was adopted to obtain 47,333,701 and 47,229,705 high quality reads from testis and ovary cDNA libraries generated from three-year-old A. sinensis. We identified 86,027 unigenes of which 30,268 were annotated in the NCBI non-redundant protein database and 28,281 were annotated in the Swiss-prot database. Among the annotated unigenes, 26,152 and 7,734 unigenes, respectively, were assigned to gene ontology categories and clusters of orthologous groups. In addition, 12,557 unigenes were mapped to 231 pathways in the Kyoto Encyclopedia of Genes and Genomes Pathway database. A total of 1,896 unigenes, potentially differentially expressed between the two gonad types, were found, with 1,894 predicted to be up-regulated in ovary and only two in testis. Fifty-five potential gametogenesis-related genes were screened in the transcriptome and 34 genes with significant matches were found. Besides, more paralogs of 11 genes in three gene families (sox, apolipoprotein and cyclin) were found in A. sinensis compared to their orthologs in the diploid Danio rerio. In addition, 12,151 putative simple sequence repeats (SSRs) were detected.
Conclusions
This study provides the first de novo transcriptome analysis currently available for A. sinensis. The transcriptomic data represents the fundamental resource for future research on the mechanism of early gametogenesis in sturgeons. The SSRs identified in this work will be valuable for assessment of genetic diversity of wild fish and genealogy management of cultured fish.
Introduction
Sturgeons are one of the most primitive vertebrates with over 200 million years of history, leading to a vital evolutionary position. Among the different sturgeon species, different degrees of ploidy caused by genome duplication events were characterized [1, 2], which is another interesting field worth for exploration on sturgeons. Besides, as the source of caviar from the roe, the sturgeon is of high economic value [3], which becomes the primary factor leading to their exceedingly endangered status [4]. According to International Union for the Conservation of Nature (IUCN 2010) data, the sturgeon has been identified as one of the most endangered group of species, with 85% being on the edge of extinction.
The Chinese sturgeon (Acipenser sinensis), is an anadromous fish distributed in Yangtze River and East China Sea [5, 6]. It is currently endangered due to habitat degradation caused by anthropogenic activities such as damming, shipping, pollution and over-fishing [3, 7]. This is compounded by the difficulty in recovery of their numbers, considering the late sexual maturity (8–18 years for males and 14–26 years for females) as well as the reproduction interval of 2–7 years. Artificial propagation has been tried to conduct on the Chinese sturgeon since the later 1980s and has shown potential for species conservation. In 2012, gonadal maturation was reached in artificially bred Chinese sturgeons, from which 23,000 larvae were hatched by in vitro fertilization [8]. However, during rearing it is impossible to distinguish males from females morphologically. In addition the gonads of artificially bred Chinese sturgeon are smaller than those of the wild [8]. These factors constitute obstacles not only to the conservation of germplasm resources but also to the efficacy of artificial propagation. To resolve these issues, it is necessary to study the mechanisms of reproduction regulation and investigate genes involved in early gametogenesis.
Gametogenesis includes spermatogenesis and oogenesis. Spermatogenesis is composed of three phases: mitosis, meiosis and spermiogenesis. Spermatogenesis begins with the proliferation of spermatogonia which develop into primary spermatocytes [9]. After two meiotic divisions, the primary spermatocytes produce four spermatids, which do not divide further but undergo the spermiogenic process to transform into elongated spermatids [10]. The differentiation from spermatogonia to primary spermatocytes with the onset of meiosis is one of the most important steps in spermatogenesis, which involves the sex steroid hormones stimulation and the endocrine regulation [11, 12]. Despite this, many studies on spermatogenesis were primarily focused on the final maturation of male gametes [13, 14].
Oogenesis commences with the formation of primordial germ-cells (PGCs), which then transform into oogonia and develop further into primary oocytes [15]. The following oocyte development has been classified as the primary oocyte stage, cortical-alveolar stage, vitellogenic stage with the onset of meiosis, and the final maturation stage [16]. Oocyte growth during the early period of oogenesis is responsible for cytoplasmic organelles replication and redistribution, RNA synthesis, and nutrient incorporation. However, research on oogenesis was concentrated mainly on the late stages of vitellogenesis, final maturation and ovulation [17, 18], while stages of primary oocyte growth was mainly unexplored. In teleost fish, the early period of primary oocyte growth may occupy a long period of the lifespan in some species like sturgeons. For example, in female Chinese sturgeons of 5-year-old, the oocytes were still in the primary oocyte growth stage [19], and might last well over a decade at this stage during artificial breeding.
Transcriptome sequencing has been proven to be an effective means of gene discovery [4, 20], especially with the availability of high-throughput next generation sequencing technologies. Transcriptome sequencing is also emerging to be a rapid and efficient method of genetic marker development, including simple sequence repeats (SSRs) or microsatellites, which are useful for numerous studies like linkage mapping and genetic diversity research [21]. This study used Illumina sequencing to provide the first characterization of the Chinese sturgeon transcriptome. We performed transcriptome sequencing on the gonads of three-year-old Chinese sturgeon. The transcriptome dataset should be beneficial for differential gene expression between gonads during early gametogenesis. SSRs detected from the transcriptome data should also be helpful for conservation of this endangered species.
Material and Methods
Ethics statement
All fish handling and experimental procedures were approved by the Animal Care and Use Committee of the Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences.
Experimental Fish and Sample Collection
One male and one female Chinese sturgeon, both 3-year-old immature individuals, were sampled at Taihu Station, Yangtze River Fisheries Research Institute, Chinese Academy of Fisheries Science. After anaesthetization by 0.05% MS-222 (Sigma, USA), testis and ovary tissues were collected and immediately immersed in the RNAlater (Ambion, Austin, TX, USA). Samples were stored at 4°C for 16 h, and then transferred to an ultralow freezer at -80°C until preparation of RNA. Small pieces of gonad were fixed in Bouin’s solution and embedded in paraffin. They were cut at 8 μm and stained with hematoxylin and eosin (HE).
RNA Isolation, Library Construction and Illumina Sequencing
Isolation of total RNAs was carried out by RNeasy Plus Mini Kit (Qiagen, Dusseldorf, Germany) according to the manufacturer’s instructions. The RNA integrity and concentration was determined by Nanodrop-2000 spectrophotometer (Thermo, USA) and Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). Genomic DNA was removed by RNase-free DNase I (Qiagen) for 30 min at 37°C. The mRNA-seq library was constructed with the mRNA-seq Sample Preparation Kit (Illumina, San Diego, CA) as described [22]. Briefly, enriched mRNA was broken into short fragments, which were used for the first-strand cDNA synthesis, followed by the second-strand cDNA synthesis. The double-strand cDNA was then end-paired, tailed and ligated with the PE Adapter Oligo Mix (Illumina). Final cDNA libraries were obtained by PCR amplification and purification, and were paired-end sequenced with the Illumina HiSeq 2000 (Biomarker Technologies Co., Ltd, Beijing, China).
De novo assembly of sequencing reads
Raw reads (2*101 bp paired-end sequencing) of the two transcriptome datasets (testis and ovary) were cleaned by filtering out adaptor-only and low quality reads. The adaptor-only reads (nt length of recognized adaptor ≤13 and the remaining adaptor-excluded nt length ≤35) were filtered out by the software WipeAadpter.pl (Biomarker Technologies Co., Ltd, Beijing, China). Reads with more than 50% of bases having a Q-value ≤20 were filtered out by the software Fastq_filter (Biomarker Technologies Co., Ltd, Beijing, China). Clean reads were then assembled with the short reads assembling program Trinity, version Trinityrnaseq_r2012-06-08 [23], with the parameters set at a similarity of 90%. Trinity contains three software modules: Inchworm, Chrysalis and Butterfly. In the process of Inchworm, overlapping k-mers (in this study, k = 25), which forms a k-mer dictionary, are generated from decomposition of each sequence read. Then the likely error-containing k-mers were removed from the library, and the k-mer most frequently appeared in the dictionary will work as the seed of the Inchworm contig. For each of the k-mer seeds, another k-mer, whose sequence could match the seed by k-1 times in either end and occurs most frequently in the dictionary, is anchored to extend the seed on the terminal base. If the neighbor k-mer is detected in neither direction, the growing of sequence terminates to produce an Inchworm contig. In the second phase of trinity, Chrysalis, the abundant contigs produced by the repeated Inchworm processes are built into de Bruijn graphs through k-1 overlaps. In the last step, Butterfly, the fragmented de Bruijn graphs are trimmed, compacted and reconciled to final linear transcripts [23]. Redundant sequences were eliminated, and the longest transcripts were recognized as unigenes, which were grouped together to conduct the final assembly for the following annotation.
Transcriptome annotation and ontology
Open reading frames (ORFs) of transcript and unigene sequences were predicted by the TransDecoder package (http://transdecoder.sourceforge.net/), with the minimum ORF length of 100 bp. Unigene sequences were assigned to the NCBI Nt database (downloaded in August, 2013) (BLASTn), the NCBI non-redundant (Nr) protein databases (downloaded in August, 2013), the Swiss-Prot protein database and the Translated EMBL Nucleotide Sequence Database (TrEMBL) (downloaded in August, 2013) (BLASTx) with an E-value of 1e-5. Each unigene sequence was allocated a gene name by BLAST hit with the highest score. The ‘‘getorf” program of EMBOSS software package (version 1.20.0) [24] was employed to predict the Open reading frames (ORFs), and the longest ORF was selected for each unigene by defaulting parameters.
Homology searches were performed by comparison against the NCBI Nr protein database with the Blastx algorithm (E-value cutoff of 1e-5) [23]. With Nr annotation, the Blast2GO program [24] was employed to produce Gene Ontology (GO) (database downloaded in March 2013) annotations. Functions of the sequences were also predicted by query of the Clusters of Orthologous Groups (COG) database (http://www.ncbi.nlm.nih.gov/COG/, database downloaded in April 2013) (BLASTx, E-value cutoff of 1e-5). The assembled sequences were assigned to the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (database downloaded in October 2011) using the online KEGG Automatic Annotation Server (KAAS) (http://www.genome.jp/kegg/kaas/) (BLASTx, E-value cutoff of 1e-5). KEGG Orthology (KO) assignment was obtained with the bi-directional best hit (BBH) method [25]. KO assignments and KEGG pathways that are fulfilled with the KO assignments were combined to constitute the final output of KEGG analysis.
Digital gene expression analysis
Numbers of reads in the RNA-Seq analyses were normalized with reads per kilo base of transcripts per million (RPKM) to compute the gene expression levels [26]. Detection of differentially expressed genes were performed by EBseq software (version 1.1.7) [27] in pair-wise comparison. The Benjamini–Hochberg false discovery rate (FDR < 0.01) was adopted for multiple testing correction of the result. Genes were defined as differentially expressed by parameters of FDR <0.001 and the absolute value of the log2 ratio >1 (The RPKM values of the gene in one sample was at least two fold that in another sample).
Microsatellite markers
Unigenes of length >1,000 bp were submitted to the software MISA (MIcroSAtellite; http://pgrc.ipk-gatersleben.de/misa, version 1.0) for SSRs detection. Six types of SSRs were examined: mono-, di-, tri-, tetra-, penta- and hexanucleotide repeats, and the compound SSR [the sequence containing more than one type of repeat units, e.g., (GT)n(AT)m]. Standards of the repeat unit numbers were set as follows: mono-10, dimer-6, trimer-5, tetramer-5, pentamer-5, and hexamer-5.
Relative real-time PCR validation
To evaluate the validity of Illumina analysis and assess the expression profiles with respect to specific mRNA abundances, 10 putative genes were chosen and detected by qRT-PCR. The cDNA templates used were reverse-transcribed with PrimeScript RT reagent Kit (Takara, Japan) as instructed. Relative real-time PCR was performed in a volume of 20 μL with a dsDNA-binding dye, SYBR green real-time PCR master mix (BioRad) on a DNA Engine Chromo 4 real-time system (BioRad) as described [28]. The primers used are listed in S6 Table. Each sample was analyzed in triplicates. The relative expression level of target genes was measured with the 2−ΔΔCT method and normalized by the median expression of β-actin. The data was presented as mean ± SD. The difference was considered statistically significant at P-value < 0.05 assessed by the Student's t-test.
Results
Sequence analysis and assembly
To obtain gonad transcriptome information of the Chinese sturgeon, cDNA libraries were constructed from the testis of an immature male sturgeon and the ovary of an immature female sturgeon, and sequencing runs were performed on the Illumina HiSeq 2000. The testis was composed mainly of the spermatogonia and primary spermatocytes, and the ovary was full of pre-vitellogenic oocytes (S1 Fig). The analysis pipeline began with preprocessing and ended with functional annotation and genetic markers (Fig 1). After stringent quality assessment and data filtering, 47,333,701 and 47,229,705 high quality reads from the testis and ovary, respectively, were selected for further analysis. The GC percentages were 51.73% (with 100% Q20 bases) from testis and 44.89% (with 100% Q20 bases) from ovary (Table 1). All sequence reads generated have been submitted to the NCBI Genbank under accession number SRP035284.
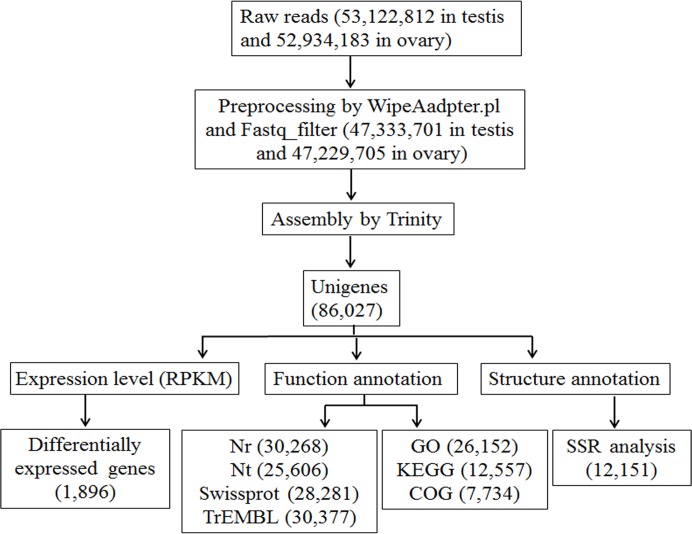
Fig 1. Transcriptome assembly and analysis pipeline.
Clean reads were obtained by pre-processing the raw reads with WipeAadpter.pl software and Fastq_filter software. A total of 86,027 unigenes were acquired by Trinity assembly. The expression levels of the unigenes were normalized with RPKM, through which 1,896 genes differentially expressed between ovary and testis were identified. The unigenes were used for functional annotation and SSR detection.
Table 1. Assembly statistics of the gonad transcriptome using the Trinity software package.
| Total number of clean reads in testis library | 47,333,701 |
| Total number of clean reads in ovary library | 47,229,705 |
| GC percentage in testis library (%) | 51.73 |
| GC percentage in ovary library (%) | 44.89 |
| Total number of transcripts a | 176,434 |
| Mean length of transcripts (bp) | 950 |
| N50 length of transcripts (bp) | 1,621 |
| Total number of unigenes b | 86,027 |
| Mean length of unigenes (bp) | 706 |
| N50 length of unigenes (bp) | 1,221 |
a Sequences constructed from Inchworm contigs by Chrysalis module and Butterfly module.
b The longest transcripts in the cluster units.
The Trinity program was used for the assembly of the RNA-Seq short reads, and a total of 12,802,085 contigs were assembled with the N50 length of 47 bp and the mean length of 45.25 bp (Table 1). A total of 176,434 transcripts were gained with the N50 length of 1,621 bp and an avarage length of 950 bp. The N50 length and mean length of the 86,027 unigenes produced was 1,221 bp and 706 bp, respectively (Table 1). Additionally, the transcript and unigene sequences were assigned with an ORF predictor TransDecoder, from which 42,227 (23.96%) transcripts (S1 Table) and 13,187 (15.33%) unigenes (S2 Table) were determined to contain complete ORFs.
Sequence annotation
BlastX was used to search various protein databases, and the Nr, Nt, Swiss-Prot, KEGG, COG, GO and TrEMBL databases were employed for annotation of the 36,157 unigene sequences (Table 2). Significant matches were found for 12,897 (83.71%) unigenes (≥1000 bp) in the Nr database, 11,809 (70.81%) in the Nt database, and 12,604 (78.81%) in the Swiss-Prot database. In the KEGG, COG, GO, and TrEMBL databases, there were 5,943 (34.72%), 4,479 (21.39%), 11,962 (72.33%), and 12,885 (84.01%) significantly matched unigenes (≥1000 bp), respectively. The Nr database queries revealed A. sinensis sequences to closely match sequences of Danio rerio (17.30%), Maylandia zebra (10.10%), Oreochromis niloticus (7.82%), Xenopus (Silurana) tropicalis (7.31%), and Chelonia mydas (6.93%).
Table 2. Functional annotation of unigenes of the Acipenser sinensis transcriptome.
| Databases | All Annotated transcripts | ≤300 (bp) | 300–1000 (bp) | ≥1000 (bp) |
|---|---|---|---|---|
| nr | 30,268 | 5,550 | 11,821 | 12,897 |
| nt | 25,606 | 4,147 | 9,650 | 11,809 |
| Swissprot | 28,281 | 4,865 | 10,812 | 12,604 |
| KEGG | 12,557 | 2,127 | 4,487 | 5,943 |
| COG | 7,734 | 944 | 2,311 | 4,479 |
| GO | 26,152 | 4,412 | 9,778 | 11,962 |
| TrEMBL | 30,377 | 5,631 | 11,861 | 12,885 |
| All_Annotated | 36,157 | 7,404 | 14,873 | 13,880 |
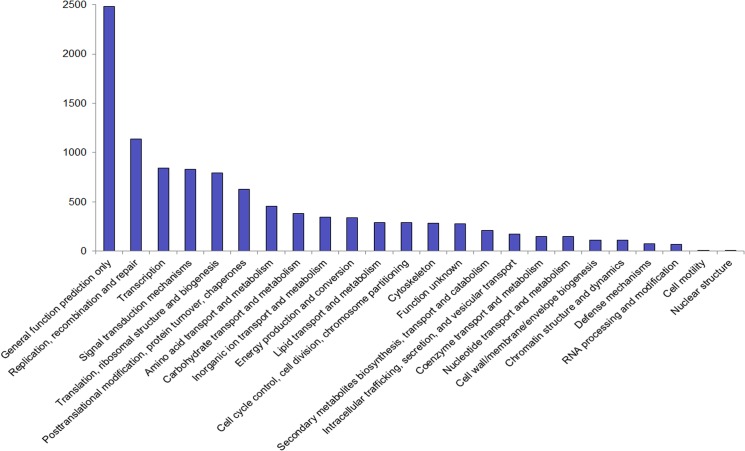
Functional prediction and classification of the unigene sequences were achieved by search against the COG database and a classification of 24 categories was obtained accordingly (Fig 2). The cluster for ‘general function prediction only’ constituted the major group (2,483; 23.79%), followed by ‘replication, recombination, and repair’ (1,135; 10.87%); ‘transcription’ (846; 8.10%); ‘signal transduction mechanisms’ (828; 7.93%); and ‘translation, ribosomal structure, and biogenesis’ (795; 7.62%). Only nine unigenes were allocated to ‘cell mobility’ and two to ‘nuclear structure’. No unigenes were found to be related to ‘extracellular structures’ (Fig 2).
Fig 2. Clusters of orthologous groups (COG) classification.
4,479 unigenes with Nr hits were grouped into 24 COG classifications.
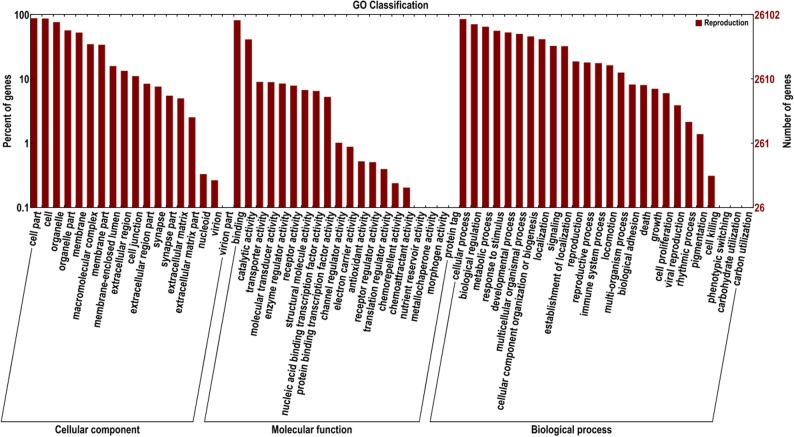
In the GO annotation, the 26,152 unigenes were allocated one or more GO terms based on sequence similarity. The three main categories of GO annotations were 130,517 (37.50%) for cellular components, 46,967 (13.50%) for molecular function, and 170,512 (50%) for biological processes (Fig 3). For cellular components, genes involved in ‘cell part’ and ‘cell’ terms were the most represented. In the category of molecular function, the term ‘binding’ was in the highest proportion of annotations, followed by ‘catalytic activity’. For biological processes, the most frequent GO term was ‘cellular process’.
Fig 3. Functional annotation of assembled sequences based on gene ontology (GO) categorization.
GO analysis was performed for three main categories: cellular components, molecular function, and biological processes.
To further demonstrate the biological pathways involved in A. sinensis, the unigene sequences were mapped to the KEGG Pathway Tools. This process assigned 12,557 unigenes to a total of 231 pathways (S3 Table). These predicted pathways contained most biological pathways involved in the process of reproduction, including GnRH signaling (160 unigenes), steroid hormone biosynthesis (23), oocyte meiosis (170), regulation of actin cytoskeleton (341), DNA replication (48), RNA polymerase (32), mismatch repair (26), purine metabolism (245), adherens junction (155), cell cycle (215), Fc gamma R-mediated phagocytosis (52), and pyrimidine metabolism (123). Further information on these predicted pathways may be useful for investigations of their functions in A. sinensis.
Search for genes involved in gametogenesis
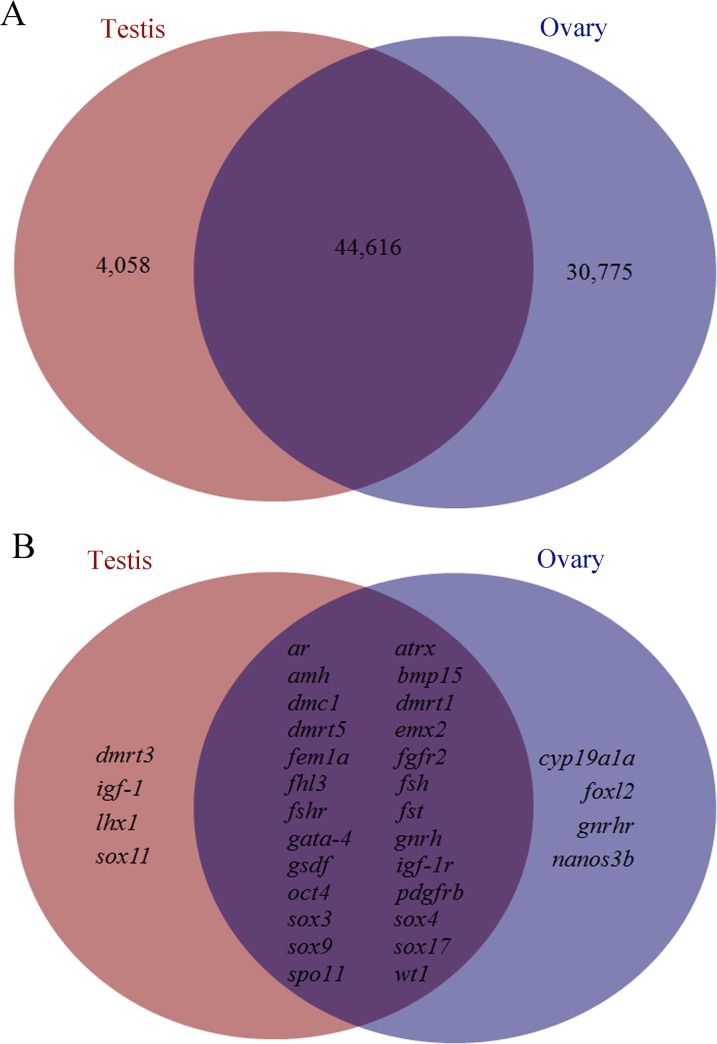
We chose 55 genes reported as active in sexual development [4, 29] to search for unigenes annotated in the transcriptome of A. sinensis (S4 Table). There were significant matches with 34 of the 55 genes. Two major sex-determining genes of fish, dmrt1 and gsdf, were also present in the gonad transcriptome of Chinese sturgeon (S4 Table). Thirty-two additional genes (S4 Table) involved in sexual development were investigated: five genes belonging to the sox subfamily, sox3, sox4, sox 9, sox 11 and sox 17; 11 transcription factor genes, bmp15, emx2, fem1a, fhl3, foxl2, gata4, lhx1, nanos3b, oct4, spo11 and wt1; seven receptors ar, atrx, fgfr2, fshr, gnrhr, igf-1r and pdgfrb; two hormone genes, gnrh and fsh; two genes belonging to the double sex and mab-3 (DM) domain, dmrt3 and dmrt5; the signaling molecule igf-1; the recombinase dmc1; and the steroidogenic enzymes amh, fst and cyp19a1a. The expression levels (RPKM) of these transcripts are listed in S4 Table. Among these transcripts, dmrt3, igf-1, lhx1, and sox11 were found to be present only in the testis transcriptome, while cyp19a1a, foxl2, gnrhr and nanos3b were present only in the ovary transcriptome (Fig 4B). The remaining genes were transcribed in both gonads. A total of 4,058 unigenes were transcribed exclusively in testis (RPKM>0), and 30,775 unigenes were found only in ovary, with 44,616 unigenes present in both testis and ovary (Fig 4A).
Fig 4. The distribution of transcripts between testis and ovary.
(A) The numbers of all transcripts present in the testis, ovary, or both. (B) The distribution of transcripts with potential function in sexual development in testis and ovary.
Differentially expressed genes in early gametogenesis and confirmation using relative real-time PCR
EBseq software was used for analysis of genes differentially regulated in testis and ovary. Since biological replicates were unavailable, the following data are qualitative indications with no statistically significant differences. It was shown that 1,894 unigenes were up-regulated in ovary, while only two unigenes were up-regulated in testis (S5 Table). The most predominant up-regulated unigenes in the ovary were the zona pellucida (ZP) genes. In testis, one of the up-regulated unigenes (Reproduction_Unigene_BMK.74160) was the Vitrin-like gene, while the other up-regulated unigene (Reproduction_Unigene_BMK.66097) had no Nr annotation. No differential expression in testis and ovary was found for the sex-determining genes dmrt1 and gsdf.
Ten potential genes involved in reproduction regulation were chosen for validation using real-time PCR. Nine (zp3, zpax, spermidine synthase, hatching enzyme, pou2, Bucky ball, cyclin E, sox3, and gdf9) were found to be up-regulated in ovary compared to testis, with the Vitrin-like gene showing higher expression in testis than in ovary, in accordance with the transcriptome analysis (Table 3). Previous RT-PCR detection of the pou2 gene also suggested its higher transcription level in ovary [30]. These selected genes showed significant expression differences in ovary and testis, but at different levels from those found in transcriptome sequencing (Table 3).
Table 3. Real-time PCR confirmation of the relative expression of genes showing differential expression between the two gonad types.
| Unigene ID | Illumina (O/T)a | qRT-PCR (O/T)b | Nr Annotation |
|---|---|---|---|
| 75530 | 14.44 | 29.00 | zona pellucida glycoprotein 3 |
| 59852 | 13.45 | 16.50 | zona pellucida glycoprotein AX |
| 57743 | 7.22 | 7.69 | spermidine synthase |
| 54618 | 9.58 | 20.00 | hatching enzyme |
| 73936 | 7.61 | 12.50 | Pou2 |
| 75228 | 5.59 | 11.11 | bucky ball |
| 71078 | 5.11 | 20.00 | E-type cyclin |
| 60411 | 4.42 | 16.67 | SRY (sex determining region Y)-box 3 |
| 74439 | 3.90 | 12.50 | growth/differentiation factor 9 |
| 74160 | -7.47 | -10.90 | predicted: vitrin-like |
a The relative-fold expression determined by Illumina transcriptome analysis.
b The relative-fold expression validated by qRT-PCR.
O/T: The relative transcription level of genes in ovary compared to that in testis. O, Ovary; T, Testis.
Transcriptome comparison of A. sinensis, A. naccarii, and A. fulvescens
We compared the A. sinensis transcriptome data with the already published transcriptome results of A. naccarii and A. fulvescens (Table 4). For sequencing of A. naccarii and A. fulvescens, Roche’s 454 platform was used, while Illumina Hiseq 2000 platform was employed for A. sinensis. In sequencing of the three species, tissues of male and female gonads were selected, with brain tissues included in A. naccarii. In A. sinensis and A. naccarii, 12,802,085 and 42,193 contigs were assembled, respectively. In A. fulvescens, the male assembly produced 12,791 contigs, while the female assembly (the ovary library and the female gonad with heterogametic sex library) produced 32,629 contigs.
Table 4. Transcriptome comparison of three sturgeon species.
| Species | Platform | Tissue source | Clean reads | Contig | Singleton | Unigene |
|---|---|---|---|---|---|---|
| A. sinensis | Illumina | Testis | 47,333,701 | 12,802,085 | 86,027 | |
| Ovary | 47,229,705 | |||||
| A. naccarii | 454 | Testis+ brain | 153,215 | 42,193 | 13,089 | |
| Ovary+brain | 175,198 | |||||
| A.fulvescens | 454 | Testis | 134,278 | 12,791 | 99,713 | |
| Ovary | 69,366 | 32,629 b | 154,724 | |||
| Heterogametic a | 269,913 |
a Female gonad with heterogametic sex
b Contigs for female assembly with combined reads of the ovary and the female gonad with heterogametic sex
Ortholog comparison between A. sinensis and Danio rerio
Acipenser sinensis is a functional tetraploid species [1], therefore more paralogs should be present compared to diploid teleosts. Here we searched the orthologs of 11 genes in three gene families (sox, apolipoprotein and cyclin) in A. sinensis and in D. rerio, a well-studied diploid teleost model. Of the sox family, additional paralogs of sox3, sox6, and sox11, were found in A. sinensis not present in their orthologs in D. rerio (Table 5). In the apolipoprotein superfamily, three more paralogs of apolipoprotein A-I (apoA-I) and two more of apolipoprotein E (apoE), together with an additional apolipoprotein L (apoL) paralog, were found in A. sinensis. Compared to D. rerio, an additional cyclin A1, cyclin A2, and cyclin B3 paralog of the cyclin family was found in A. sinensis. Two more paralogs of cyclin B1 and cyclin-dependent kinase 12 (cdk12) of the cyclin family were present in A. sinensis (Table 5).
Table 5. Comparison of orthologs from three gene families in Chinese sturgeon and zebrafish.
| Acipenser sinensis | Danio rerio | |||
|---|---|---|---|---|
| Paralog Name | Numbers | Annotation Number | Numbers | Genbank Number |
| sox3 | 2 | Unigene_BMK.60411 | 1 | BAD11369 |
| Unigene_BMK.60413 | ||||
| sox6 | 3 | Unigene_BMK.66469 | 2 | AEO16862 |
| Unigene_BMK.66470 | AEO16863 | |||
| Unigene_BMK.66471 | ||||
| sox11 | 3 | Unigene_BMK.55711 | 2 | AAI15165 |
| Unigene_BMK.83418 | NP_571412 | |||
| Unigene_BMK.6537 | ||||
| apoA-I | 4 | Unigene_BMK.41555 | 1 | NP_571203 |
| Unigene_BMK.48306 | ||||
| Unigene_BMK.81617 | ||||
| Unigene_BMK.9655 | ||||
| apoE | 4 | Unigene_BMK.11762 | 2 | XP_005173815 |
| Unigene_BMK.4517 | AAH65592 | |||
| Unigene_BMK.64153 | ||||
| Unigene_BMK.1736 | ||||
| apoL | 4 | Unigene_BMK.43849 | 3 | XP_005171358 |
| Unigene_BMK.60451 | XP_005171359 | |||
| Unigene_BMK.18017 | XP_005171360 | |||
| Unigene_BMK.65059 | ||||
| cyclin A1 | 2 | Unigene_BMK.40637 | 1 | NP_997983 |
| Unigene_BMK.73469 | ||||
| cyclin A2 | 2 | Unigene_BMK.55138 | 1 | NP_694481 |
| Unigene_BMK.60588 | ||||
| cyclin B1 | 3 | Unigene_BMK.63792 | 1 | NP_571588 |
| Unigene_BMK.47153 | ||||
| Unigene_BMK.71665 | ||||
| cyclin B3 | 2 | Unigene_BMK.73792 | 1 | NP_001070187 |
| Unigene_BMK.62679 | ||||
| cdk12 | 3 | Unigene_BMK.79801 | 1 | XP_003200579 |
| Unigene_BMK.16306 | ||||
| Unigene_BMK.66799 | ||||
Discovery of microsatellites
To explore the SSR profile in unigenes of Chinese sturgeon, the 16,687 unigene sequences were analyzed by MISA software. In the 7,654 unigene sequences investigated, 12,151 SSRs were detected, with 2,886 (37.71%) sequences containing more than one SSR. Five types of SSRs were found, among which the mono-nucleotide repeat motif represented the largest group (65.39%), followed by the dimer (18.71%), trimer (14.14%), tetramer (1.74%), and pentamer repeat motifs (0.02%) (Table 6).
Table 6. Summary of simple sequence repeat (SSR) types in the Chinese sturgeon transcriptome.
| Repeat motif | Numbera | %b |
|---|---|---|
| Mono-nucleotide | ||
| A/T/C/G | 7,946 | 65.39 |
| Di-nucleotide | ||
| AC/GT/AG/CT | 1,548 | |
| AT/AT | 724 | |
| CG/CG | 1 | |
| Di-nucleotide Total | 2,273 | 18.71 |
| Tri-nucleotide | ||
| AAC/GTT/AAG/CTT/ AAT/ATT | 622 | |
| ACC/GGT/ACG/CGT/ACT/AGT | 76 | |
| AGC/CTG/AGG/CCT/ATC/ATG | 1,006 | |
| CCG/CGG | 14 | |
| Tri-nucleotide Total | 1,718 | 14.14 |
| Tetra-nucleotide | ||
| AAAC/GTTT/AAAG/CTTT/ AAAT/ATTT | 105 | |
| AACC/GGTT/AACT/AGTT/ | 4 | |
| AAGC/CTTG/AAGG/CCTT | 7 | |
| AATC/ATTG/AATG/ATTC/AATT/AATT | 19 | |
| ACAG/CTGT/ACAT/ATGT ACCT/AGGT | 34 | |
| ACGC/CGTG/ACGG/CCGT | 4 | |
| ACTC/AGTG/ACTG/AGTC | 7 | |
| AGAT/ATCT/AGCC/CTGG | 13 | |
| AGGC/CCTG/AGGG/CCCT | 13 | |
| ATCC/ATGG | 5 | |
| Tetra-nucleotide Total | 211 | 1.74 |
| Penta-nucleotide | ||
| ACAGT/ACTGT | 1 | |
| ACCTG/AGGTC | 1 | |
| ATCCC/ATGGG | 1 | |
| Penta-nucleotide Total | 3 | 0.02 |
| Grand Total | 12,151 | 100.00 |
aNumber of SSRs detected in unigenes
bRelative percent of SSRs with different repeat motifs among the total SSRs
Discussion
High throughput transcriptome sequencing has been widely used in numerous studies such as gene expression profiling, and simultaneous identification of mutations, sequence aberrations, and alternative splice variants [31]. Our study provides the first attempt into the investigation of transcriptome of the Chinese sturgeon. In total, 86,027 unigenes with the mean length of 705.84 bp were assembled. The unigenes obtained were further annotated with various protein databases, and were used to detect SSRs. These results should be meaningful for further investigation on early gametogenesis and conservation of the Chinese sturgeon.
In teleosts, as in other vertebrates, the regulation of reproductive process relies mainly on the neuroendocrine system, the brain–pituitary–gonad (BPG) axis [32]. In this axis, gonadotropin-releasing hormone (GnRH) promotes the synthesis and release of two specific pituitary hormones, most dominantly follicle stimulating hormone (FSH) and luteinizing hormone (LH). FSH and LH then activate receptors and stimulate synthesis of the various sex steroid hormones in the gonads to regulate steroidogenesis and gametogenesis [33, 34]. In the KEGG pathway annotation of Chinese sturgeon transcriptome in this study, a number of unigenes was assigned to the GnRH pathway (S3 Table), which was in accordance with its regulatory role during the gametogenesis in Chinese sturgeon. Besides, sbgnrh, gnrhr, fsh and fshr transcripts, except for lh and lhr (lh receptor), were found in the Chinese sturgeon transcriptome (Fig 4 and S4 Table). In our previous study, one GnRH precursor AsGnRH1 transcript (the original Nr annotation for sbgnrh found in this study) was found in the testis and ovary of 4-year-old males and females [28], which was consistent with the detection of this study. In another research on the expression pattern of FSHβ and LHβ revealed the presence of both FSHβ and LHβ in mature Chinese sturgeon; while in the immature 4-year-old male Chinese sturgeon, only FSHβ was detected [35]. It might be that LHβ will only be expressed in the maturation period, but not in the gametogenesis period, just like the expression pattern of LH reported in salmonids [36–38].
The regulation of reproduction is a complex process that requires not only the control of HPG axis but also the cooperation of many autocrine and paracrine factors, including the insulin-like growth factor 1 (Igf-1). By binding to their receptors, Igf-1 plays very important role in spermatogenesis, Leydig cell differentiation and proliferation [39, 40]. In the Chinese sturgeon transcriptome, we found igf-1 transcript transcribed only in the testis with no transcription in the ovary. The igf-1 gene was reported to be involved in early vitellogenesis and ovary maturation in the sterlet, Acipenser ruthenus [41, 42]. It is then suspected that igf-1 expression occurred later in the vitellogenesis stage in the Chinese sturgeon.
The testicular tissue was in the early spermatogenesis stage with many primary spermatocytes and a few of spermatogonia (Figure A in S1 Fig). It is thus indicated that some spermatogonia differentiated into primary spermatocytes through meiosis, while a small group of spermatogonia maintained the undifferentiated state. In mice, nanos homolog 3 (nanos3) is responsible for maintaining the undifferentiated state of spermatogonia by the control of their cell cycle [43]. Other markers of undifferentiated spermatogonia such as oct4 (also known as pou2), neurogenin3 and SRY (sex determining region Y)-box 3 (sox3) have also been identified, but with no specific function [44–46]. In the Chinese sturgeon transcriptome, nanos3b and the undifferentiated spermatogonia markers sox3 and oct4 were present, both with higher expression levels in the ovary compared to that in the testis (S4 Table). Our previous study revealed that oct4 (pou2) was transcribed in gonads of immature Chinese sturgeons, including the testes of 1-year-old males that was primarily composed [30], indicating its possible role in the early development of spermatogonia. Besides, the meiosis specific markers of DNA meiotic recombinase 1 (dmc1) and meiotic protein covalently bound to DSB (spo11) [12] were also found in the present transcriptome. The anti-mullerian hormone (amh) gene was another candidate factor involved in testis differentiation, with up-regulated mRNA expression in the testis in some teleosts including zebrafish [47], Nile tilapia [48], rainbow trout [49], sea bass [50] and Japanese flounder [51]. In this study, amh was also present in the Chinese sturgeon transcriptome, with higher transcription level in testis than in ovary (Fig 4 and S4 Table), and its role in testicular differentiation of sturgeons requires future investigation.
For the initial phases of ovarian development, limited genes were reported to be responsible. For instance, no stage specific proteins were discovered in the proteome display of immature follicles of zebrafish and the gilthead seabream [52]. In mammals, two members of TGF-β family, the growth and differentiation factor 9 (gdf9) and bone morphogenetic factor 15 (bmp15) were proved to be involved in early ovarian follicle growth. In this study, bmp15 was transcribed with higher levels in the ovary than that in the testis (S4 Table), indicating its potential role in the primary oocyte growth in the Chinese sturgeon. Cytochrome P450 aromatase, encoded by cyp19a, represents the crucial enzyme that converts androgens to estrogens in the steroidogenic pathway [53]. In our study, cyp19a1a was transcribed only in the ovary of the immature Chinese sturgeon, confirming its role in controlling conversion of estradiol-17β, the main regulator of the ovarian development [17]. The forkhead transcription factor (foxl2) was another gene transcribed only in ovary of the Chinese sturgeon (Fig 4 and S4 Table), which is the same with that in mouse embryos, chickens, and turtle [54]. The foxl2 was found to play an essential role in early ovarian development and sex determination, as well as a later role in granulosa cell differentiation with subsequent follicle depletion, and mutations of FOXL2 conduce to a variety of conditions and disease states [55]. Concerning its significant role in the ovary, it would be necessary to explore the function of foxl2 further in the Chinese sturgeon.
Among the reported sex-determining genes, transcription factor double sex and mab-3 related transcription factor 1 (dmrt1) is the only one conserved both in invertebrates and vertebrates essential for male determination [56, 57]. The dmrt1 expression was revealed to be sexually dimorphic in gonads of lake sturgeon [58] and Siberian sturgeon [59, 60], but not in the shovelnose sturgeon [61]. In this study, no significant difference in dmrt1 expression was observed between the ovary and testis of Chinese sturgeon. It has been reported that obvious sex differentiation of the Chinese sturgeon can be distinguished early in the 9-month-old sturgeons using surgical operation [19, 62]. Therefore, it is reasonable to speculate that the sexual dimorphism of dmrt1 might occur in the early stages of gonad differentiation. Besides,in teleost fish, dmrt1 is assumed to be involved in testis differentiation, considering its expression pattern during the early period of gonad development [48, 49] and on its behavior when masculinizing and feminizing treatments are used [59]. In spermatogonia, dmrt1 was critical for determining whether spermatogonia go through mitosis and spermatogonial differentiation or meiosis [63]. Thus it would be worth the effort to examine the specific role dmrt1 plays during the early gametogenesis in Chinese sturgeon. Two other DM domain genes dmrt3 and dmrt5 were also found in the Chinese sturgeon transcriptome (Fig 4 and S4 Table). The dmrt3 was reported to function cooperatively with dmrt1 after gonadal differentiation in Takifugu rubripes [64]. In zebrafish, dmrt5 was expressed in developing brain and germ cells, evidenced their potential involvement in the HPG axis [65].
sox9, an important gene expressed during testis development in mammals [66], was found in the gonad transcriptome of Chinese sturgeon. Together with dmrt1, sox9 is a key SRY target in mammalian testis development, but few studies of sox9 have been conducted in fish [20]. A study of sox9 in the Siberian sturgeon revealed it to play a role in late testis differentiation, but not to be a key factor throughout the development of male gonad [60]. Similarly to the expression of dmrt1, that of sox9 in the Chinese sturgeon did not differ significantly in ovary and testis. Further studies were needed to investigate their roles in early gonad differentiation in younger fish. The sex-determination gene gsdf was also detected in the Chinese sturgeon transcriptome, again with no significant expression difference between the testis and ovary. Whether the gsdf gene, as shown to be sex-determining in Oryzias luzonensis [67], plays the same role in the Chinese sturgeon needs further exploration. Furthermore, recombinant Gsdf promotes the spermatogonia proliferation in rainbow trout [68] and gsdf gene expression is relevant to early testicular differentiation in medaka [69]. These results strongly indicate a potential function of gsdf in the early gametogenesis in Chinese sturgeon.
In C. elegans, fem-1 is part of the signal transduction pathway responsible for sex determination [70], and normal masculinization of somatic and germline tissue [71]. The orthologs of fem-1 in C. elegans (fem1a) were also identified in mouse and human [72, 73]. In addition, transcriptome sequencing in Pinctada margaritifera and Macrobrachium nipponense indicated the fem1a gene to be a sex determination candidate gene [74, 75]. In the Chinese sturgeon transcriptome, no differential expression of fem1a was found between testis and ovary, and its function remains unclear in this species.
In the transcriptome annotation, 13 other genes involved in gametogenesis were found (ar, atrx, emx2, gata-4, fgfr2, fhl3, lhx1, sox4, sox11, sox17 and wt1) (Fig 4 and S4 Table). As their specific functions have not been studied in the Chinese sturgeon yet, they are recommended for additional investigations in the future. Of the 22 genes reported in the Adriatic sturgeon transcriptome [4], 8 (dax1, sox1, sox6, sox14, rspo, sf-1, fgf9, and lhx9) were not observed in the Chinese sturgeon transcriptome. A transcriptome screen for sex differentiation genes in the lake sturgeon identified 12 (sox2, sox4, sox17, sox21, sox9, dmrt1, rspo-1, wt1, wnt4, foxl2, tra-1, fem1) [58], of which five (sox2, sox21, rspo-1, wnt4 and tra-1) were not detected in our study. These differences could be caused by differences in transcriptome sequencing methods and the species used, as well as by the diversity of the developmental stages of the gonads analyzed. Furthermore, only 23.96% of transcript sequences and 15.33% of unigene sequences presented complete ORFs, which suggested that traditional cDNA library and Sanger’s sequencing method were needed for complete transcriptome sequencing. In other words, not all genes transcribed in the gonad transcriptome were annotated. This might be an additional reason why these genes whose function is related to gonad development were not found in the Chinese sturgeon transcriptome.
The detected genes were primarily up-regulated in the ovary compared with the testis. This is similar to the transcriptome of Sebastiscus marmoratus, in which differentially expressed genes were predominantly present in the female [76]. The acellular envelope of the developing oocyte composed of 2–4 isoforms of ZP proteins [77]. In our previous study, three zp3 genes were characterized in A. sinensis [78]. In this study, more types of zp genes were identified that were the most significantly differentially expressed unigenes in ovary (S5 Table), which is consistent with their essential role in the protection of the oocyte. The function of other differentially expressed unigenes is a topic for further research. The specific relative-fold expression of the differentially expressed genes in ovary and testis were different in the RNA-seq data from that obtained by real-time PCR. It may be that biological replicates, which were more valuable and accurate than was increasing the sequencing depth for detecting differently expressed genes, were not included in the transcriptome sequencing [79].
Chromosome numbers in sturgeon species are variable [1, 80]. Acipenser sinensis is believed to be a functional tetraploid [80]. Compared to the diploid D. rerio, A. sinensis tends to have a greater number of paralogs in the superfamilies of sox, apolipoprotein and cyclin (Table 4), possibly the result of genome polyploidization. Generally, genes of the sox family share conserved domains including the High Mobility Group Box; therefore the unigenes we obtained by BLASTX against the NCBI nr database could show multiple matches with sox genes with higher scores. As a result, the full-length cDNA transcripts of these unigenes should be cloned for validation in further studies. In addition, the detailed transcriptome investigation of the functional tetraploid Chinese sturgeon in this study is important for evaluating the functional reduction of ploidy when the genome is sequenced or when the transcriptome of functional diploid or octaploid sturgeon species is available [4].
SSRs are tandem repeat DNA sequences that constitute an important part of eukaryote genomes. Being highly polymorphic, they are increasingly used as marker systems in molecular genetics studies, including research involving genetic diversity assessment, comparative genomics, gene flow characterization, and genetic linkage mapping [81]. For the conservation of the Chinese sturgeon, SSRs can serve as effective genetic markers for quantifying genetic diversity within and among populations of this endangered species. In this study, a set of SSRs was identified in which 65.39% were mononucleotide repeat motifs that might be caused by sequencing. Therefore, only the di-, tri-, tetra-, and penta-repeat motifs (Table 6) found would be suitable for polymorphic microsatellite loci identification. In A. dabryanus, a set of polymorphic microsatellite loci with the di-nucleotide repeat motif were identified [82]. Following the mono-nucleotide repeat motif, the di-nucleotide repeat motif accounted for the second largest group in the A. sinensis transcriptome (18.71%), suggesting the existence of diverse di-nucleotide repeat motif loci in A. sinensis similar to those of A. dabryanus. SSRs identified from the unigenes are useful for description of genealogy and assessment of genetic diversity. The dataset will make contributions to the identification of the molecular mechanism controlling sexual dimorphism and sexual development regulation of A. sinensis, as well as to the better conservation of this endangered species.
Supporting Information
Labels: O, pre-vitellogenic oocyte; PSC, primary spermatocyte; SG, spermatogonia.
(TIF)
Sequence Headers containing "type:complete" represent transcript sequences with complete ORFs.
(TXT)
Sequence Headers containing "type:complete" represent unigene sequences with complete ORFs.
(TXT)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Acknowledgments
This work was supported by grants from the National Nonprofit Institute Research Grant of Chinese Academy of Fisheries (2013A0403), the National Basic Research Program of China (973 Program) (2015CB150702) and the National Natural Science Foundation of China (31472286 and 31172413). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding Statement
This work was supported by grants from the National Nonprofit Institute Research Grant of Chinese Academy of Fisheries (2013A0403), the National High Technology Research and Development Program of China (2011AA100401), the National Natural Science Foundation of China (31472286 and 31172413), the National Basic Research Program of China (973 Program) (2015CB150702), and the National Nonprofit Institute Research Grant of Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (2013JBFZ01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ludwig A, Belfiore NM, Pitra C, Svirsky V, Jenneckens I. Genome duplication events and functional reduction of ploidy levels in sturgeon (Acipenser, Huso and Scaphirhynchus). Genetics. 2001;158(3):1203–15. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fontana F, Congiu L, Mudrak VA, Quattro JM, Smith TI, Ware K, et al. Evidence of hexaploid karyotype in shortnose sturgeon. Genome / National Research Council Canada = Genome / Conseil national de recherches Canada. 2008;51(2):113–9. 10.1139/g07-112 PubMed . [DOI] [PubMed] [Google Scholar]
- 3. Ludwig A. Identification of Acipenseriformes species in trade. J Appl Ichthyol 2008;24(S1):2–19. [Google Scholar]
- 4. Vidotto M, Grapputo A, Boscari E, Barbisan F, Coppe A, Grandi G, et al. Transcriptome sequencing and de novo annotation of the critically endangered Adriatic sturgeon. BMC genomics. 2013;14:407 10.1186/1471-2164-14-407 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei QW, Ke FE, Zhang JM, Zhuang P, Luo JD, Zhou RQ, et al. Biology, fisheries, and conservation of sturgeons and paddlefish, in China. Environ Biol Fish. 1997;48:241–55. [Google Scholar]
- 6. Birstein VJ, Bemis WE, Waldman JR. The threatened status of acipenseriform species: A summary. Environmental Biology of Fishes. 1997;48(1–4):427–35. 10.1023/A:1007382724251 PubMed . [DOI] [Google Scholar]
- 7. Zhang H, Wei QW, Kyanrd BE, Du H, Yang DG, Chen XH. Spatial structure and bottom characteristics of the only remaining spawning area of Chinese sturgeon in the Yangtze River. J Appl Ichthyol. 2011;27:251–6. [Google Scholar]
- 8. Wei QW, Li LX, Du H, Zhang XY, Xiong W, Zhang H, et al. Research on technology for controlled propagation of cultured Chinese sturgeon(Acipenser sinensis). Journal of Fishery Sciences of China (In Chinese). 2013;20(1):1–11. [Google Scholar]
- 9. de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. Journal of andrology. 2000;21(6):776–98. PubMed . [PubMed] [Google Scholar]
- 10. Russell LD, Ettlin RA, Hikim APS, Clegg ED. Histological and histopathological evaluation of the testis. Int J Androl. 1993;16(1):83. [Google Scholar]
- 11. Rossi P, Dolci S. Paracrine mechanisms involved in the control of early stages of mammalian spermatogenesis. Frontiers in endocrinology. 2013;4:181 10.3389/fendo.2013.00181 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulz RW, de Franca LR, Lareyre JJ, Le Gac F, Chiarini-Garcia H, Nobrega RH, et al. Spermatogenesis in fish. General and comparative endocrinology. 2010;165(3):390–411. 10.1016/j.ygcen.2009.02.013 PubMed . [DOI] [PubMed] [Google Scholar]
- 13. Schlatt S, Ehmcke J. Regulation of spermatogenesis: an evolutionary biologist's perspective. Seminars in cell & developmental biology. 2014;29:2–16. 10.1016/j.semcdb.2014.03.007 PubMed . [DOI] [PubMed] [Google Scholar]
- 14. Cheng CY, Mruk DD. The biology of spermatogenesis: the past, present and future. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2010;365(1546):1459–63. 10.1098/rstb.2010.0024 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miura C, Higashino T, Miura T. A progestin and an estrogen regulate early stages of oogenesis in fish. Biology of reproduction. 2007;77(5):822–8. 10.1095/biolreprod.107.061408 PubMed . [DOI] [PubMed] [Google Scholar]
- 16. Çakıcı Ö, Üçüncü Sİ. Oocyte development in the zebrafish, Danio rerio (Teleostei: Cyprinidae). EU Journal of Fisheries & Aquatic Sciences. 2007;24(1–2):137–41. [Google Scholar]
- 17. Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50 Suppl 1:S195–219. Epub 2008/05/17. DGD1019 [pii] 10.1111/j.1440-169X.2008.01019.x PubMed . [DOI] [PubMed] [Google Scholar]
- 18. Patino R, Yoshizaki G, Thomas P, Kagawa H. Gonadotropic control of ovarian follicle maturation: the two-stage concept and its mechanisms. Comparative biochemistry and physiology Part B, Biochemistry & molecular biology. 2001;129(2–3):427–39. PubMed . [DOI] [PubMed] [Google Scholar]
- 19. Chen XH, Wei QW, Yang DG, Zhu YJ. Observations on the formation and development of the primary germinal tissue of cultured Chinese sturgeon, Acipenser sinensis . J Appl Ichthyol. 2006;22:358–60. 10.1111/j.1439-0426.2007.00984.x PubMed . [DOI] [Google Scholar]
- 20. Sun FY, Liu SK, Gao XY, Jiang YL, Perera D, Wang XL, et al. Male-biased genes in catfish as revealed by RNA-seq analysis of the testis transcriptome. PloS one. 2013;8(7):e68452 10.1371/journal.pone.0068452 PubMed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luikart G, England PR, Tallmon D, Jordan S, Taberlet P. The power and promise of population genomics: from genotyping to genome typing. Nature reviews Genetics. 2003;4(12):981–94. 10.1038/nrg1226 PubMed . [DOI] [PubMed] [Google Scholar]
- 22. Liu M, Qiao G, Jiang J, Yang H, Xie L, Xie J, et al. Transcriptome sequencing and de novo analysis for Ma bamboo (Dendrocalamus latiflorus Munro) using the Illumina platform. PloS one. 2012;7(10):e46766 10.1371/journal.pone.0046766 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–U130. 10.1038/Nbt.1883 PubMed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rice P, Longden I, Bleasby A. EMBOSS: the European molecular biology open software suite. Trends in genetics: TIG. 2000;16(6):276–7. PubMed . [DOI] [PubMed] [Google Scholar]
- 25. Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic acids research. 2007;35(Web Server issue):W182–5. 10.1093/nar/gkm321 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature methods. 2008;5(7):621–8. 10.1038/nmeth.1226 PubMed . [DOI] [PubMed] [Google Scholar]
- 27. Leng N, Dawson JA, Thomson JA, Ruotti V, Rissman AI, Smits BM, et al. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29(8):1035–43. 10.1093/bioinformatics/btt087 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yue H, Ye H, Chen X, Cao H, Li C. Molecular cloning of cDNA of gonadotropin-releasing hormones in the Chinese sturgeon (Acipenser sinensis) and the effect of 17beta-estradiol on gene expression. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2013;166(4):529–37. 10.1016/j.cbpa.2013.08.011 PubMed . [DOI] [PubMed] [Google Scholar]
- 29. Forconi M, Canapa A, Barucca M, Biscotti MA, Capriglione T, Buonocore F, et al. Characterization of sex determination and sex differentiation genes in Latimeria. PloS one. 2013;8(4):e56006 10.1371/journal.pone.0056006 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ye H, Du H, Chen XH, Cao H, Liu T, Li CJ. Identification of a pou2 ortholog in Chinese sturgeon, Acipenser sinensis and its expression patterns in tissues, immature individuals and during embryogenesis. Fish physiology and biochemistry. 2012;38(4):929–42. 10.1007/s10695-011-9579-8 PubMed . [DOI] [PubMed] [Google Scholar]
- 31. Morozova O, Hirst M, Marra MA. Applications of new sequencing technologies for transcriptome analysis. Annual review of genomics and human genetics. 2009;10:135–51. 10.1146/annurev-genom-082908-145957 PubMed . [DOI] [PubMed] [Google Scholar]
- 32. Weltzien FA, Andersson E, Andersen O, Shalchian-Tabrizi K, Norberg B. The brain-pituitary-gonad axis in male teleosts, with special emphasis on flatfish (Pleuronectiformes). Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2004;137(3):447–77. 10.1016/j.cbpb.2003.11.007 PubMed . [DOI] [PubMed] [Google Scholar]
- 33. Chang JP, Johnson JD, Sawisky GR, Grey CL, Mitchell G, Booth M, et al. Signal transduction in multifactorial neuroendocrine control of gonadotropin secretion and synthesis in teleosts-studies on the goldfish model. General and comparative endocrinology. 2009;161(1):42–52. 10.1016/j.ygcen.2008.09.005 PubMed . [DOI] [PubMed] [Google Scholar]
- 34. Taranger GL, Carrillo M, Schulz RW, Fontaine P, Zanuy S, Felip A, et al. Control of puberty in farmed fish. General and comparative endocrinology. 2010;165(3):483–515. 10.1016/j.ygcen.2009.05.004 PubMed . [DOI] [PubMed] [Google Scholar]
- 35. Cao H, Zhou L, Zhang YZ, Wei QW, Chen XH, Gui JF. Molecular characterization of Chinese sturgeon gonadotropins and cellular distribution in pituitaries of mature and immature individuals. Molecular and cellular endocrinology. 2009;303(1–2):34–42. 10.1016/j.mce.2009.01.015 PubMed . [DOI] [PubMed] [Google Scholar]
- 36. Weil C, Bougoussa-Houadec M, Gallais C, Itoh S, Sekine S, Valotaire Y. Preliminary evidence suggesting variations of GtH 1 and GtH2 mRNA levels at different stages of gonadal development in rainbow trout, Oncorhynchus mykiss . General and comparative endocrinology. 1995;100(3):327–33. 10.1006/gcen.1995.1163 PubMed . [DOI] [PubMed] [Google Scholar]
- 37. Breton B, Govoroun M, Mikolajczyk T. GTH I and GTH II secretion profiles during the reproductive cycle in female rainbow trout: relationship with pituitary responsiveness to GnRH-A stimulation. General and comparative endocrinology. 1998;111(1):38–50. 10.1006/gcen.1998.7088 PubMed . [DOI] [PubMed] [Google Scholar]
- 38. Gomez JM, Weil C, Ollitrault M, Le Bail PY, Breton B, Le Gac F. Growth hormone (GH) and gonadotropin subunit gene expression and pituitary and plasma changes during spermatogenesis and oogenesis in rainbow trout (Oncorhynchus mykiss). General and comparative endocrinology. 1999;113(3):413–28. 10.1006/gcen.1998.7222 PubMed . [DOI] [PubMed] [Google Scholar]
- 39. Villalpando I, Lira E, Medina G, Garcia-Garcia E, Echeverria O. Insulin-like growth factor 1 is expressed in mouse developing testis and regulates somatic cell proliferation. Experimental biology and medicine. 2008;233(4):419–26. 10.3181/0708-RM-212 PubMed . [DOI] [PubMed] [Google Scholar]
- 40. Huang YH, Chin CC, Ho HN, Chou CK, Shen CN, Kuo HC, et al. Pluripotency of mouse spermatogonial stem cells maintained by IGF-1- dependent pathway. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23(7):2076–87. 10.1096/fj.08-121939 PubMed . [DOI] [PubMed] [Google Scholar]
- 41. Wuertz S, Gessner J, Kirschbaum F, Kloas W. Expression of IGF-I and IGF-I receptor in male and female sterlet, Acipenser ruthenus—evidence for an important role in gonad maturation. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2007;147(1):223–30. 10.1016/j.cbpa.2006.12.031 PubMed . [DOI] [PubMed] [Google Scholar]
- 42. Wuertz S, Nitsche A, Jastroch M, Gessner J, Klingenspor M, Kirschbaum F, et al. The role of the IGF-I system for vitellogenesis in maturing female sterlet, Acipenser ruthenus Linnaeus, 1758. General and comparative endocrinology. 2007;150(1):140–50. 10.1016/j.ygcen.2006.07.005 PubMed . [DOI] [PubMed] [Google Scholar]
- 43. Lolicato F, Marino R, Paronetto MP, Pellegrini M, Dolci S, Geremia R, et al. Potential role of Nanos3 in maintaining the undifferentiated spermatogonia population. Dev Biol. 2008;313(2):725–38. 10.1016/j.ydbio.2007.11.011 PubMed . [DOI] [PubMed] [Google Scholar]
- 44. Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, et al. Identification and characterization of stem cells in prepubertal spermatogenesis in mice. Dev Biol. 2003;258(1):209–25. PubMed . [DOI] [PubMed] [Google Scholar]
- 45. Raverot G, Weiss J, Park SY, Hurley L, Jameson JL. Sox3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis. Dev Biol. 2005;283(1):215–25. 10.1016/j.ydbio.2005.04.013 PubMed . [DOI] [PubMed] [Google Scholar]
- 46. Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, et al. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269(2):447–58. 10.1016/j.ydbio.2004.01.036 PubMed . [DOI] [PubMed] [Google Scholar]
- 47. Rodriguez-Mari A, Yan YL, Bremiller RA, Wilson C, Canestro C, Postlethwait JH. Characterization and expression pattern of zebrafish Anti-Mullerian hormone (Amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene expression patterns: GEP. 2005;5(5):655–67. 10.1016/j.modgep.2005.02.008 PubMed . [DOI] [PubMed] [Google Scholar]
- 48. Ijiri S, Kaneko H, Kobayashi T, Wang DS, Sakai F, Paul-Prasanth B, et al. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus . Biology of reproduction. 2008;78(2):333–41. 10.1095/biolreprod.107.064246 PubMed . [DOI] [PubMed] [Google Scholar]
- 49. Vizziano D, Randuineau G, Baron D, Cauty C, Guiguen Y. Characterization of early molecular sex differentiation in rainbow trout, Oncorhynchus mykiss . Developmental dynamics: an official publication of the American Association of Anatomists. 2007;236(8):2198–206. 10.1002/dvdy.21212 PubMed . [DOI] [PubMed] [Google Scholar]
- 50. Halm S, Rocha A, Miura T, Prat F, Zanuy S. Anti-Mullerian hormone (AMH/AMH) in the European sea bass: its gene structure, regulatory elements, and the expression of alternatively-spliced isoforms. Gene. 2007;388(1–2):148–58. 10.1016/j.gene.2006.10.018 PubMed . [DOI] [PubMed] [Google Scholar]
- 51. Yoshinaga N, Shiraishi E, Yamamoto T, Iguchi T, Abe S, Kitano T. Sexually dimorphic expression of a teleost homologue of Mullerian inhibiting substance during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus . Biochemical and biophysical research communications. 2004;322(2):508–13. 10.1016/j.bbrc.2004.07.162 PubMed . [DOI] [PubMed] [Google Scholar]
- 52. Ziv T, Gattegno T, Chapovetsky V, Wolf H, Barnea E, Lubzens E, et al. Comparative proteomics of the developing fish (zebrafish and gilthead seabream) oocytes. Comparative biochemistry and physiology Part D, Genomics & proteomics. 2008;3(1):12–35. 10.1016/j.cbd.2007.06.004 PubMed . [DOI] [PubMed] [Google Scholar]
- 53. Goto-Kazeto R, Kight KE, Zohar Y, Place AR, Trant JM. Localization and expression of aromatase mRNA in adult zebrafish. General and comparative endocrinology. 2004;139(1):72–84. 10.1016/j.ygcen.2004.07.003 PubMed . [DOI] [PubMed] [Google Scholar]
- 54. Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144(7):3237–43. 10.1210/en.2002-0095 PubMed . [DOI] [PubMed] [Google Scholar]
- 55. Pisarska MD, Barlow G, Kuo FT. Minireview: roles of the forkhead transcription factor FOXL2 in granulosa cell biology and pathology. Endocrinology. 2011;152(4):1199–208. 10.1210/en.2010-1041 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haag ES, Doty AV. Sex determination across evolution: connecting the dots. PLoS biology. 2005;3(1):e21 10.1371/journal.pbio.0030021 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Volff JN, Zarkower D, Bardwell VJ, Schartl M. Evolutionary dynamics of the DM domain gene family in metazoans. Journal of molecular evolution. 2003;57 Suppl 1:S241–9. 10.1007/s00239-003-0033-0 PubMed . [DOI] [PubMed] [Google Scholar]
- 58. Hale MC, Jackson JR, Dewoody JA. Discovery and evaluation of candidate sex-determining genes and xenobiotics in the gonads of lake sturgeon (Acipenser fulvescens). Genetica. 2010;138(7):745–56. 10.1007/s10709-010-9455-y PubMed . [DOI] [PubMed] [Google Scholar]
- 59. Berbejillo J, Martinez-Bengochea A, Bedo G, Brunet F, Volff JN, Vizziano-Cantonnet D. Expression and phylogeny of candidate genes for sex differentiation in a primitive fish species, the Siberian sturgeon, Acipenser baerii . Molecular reproduction and development. 2012;79(8):504–16. 10.1002/mrd.22053 PubMed . [DOI] [PubMed] [Google Scholar]
- 60. Berbejillo J, Martinez-Bengochea A, Bedo G, Vizziano-Cantonnet D. Expression of dmrt1 and sox9 during gonadal development in the Siberian sturgeon (Acipenser baerii). Fish physiology and biochemistry. 2013;39(1):91–4. 10.1007/s10695-012-9666-5 PubMed . [DOI] [PubMed] [Google Scholar]
- 61. Amberg JJ, Goforth R, Stefanavage T, Sepulveda MS. Sexually dimorphic gene expression in the gonad and liver of shovelnose sturgeon (Scaphirhynchus platorynchus). Fish physiology and biochemistry. 2010;36(4):923–32. 10.1007/s10695-009-9369-8 PubMed . [DOI] [PubMed] [Google Scholar]
- 62. Chen XH, Wei QW, Yang DG, Zhu YJ, Liu Y. Histological studies on gonadal origin and differentiation of cultured Acipenser sinensis (In Chinese). J Fish China. 2004;28(6):633–9. [Google Scholar]
- 63. Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 Is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Developmental cell. 2010;19(4):612–24. 10.1016/j.devcel.2010.09.010 PubMed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamaguchi A, Lee KH, Fujimoto H, Kadomura K, Yasumoto S, Matsuyama M. Expression of the DMRT gene and its roles in early gonadal development of the Japanese pufferfish Takifugu rubripes . Comparative biochemistry and physiology Part D, Genomics & proteomics. 2006;1(1):59–68. 10.1016/j.cbd.2005.08.003 PubMed . [DOI] [PubMed] [Google Scholar]
- 65. Guo Y, Li Q, Gao S, Zhou X, He Y, Shang X, et al. Molecular cloning, characterization, and expression in brain and gonad of Dmrt5 of zebrafish. Biochemical and biophysical research communications. 2004;324(2):569–75. 10.1016/j.bbrc.2004.09.085 PubMed . [DOI] [PubMed] [Google Scholar]
- 66. Jiang T, Hou CC, She ZY, Yang WX. The SOX gene family: function and regulation in testis determination and male fertility maintenance. Molecular biology reports. 2013;40(3):2187–94. 10.1007/s11033-012-2279-3 PubMed . [DOI] [PubMed] [Google Scholar]
- 67. Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, et al. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis . Genetics. 2012;191(1):163–70. 10.1534/genetics.111.137497 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sawatari E, Shikina S, Takeuchi T, Yoshizaki G. A novel transforming growth factor-beta superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss). Dev Biol. 2007;301(1):266–75. 10.1016/j.ydbio.2006.10.001 PubMed . [DOI] [PubMed] [Google Scholar]
- 69. Shibata Y, Paul-Prasanth B, Suzuki A, Usami T, Nakamoto M, Matsuda M, et al. Expression of gonadal soma derived factor (GSDF) is spatially and temporally correlated with early testicular differentiation in medaka. Gene expression patterns: GEP. 2010;10(6):283–9. 10.1016/j.gep.2010.06.005 PubMed . [DOI] [PubMed] [Google Scholar]
- 70. Doniach T, Hodgkin J. A sex-determining gene, fem-1, required for both male and hermaphrodite development in Caenorhabditis elegans . Dev Biol. 1984;106(1):223–35. PubMed . [DOI] [PubMed] [Google Scholar]
- 71. Hodgkin J. Sex determination in the nematode C. elegans: analysis of tra-3 suppressors and characterization of fem genes. Genetics. 1986;114(1):15–52. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Krakow D, Sebald E, King LM, Cohn DH. Identification of human FEM1A, the ortholog of a C. elegans sex-differentiation gene. Gene. 2001;279(2):213–9. PubMed . [DOI] [PubMed] [Google Scholar]
- 73. Ventura-Holman T, Seldin MF, Li W, Maher JF. The murine fem1 gene family: homologs of the Caenorhabditis elegans sex-determination protein FEM-1. Genomics. 1998;54(2):221–30. 10.1006/geno.1998.5569 PubMed . [DOI] [PubMed] [Google Scholar]
- 74. Teaniniuraitemoana V, Huvet A, Levy P, Klopp C, Lhuillier E, Gaertner-Mazouni N, et al. Gonad transcriptome analysis of pearl oyster Pinctada margaritifera: identification of potential sex differentiation and sex determining genes. BMC genomics. 2014;15:491 10.1186/1471-2164-15-491 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jin S, Fu H, Zhou Q, Sun S, Jiang S, Xiong Y, et al. Transcriptome analysis of androgenic gland for discovery of novel genes from the oriental river prawn, Macrobrachium nipponense, using Illumina Hiseq 2000. PloS one. 2013;8(10):e76840 10.1371/journal.pone.0076840 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sun L, Wang C, Huang L, Wu M, Zuo Z. Transcriptome analysis of male and female Sebastiscus marmoratus . PloS one. 2012;7(11):e50676 10.1371/journal.pone.0050676 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Modig C, Modesto T, Canario A, Cerda J, von Hofsten J, Olsson PE. Molecular characterization and expression pattern of zona pellucida proteins in gilthead seabream (Sparus aurata). Biology of reproduction. 2006;75(5):717–25. 10.1095/biolreprod.106.050757 PubMed . [DOI] [PubMed] [Google Scholar]
- 78. Li CJ, Wei QW, Chen XH, Zhou L, Cao H, Gan F, et al. Molecular characterization and expression pattern of three zona pellucida 3 genes in the Chinese sturgeon, Acipenser sinensis . Fish physiology and biochemistry. 2011;37(3):471–84. 10.1007/s10695-010-9448-x PubMed . [DOI] [PubMed] [Google Scholar]
- 79. Liu Y, Zhou J, White KP. RNA-seq differential expression studies: more sequence or more replication? Bioinformatics. 2014;30(3):301–4. 10.1093/bioinformatics/btt688 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang SM, Yan Y, Deng H, Wang DQ, Wei QW, Wu QJ. Genome size, ploidy characters of several species of sturgeons and paddlefishes with comment on cellular evolution of Acipenseriformes (In Chinese). Acta Zool Sin. 1999;45(2):200–6. [Google Scholar]
- 81. Li YC, Korol AB, Fahima T, Beiles A, Nevo E. Microsatellites: genomic distribution, putative functions and mutational mechanisms: a review. Molecular ecology. 2002;11(12):2453–65. PubMed . [DOI] [PubMed] [Google Scholar]
- 82. Zhang SH, Luo H, Du H, Wang DQ, Wei QW. Isolation and characterization of twenty-six microsatellite loci for the tetraploid fish Dabry’s sturgeon (Acipenser dabryanus). Conservation Genet Resour. 2013;5:409–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Labels: O, pre-vitellogenic oocyte; PSC, primary spermatocyte; SG, spermatogonia.
(TIF)
Sequence Headers containing "type:complete" represent transcript sequences with complete ORFs.
(TXT)
Sequence Headers containing "type:complete" represent unigene sequences with complete ORFs.
(TXT)
(XLSX)
(XLSX)
(XLSX)
(DOCX)