Abstract
The purpose of this paper was to evaluate the levels of IL-9 in patients with SLE and RA compared with controls and the association of IL-9 levels with clinical and laboratory parameters. IL-9 levels were assessed in 117 SLE patients, 67 RA patients, and 24 healthy controls by ELISA. Clinical and laboratory parameters were recorded. The IL-9 serum levels were significantly higher in RA patients (4,77 ± 3,618 pg/mL) and in SLE patients (12,26 ± 25,235 pg/mL) than in healthy individuals (1,22 ± 0,706 pg/mL) (p < 0,001). In SLE patients, there were no statistically significant associations or correlations between the levels of IL-9 and SLEDAI or other clinical and laboratorial parameters, with the exception of disease time, which showed a statistically significant negative correlation with IL-9 levels (r = −0,1948; p = 0,0378). In RA patients, no association or statistically significant correlation was observed with disease duration, DAS28, HAQ, rheumatoid factor positivity, or erosions on radiography. These data demonstrated increased serum levels of IL-9 in SLE and RA patients, but further studies are needed to clarify the precise role of this cytokine and its potential use as therapeutic target.
1. Introduction
The imbalance between effector and regulatory cell populations (Treg) is of critical importance in the pathogenesis of various autoimmune disorders. According to the current paradigm, the proinflammatory axis of Th1 and Th17 cells is counter balanced by the cell populations Th2 cells and Treg [1].
Interleukin-9 (IL-9) is a member of the gamma-chain family of cytokines, first described as a member of a growing number of cytokines that have crucial roles in the development, proliferation, survival, and differentiation of multiple cell lineages of both the innate and adaptive immune systems [2].
IL-9 production was first associated with the Th2 phenotype, and many of the preliminary functions of IL-9 were tested in models of Th2-associated immunity. However, it is now known that, under specific conditions, regulatory (Tregs), Th1, and Th17 subset of T cells also express IL-9. Recently, it has been shown that IL-9-producing CD4+ T cells may represent the T helper subset Th9 cells. In vivo T cells produce IL-9 in both proinflammatory and anti-inflammatory environments [3].
There is some evidence to suggest that IL-9 production in Th17 cells is pathogenic during autoimmunity, particularly in type 1 diabetes, asthma, and experimental autoimmune encephalomyelitis (EAE) [4–9]. There are multiple cell types responsive to IL-9 including mast cells, T cells, antigen-presenting cells, and epithelial cells of the lung and the gut. In mast cells, IL-9 appears to promote their recruitment and expansion. IL-9 also appears to directly cause the production of TGFβ in antigen-presenting cells. This leads to a decrease in lipopolysaccharide-induced oxidative burst and to TNF-α release [10, 11]. In addition to indirect effects on T cells through antigen-presenting cells, IL-9 appears to increase the suppressive effect of Treg cells and enhance the proliferation and/or accumulation of Th17 cells [6].
Recently, evidence is emerging that inappropriate regulation of Th17 cells plays a fundamental role in the development of many autoimmune diseases, including systemic lupus erythematosus (SLE) [12, 13] and rheumatoid arthritis (RA) [14]. Since IL-9 is a cytokine related to Th17 pathway, it is believed that this cytokine may be also associated with the pathogenesis of these diseases. Nevertheless, few published studies have evaluated the role of IL-9 in patients with RA [15] or SLE [16]. The purpose of this paper was to evaluate the levels of IL-9 in patients with SLE and RA compared with controls and the association of IL-9 levels with clinical and laboratory parameters.
2. Patients and Methods
2.1. Patients
Serum samples were obtained from 117 patients with SLE and 67 patients with RA who fulfilled the classification criteria of the American College of Rheumatology (ACR) [17, 18], from the Department of Rheumatology at Hospital das Clínicas da Universidade Federal de Pernambuco (UFPE). The mean age of the patients with SLE was 37,5 ± 10,41 years (range: 19 to 61 years) and for those with RA was 52,6 ± 10,15 years (range: 28 to 75 years). About 24 healthy controls were recruited in the study (all females; mean age: 35,1 ± 9,138 years, range: from 20 to 55 years). Informed consent was obtained from each patient before being included in the study. The study was approved by the ethics committee of the UFPE.
2.2. Collection of Clinical and Laboratory Data
The demographic, clinical, and laboratory information of the patients with SLE such as age, disease duration, gender, medication use, activity (SLEDAI) and organ damage scores (SLICC), systemic involvement and number of classification criteria, complement levels, and autoantibodies positivity were collected at the time of blood sampling and are summarized in Table 1. For the RA patients, besides demographic data, tender joint count, swollen joint count, DAS28 and CDAI values, erythrocyte sedimentation rate (ESR), and presence of rheumatoid factor (RF) were collected and are summarized in Table 1.
Table 1.
Demographic, clinical, and laboratory data of SLE and RA patients and controls.
| SLE (n = 117) | % | RA (n = 67) | % | Controls (n = 24) | % | |
|---|---|---|---|---|---|---|
| Age (years, average ± SD) | 37,5 ± 10,41 | 52,65 ± 10,150 | 35,1 ± 9,138 | |||
| Disease time (months, average ± SD) | 105,48 ± 93,83 | 135,70 ± 103,39 | — | |||
| IL-9 levels (pg/mL) | 12,26 ± 25,235 | 4,77 ± 3,618 | 1,22 ± 0,706 | |||
| Gender | ||||||
| Female | 114 | 97,44 | 64 | 95,52 | 24 | 100 |
| Male | 3 | 2,56 | 3 | 4,48 | 0 | 0 |
| Active nephritis | 24 | 20,51 | — | — | — | — |
| Rash | 13 | 11,11 | — | — | — | — |
| Alopecia | 12 | 10,26 | — | — | — | — |
| Anti-DNA positive | 16 | 14,16 | — | — | — | — |
| SLEDAI | 3,31 ± 3,804 | |||||
| SLICC | 1,008 ± 1,276 | — | — | — | — | |
| Positive RF | — | — | 48 | 72,73 | — | — |
| Erosions∗ | — | — | 35 | 52,24 | — | — |
| DAS28 | — | — | 4,84 ± 1,353 | — | — | |
| CDAI | — | — | 20,75 ± 13,453 | — | — | |
| ESR (mm/h) | — | — | 41 ± 22,000 | — | — | |
| Treatment | — | — | ||||
| Corticosteroids | 90 | 77,6 | 51 | 76,1 | — | — |
| Antimalarials | 70 | 60,3 | 13 | 19,4 | — | — |
| Methotrexate | — | — | 44 | 65,7 | — | — |
| Leflunomide | — | — | 26 | 38,8 | — | — |
| Azathioprine | 38 | 32,8 | — | — | — | — |
| Mycophenolate mofetil | 12 | 10,3 | — | — | — | — |
| Biological therapy | — | — | 5 | 7,5 | — | — |
∗Analysis for 44 patients.
2.3. Measurement of Serum IL-9 Levels
Cytokines in the sera were assayed with ELISA kit according to the manufacturer's recommendation (eBiosciences). The lower limits of detection for ELISA IL-9 kit were 1 pg/mL.
2.4. Statistical Analysis
Associations of serum IL-9 levels with clinical and laboratory parameters of RA and SLE patients were analyzed by univariate comparisons using nonparametric tests (Mann-Whitney tests). p < 0.01 was considered as an indicator of a significant association and p < 0.05 as an indicator of a suggestive association. The results are shown considering the mean value. All quantitative data were plotted with Graph Pad Prism 3.02 software.
3. Results
Serum IL-9 levels were significantly higher in RA patients (4,77 ± 3,618 pg/mL) and in SLE patients (12,26 ± 25,235 pg/mL) than in healthy individuals (1,22 ± 0,706 pg/mL) (p < 0,001) (Figure 1). No difference was observed between the levels of IL-9 in patients with SLE and RA, although SLE patients presented higher levels (p = 0,195). There were no statistically significant associations or correlations between the levels of IL-9 and SLEDAI, number of ACR criteria, organ damage, clinical manifestations, complement consumption, and ANA or anti-DNA positivity, with the exception of disease time, which showed a statistically significant negative correlation with IL-9 levels (r = −0,1948; p = 0,0378) (Figure 2). Regarding the treatment, there were no statistical differences between the various regimens used in both diseases and serum levels of IL-9. In RA patients, no association or statistically significant correlation was observed with disease duration, DAS28, HAQ, rheumatoid factor positivity, or erosions on radiography (Figure 3).
Figure 1.
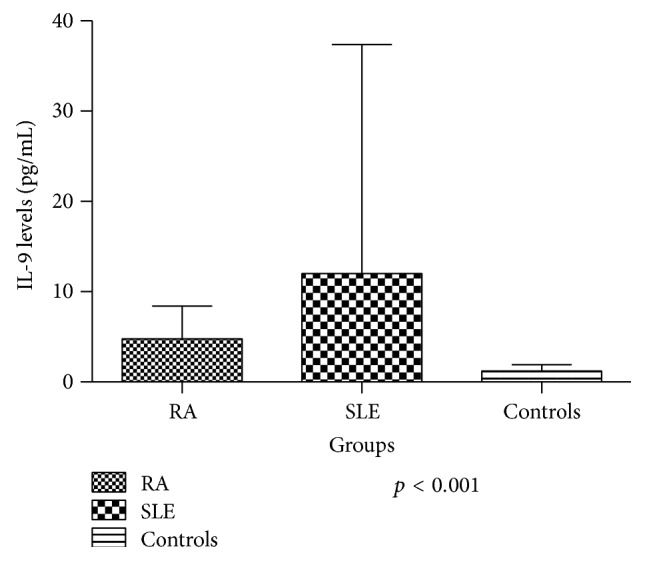
Comparison of the mean levels of IL-9 in patients with RA (n = 67), SLE (n = 117), and controls (n = 24).
Figure 2.
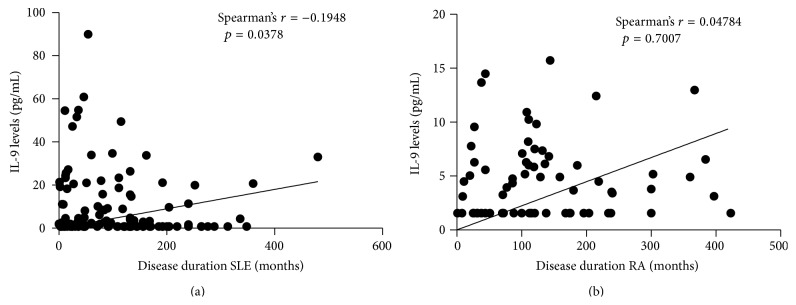
Correlation between disease duration and IL-9 levels in patients with SLE and RA.
Figure 3.
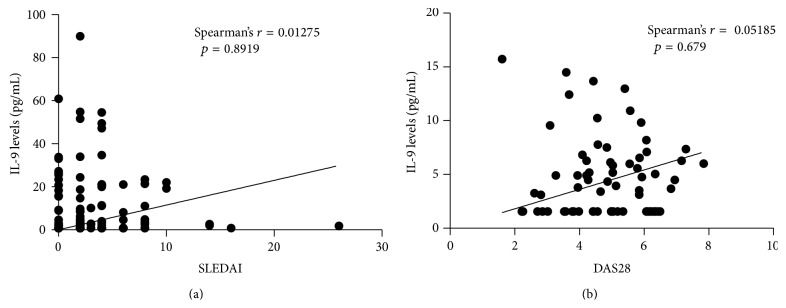
Correlation between disease activity and IL-9 levels in patients with SLE (a) and RA (b).
4. Discussion
The present study revealed that the IL-9 levels are significantly increased in SLE and RA patients compared to healthy controls; however, it has not demonstrated association with clinical or laboratory parameters in both diseases, with the exception of a negative correlation with disease duration in SLE patients.
Described for the first time in the late 1980s as a member of a growing number of cytokines that had pleiotropic functions in the immune system, it remains an understudied cytokine [19]. It has been most frequently associated with allergic inflammation [20, 21] and immunity to extracellular parasites [22, 23], although developing literature has demonstrated a role for IL-9 or IL-9-responsive cells in Th1/Th17-mediated inflammation and in T regulatory cell responses [7, 24]. The factors required for IL-9 production in T cells are being elucidated and require the integration of signals from multiple cytokines [19].
Very few studies in recent years have evaluated IL-9 in the context of rheumatic diseases such as SLE and RA. Hughes-Austin et al. [15] measured twenty-five cytokines/chemokines of first-degree relatives of RA probands, a population without RA but at increased risk for its future development. At this population, the levels of IL-9 were associated with rheumatoid factor or anti-CCP positivity. In another study, Ouyang et al. [16] showed that the plasma concentration and mRNA levels of IL-9 were significantly elevated in SLE patients compared with healthy controls and this elevation correlated with disease activity and severity. Additionally, the percentages of CD4+IL-9+ T cells and serum IL-9 levels in eight untreated active SLE patients were decreased at 1, 2, and 3 weeks after treatment with methylprednisolone, suggesting an important role of IL-9 in the pathogenesis of SLE. Burkhardt et al. [25] evaluated the association of X-chromosomal genes with RA. Two single-nucleotide polymorphisms (SNP) were associated with RA: TIMP1 that was associated with RA in general (p = 0.035) and IL-9 receptor (IL9R) that was associated with anti-CCP-positive RA patients (p = 0.037) and with male RA patients (p = 0.010).
Although it was originally defined as a Th2 cytokine, other T helper subsets also appear to have the potential for IL-9 production. Th17 cells, which are defined by secretion of IL-17A and IL-17F, may also secrete IL-9 in vitro and ex vivo [5, 6]. Human Th17 cells can secrete IL-9, and long-term Th17 cultures have the ability to coexpress IL-17A and IL-9 [4]. In contrast, IL-23, a cytokine required for maintenance of the IL-17-secreting phenotype, has inhibitory effects on IL-9 production [6]. T regulatory cells may also produce IL-9. A study linking mast cells to peripheral tolerance demonstrated that natural Tregs (nTregs) and inducible Tregs (iTregs), both Foxp3+ populations, secrete IL-9 [26]. There is conflicting evidence regarding production of IL-9 from human Treg cells [4].
Recently, the Th9 cells were described as a new subset of effector T cells that develop from naive T cells in the presence of transforming growth factor β (TGFβ) and interleukin-4 (IL-4). Cells cultured under these conditions are primed for the production of IL-9 and require transcription factors that include STAT6 (signal transducer and activator of transcription 6), PU.1, IRF4 (interferon response factor 4), and GATA3 [27–29]. Other recent finding is the concept that contrary to expectations based on previous results obtained in vitro, it is not the T cell the main cell type that expresses IL-9 in vivo [30] but a previously unknown type of innate lymphoid cell, called the “ILC2 cell.”
The established functions of Th9 cells have been divided into type II immunity, associated with Th2 cells, and autoimmunity, associated with Th1 and Th17 cells. It has not been established if there is any immune response that is strictly dependent on Th9 cells, or if IL-9-secreting T cells function in vivo as a helper cell of T helper cells. A recent Chinese study demonstrated that the expression of Th9 cells (CD3+CD8+IL-9+) in RA patients was significantly higher than in normal controls, and this expression was positively correlated with high disease activity, elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), number of tender joints, the number of swollen joints, and RF [31].
Pathogenic Th17 cells mediate pannus growth, osteoclastogenesis, and synovial neoangiogenesis in RA and the imbalance between Th17 and Treg cells has been identified as a crucial event in the pathogenesis of RA [32]. Since Th9 cells through IL-9 could mediate recruitment of autoimmune Th17 cells [33], this could be a possible explanation of the role of IL-9 in the pathogenesis of RA.
Some articles described IL-9 ability to promote the survival of a large number of different cell types, including T cells, mast cells and eosinophils, neurons, tumor, and epithelial cells [34–39]. Exposure of such effector cells to IL-9 either in vivo as a consequence of IL-2-mediated induction of IL-9 in ILC2 cells or during in vitro culture might result in improved survival, that could facilitate enhanced functional responses of all T cells that express IL-9 receptor and thus mediate a diversity of different functions. This can help to explain diverse IL-9-mediated phenotypes as well as enhanced antibody production by B cells [30]. Since SLE is a disorder mediated by autoantibodies, this could be an explanation for the negative correlation found between disease duration and levels of IL-9 in our study (higher levels of IL-9 in the onset of disease), since the early event in the disease process is the breakdown of B cell tolerance and enhanced autoantibodies production [40].
In a very recent work, Yang et al. [41] demonstrated increased IL-9 levels in the spleens and kidneys of MRL/lpr mice and this was closely related to the production of antibodies against double-stranded DNA (dsDNA). IL-9 appears to promote B cell proliferation and immunoglobulin production, which could be blocked by inhibition of signal transducer and activator of transcription 3 (STAT3). In vivo treatment with neutralizing anti-IL-9 antibody decreased serum anti-dsDNA antibody titers and alleviated lupus nephritis in MRL/lpr mice, suggesting that IL-9 is a potential therapeutic target for SLE.
Although IL-9 was discovered decades ago, it remains one of the most enigmatic cytokines identified so far, in particular because its functional activities remain far from clear, and the literature frequently provides conflicting results on efforts to identify additional functions for IL-9. Few studies have evaluated the expression of Th9 cells or the levels of IL-9 in patients with autoimmune diseases, and it is unclear if there is an association with the pathogenesis of these diseases or if it is just an epiphenomenon [31, 42]. As IL-9 is a pleiotropic cytokine, Th9 cells might contribute to both protective immunity and immunopathological disease through a myriad of pathways. However, it is important to remember that Th9 cells are not the only source of IL-9 [43]. In allergic diseases, IL-9 production can also occur in mast cells, in part through stimuli such as histamine, IL-1, antigen-specific immunoglobulin E and antigen, and IL-9 itself, and can help to facilitate their growth and expansion.
Further work would be needed to demonstrate the extent to which mast-cell-derived IL-9 can influence immune responses in comparison to that from various T cell subsets. Despite the broad ability of IL-9 to impact multiple types of inflammation, it is not clear how it functions in the context of autoimmune diseases. Some evidence suggests that it may have pathogenic role of this cytokine in SLE and RA. Further studies, with a larger number of patients, should be performed to evaluate not only its pathogenic role but also the possible therapeutic target in these diseases.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Andréa Tavares Dantas and Claudia Diniz Lopes Marques contributed equally to this work.
References
- 1.Siegmund K., Feuerer M., Siewert C., et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106(9):3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rochman Y., Spolski R., Leonard W. J. New insights into the regulation of T cells by gamma(c) family cytokines. Nature Reviews Immunology. 2009;9(7):480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stassen M., Schmitt E., Bopp T. From interleukin-9 to T helper 9 cells. Annals of the New York Academy of Sciences. 2012;1247(1):56–68. doi: 10.1111/j.1749-6632.2011.06351.x. [DOI] [PubMed] [Google Scholar]
- 4.Beriou G., Bradshaw E. M., Lozano E., et al. TGF-β induces IL-9 production from human Th17 cells. The Journal of Immunology. 2010;185(1):46–54. doi: 10.4049/jimmunol.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowak E. C., Weaver C. T., Turner H., et al. IL-9 as a mediator of Th17-driven inflammatory disease. The Journal of Experimental Medicine. 2009;206(8):1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elyaman W., Bradshaw E. M., Uyttenhove C., et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jäger A., Dardalhon V., Sobel R. A., Bettelli E., Kuchroo V. K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. The Journal of Immunology. 2009;183(11):7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visekruna A., Ritter J., Scholz T., et al. Tc9 cells, a new subset of CD8+ T cells, support Th2-mediated airway inflammation. European Journal of Immunology. 2013;43(3):606–618. doi: 10.1002/eji.201242825. [DOI] [PubMed] [Google Scholar]
- 9.Sismanopoulos N., Delivanis D. A., Alysandratos K. D., et al. IL-9 induces VEGF secretion from human mast cells and IL-9/IL-9 receptor genes are overexpressed in atopic dermatitis. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0033271.e33271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grohmann U., van Snick J., Campanile F., et al. IL-9 protects mice from gram-negative bacterial shock: suppression of TNF-alpha, IL-12, and IFN-gamma, and induction of IL-10. The Journal of Immunology. 2000;164(8):4197–4203. doi: 10.4049/jimmunol.164.8.4197. [DOI] [PubMed] [Google Scholar]
- 11.Pilette C., Ouadrhiri Y., van Snick J., et al. IL-9 inhibits oxidative burst and TNF-α release in lipopolysaccharide-stimulated human monocytes through TGF-β . The Journal of Immunology. 2002;168(8):4103–4111. doi: 10.4049/jimmunol.168.8.4103. [DOI] [PubMed] [Google Scholar]
- 12.Chen D.-Y., Chen Y.-M., Wen M.-C., Hsieh T.-Y., Hung W.-T., Lan J.-L. The potential role of Th17 cells and Th17-related cytokines in the pathogenesis of lupus nephritis. Lupus. 2012;21(13):1385–1396. doi: 10.1177/0961203312457718. [DOI] [PubMed] [Google Scholar]
- 13.Wong C. K., Lit L. C. W., Tam L. S., Li E. K. M., Wong P. T. Y., Lam C. W. K. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clinical Immunology. 2008;127(3):385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Kim J., Kang S., Kwon G., Koo S. Elevated levels of T helper 17 cells are associated with disease activity in patients with rheumatoid arthritis. Annals of Laboratory Medicine. 2013;33(1):52–59. doi: 10.3343/alm.2013.33.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes-Austin J. M., Deane K. D., Derber L. A., et al. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: studies of the Aetiology of Rheumatoid Arthritis (SERA) Annals of the Rheumatic Diseases. 2013;72(6):901–907. doi: 10.1136/annrheumdis-2012-201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang H., Shi Y., Liu Z., et al. Increased Interleukin-9 and CD4+IL-9+ T cells in patients with Systemic lupus erythematosus. Molecular Medicine Reports. 2013;7(3):1031–1037. doi: 10.3892/mmr.2013.1258. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism. 1997;40(9):p. 1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Arnett F. C., Edworthy S. M., Bloch D. A., et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Goswami R., Kaplan M. H. A brief history of IL-9. Journal of Immunology. 2011;186(6):3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Temann U. A., Ray P., Flavell R. A. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. TJournal of Clinical Investigation. 2002;109(1):29–39. doi: 10.1172/jci200213696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Temann U.-A., Geba G. P., Rankin J. A., Flavell R. A. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. The Journal of Experimental Medicine. 1998;188(7):1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes E. E., Groschwitz K., Abonia J. P., et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. The Journal of Experimental Medicine. 2008;205(4):897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faulkner H., Renauld J.-C., Van Snick J., Grencis R. K. Interleukin-9 enhances resistance to the intestinal nematode Trichuds muris. Infection and Immunity. 1998;66(8):3832–3840. doi: 10.1128/iai.66.8.3832-3840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan C., Aziz M. K., Lovaas J. D., et al. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. Journal of Immunology. 2010;185(11):6795–6801. doi: 10.4049/jimmunol.1001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkhardt J., Petit-Teixeira E., Teixeira V. H., et al. Association of the X-chromosomal genes TIMP1 and IL9R with rheumatoid arthritis. The Journal of Rheumatology. 2009;36(10):2149–2157. doi: 10.3899/jrheum.090059. [DOI] [PubMed] [Google Scholar]
- 26.Lu L.-F., Lind E. F., Gondek D. C., et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442(7106):997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 27.Dardalhon V., Awasthi A., Kwon H., et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nature Immunology. 2008;9(12):1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staudt V., Bothur E., Klein M., et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33(2):192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Veldhoen M., Uyttenhove C., van Snick J., et al. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature Immunology. 2008;9(12):1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm C., Turner J.-E., van Snick J., Stockinger B. The many lives of IL-9: a question of survival? Nature Immunology. 2012;13(7):637–641. doi: 10.1038/ni.2303. [DOI] [PubMed] [Google Scholar]
- 31.Ran C., Jian-Hua X. The abnormality and clinical significance of T helper 9 cells and interleukin-9 in patients with rheumatoid arthritis. Chinese Journal of Rheumatology. 2012;32(2) [Google Scholar]
- 32.Alunno A., Manetti M., Caterbi S., et al. Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediators of Inflammation. 2015;2015:12. doi: 10.1155/2015/751793.751793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt E., Klein M., Bopp T. Th9 cells, new players in adaptive immunity. Trends in Immunology. 2014;35(2):61–68. doi: 10.1016/j.it.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 34.van Snick J., Goethals A., Renauld J. C., et al. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40) The Journal of Experimental Medicine. 1989;169(1):363–368. doi: 10.1084/jem.169.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hultner L., Druez C., Moeller J., et al. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9) European Journal of Immunology. 1990;20(6):1413–1416. doi: 10.1002/eji.1830200632. [DOI] [PubMed] [Google Scholar]
- 36.Gounni A. S., Gregory B., Nutku E., et al. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood. 2000;96(6):2163–2171. [PubMed] [Google Scholar]
- 37.Fontaine R. H., Cases O., Lelièvre V., et al. IL-9/IL-9 receptor signaling selectively protects cortical neurons against developmental apoptosis. Cell Death and Differentiation. 2008;15(10):1542–1552. doi: 10.1038/cdd.2008.79. [DOI] [PubMed] [Google Scholar]
- 38.Knoops L., Renauld J.-C. IL-9 and its receptor: from signal transduction to tumorigenesis. Growth Factors. 2004;22(4):207–215. doi: 10.1080/08977190410001720879. [DOI] [PubMed] [Google Scholar]
- 39.Singhera G. K., MacRedmond R., Dorscheid D. R. Interleukin-9 and -13 inhibit spontaneous and corticosteroid induced apoptosis of normal airway epithelial cells. Experimental Lung Research. 2008;34(9):579–598. doi: 10.1080/01902140802369372. [DOI] [PubMed] [Google Scholar]
- 40.Anolik J. H. B cell biology: implications for treatment of systemic lupus erythematosus. Lupus. 2013;22(4):342–349. doi: 10.1177/0961203312471576. [DOI] [PubMed] [Google Scholar]
- 41.Yang J., Li Q., Yang X., Li M. Interleukin-9 is associated with elevated anti-double-stranded DNA antibodies in lupus-prone mice. Molecular Medicine. 2015 doi: 10.2119/molmed.2014.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramming A., Schulze-Koops H., Skapenko A. Th9 cells. Functionally important or just an epiphenomenon? Zeitschrift fur Rheumatologie. 2012;71(5):417–419. doi: 10.1007/s00393-011-0942-z. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan M. H., Hufford M. M., Olson M. R. The development and in vivo function of T helper 9 cells. Nature Reviews Immunology. 2015;15(5):295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]


