Abstract
Background
Strychnine-sensitive glycine receptors (GlyRs) are expressed throughout the brain and spinal cord and are among the strongly supported protein targets of alcohol. This is based largely on studies of the α1-subunit; however, α2- and α3-GlyR subunits are as or more abundantly expressed than α1-GlyRs in multiple forebrain brain areas considered to be important for alcohol-related behaviors, and uniquely some α3-GlyRs undergo RNA editing. Nanomolar and low micromolar concentrations of zinc ions potentiate GlyR function, and in addition to zinc’s effects on glycine-activated currents, we have recently shown that physiological concentrations of zinc also enhance the magnitude of ethanol (EtOH)’s effects on α1-GlyRs.
Methods
Using 2-electrode voltage-clamp electrophysiology in oocytes expressing either α2- or α3-GlyRs, we first tested the hypothesis that the effects of EtOH on α2- and α3-GlyRs would be zinc dependent, as we have previously reported for α1-GlyRs. Next, we constructed an α3P185L-mutant GlyR to test whether RNA-edited and unedited GlyRs contain differences in EtOH sensitivity. Last, we built a homology model of the α3-GlyR subunit.
Results
The effects of EtOH (20 to 200 mM) on both subunits were greater in the presence than in the absence of 500 nM added zinc. The α3P185L-mutation that corresponds to RNA editing increased sensitivity to glycine and decreased sensitivity to EtOH.
Conclusions
Our findings provide further evidence that zinc is important for determining the magnitude of EtOH’s effects at GlyRs and suggest that by better understanding zinc/EtOH interactions at GlyRs, we may better understand the sites and mechanisms of EtOH action.
Keywords: Glycine Receptor, Ethanol, Zinc
Although ethanol (EtOH) is a prevalently used drug, the sites and mechanisms of action by which it produces its intoxicating effects are not thoroughly understood. One widely accepted idea is that EtOH acts at protein targets in the body. Among the strongly supported protein targets of EtOH are strychnine-sensitive glycine receptors (GlyRs) (Harris et al., 2008), which belong to the Cys-loop superfamily of ligand-gated ion channels.
There are 4 GlyR alpha subunits (α1, α2, α3, and α4) and 1 GlyR beta subunit (Lynch, 2004). GlyRs can assemble to form α-homomeric or αβ-heteromeric chloride channels that mediate inhibition in the central nervous system (CNS). They are localized most abundantly in the spinal cord and brainstem (Legendre, 2001), but are also expressed throughout the brain (Baer et al., 2009; Jonsson et al., 2009, 2012).
GlyRs are not only modulated by alcohols, but also by volatile anesthetics, and inhaled drugs of abuse (Beckstead et al., 2002; Mihic et al., 1997). In addition to these exogenous agents, endogenous cations such as zinc also allosterically modulate GlyR function. Unlike the exogenous sedative hypnotics that strictly enhance GlyR function, zinc produces biphasic effects on GlyR function such that nanomloar and low micromolar concentrations enhance glycine-activated currents, whereas higher micromolar concentrations of zinc inhibit GlyR function (Bloomenthal et al., 1994; Laube et al., 2000; Miller et al., 2005a). In addition, recent findings in the GlyR α1-subunit show that zinc at physiological concentrations enhances the magnitude of EtOH’s effects on GlyR function (McCracken et al., 2010b, 2013).
Although the effects of EtOH on the α1-GlyR subunit have been relatively well studied, less work has focused on the GlyR α2-subunit, and presently, there are no published reports of EtOH modulation of the GlyR α3-subunit despite that both α2- and α3-GlyR subunits are expressed in a number of brain regions involved in EtOH-related behaviors (Jonsson et al., 2009, 2012). More specifically, in limbic and motivation centers of the brain, such as the amygdala and nucleus accumbens, studies of gene and receptor membrane expression demonstrate that there is as much or greater abundance of α2- and α3-subunits than α1-subunits in these regions (Delaney et al., 2010; Jonsson et al., 2009, 2012). Unlike GlyRs expressed in the spinal cord, which are predominantly α1β-heteromers, recent studies of GlyRs in brain suggest that they are likely to include populations of α2- or α3-homomers (Adermark et al., 2011; Chen et al., 2011; Eichler et al., 2009; Muller et al., 2008; Weltzien et al., 2012). Therefore, studies investigating these GlyRs are of physiological relevance.
In addition to differences in brain region expression among GlyR alpha subunits, there is emergent evidence suggesting functional differences. For example, subpopulations, particularly of α3-subunits, undergo RNA editing that results in α3P185L-receptors (Meier et al., 2005). These edited subunits confer high sensitivity to agonists such as glycine (Legendre et al., 2009), which may be important for tonic inhibition (Eichler et al., 2009). Recent studies of RNA editing focus on α3-GlyRs, but there is evidence for a similar posttranscriptional modification in α2-GlyRs (Eichler et al., 2008).
Due to their localization in limbic, motivation, and reward-related brain areas, thorough studies of the effects of EtOH at homomeric α2- and α3-GlyRs are necessary and relevant for better understanding the mechanisms by which EtOH exerts its physiological effects. In addition, because zinc exists in the CNS at tonic baseline nanomolar concentrations (Frederickson et al., 2006), which are sufficient for enhancement of GlyR function and critical for determining the magnitude of EtOH’s effects on α1-GlyRs (McCracken et al., 2010b), it is important to investigate the role of zinc in EtOH modulation of α2- and α3-GlyR subunits. Furthermore, studies of RNA-edited GlyRs in CNS inhibition (Eichler et al., 2009; Legendre et al., 2009; Meier et al., 2005) suggest that comparisons of EtOH sensitivity between edited and unedited receptors may be important for a thorough understanding of EtOH action.
In this study, we tested the hypothesis that the magnitude of EtOH’s effects on α2- and α3-GlyR subunits like α1-subunits would be enhanced in the presence of physiologically relevant (nanomolar) concentrations of zinc. Because the effects of EtOH on α3-GlyRs have not been previously studied, sensitivity to EtOH was first confirmed in this subunit. A secondary goal of this study was to determine whether or not there are differences in EtOH sensitivity between edited and unedited α3-GlyRs.
Materials and Methods
Materials
All chemical reagents (tricine, zinc chloride, EtOH, glycine) and modified Barth’s solution (MBS) buffer constituents (NaCl, KCl, NaHCO3, HEPES, MgSO4) were purchased from Sigma-Aldrich (St. Louis, MO). Xenopus laevis frogs were purchased from Xenopus Express (Brooksville, FL).
cDNA Constructs and Site-Directed Mutagenesis
GlyR α2 (human)- and α3 (rat)-subunit clones (both in pCis2 vectors) were obtained from Dr. N. Harrison (Columbia University, New York, NY) and Dr. H. Betz (Max-Planck Institute for Brain Research, Frankfurt, Germany), respectively. A mutant α3-GlyR was created by substituting the proline at position 185 with leucine to create an α3P185L-mutant GlyR that corresponds to RNA-edited GlyRs occurring in vivo. The mutagenesis reaction was carried out using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and commercially engineered mutagenic primers (Integrated DNA Technologies, San Diego, CA). Successful mutagenesis was confirmed using DNA sequencing at the University of Texas DNA Core Facility (Austin, TX).
Xenopus Oocyte Preparation
Partial ovariectomies were performed on sexually mature female X. laevis. Manual isolation of individual oocytes from harvested ovary fragments, cDNA injection of isolated oocytes, and incubation of isolated oocytes were performed as previously described (McCracken et al., 2010b). Electrophysiology recordings were performed approximately 1 to 7 days following cDNA injection.
Two-Electrode Voltage-Clamp Electrophysiology
For electrophysiology recordings, oocytes were impaled in the animal poles with 2 high-resistance (>1 MΩ) glass electrodes containing 3 M KCl and were voltage clamped at −70 mV using a Warner OC-725C oocyte clamp (Warner Instruments, Hamden, CT). A Masterflex USA peristalsis pump (Cole-Parmer Instrument Corporation, Vernon Hills, IL) was used to deliver MBS to oocytes via bath perfusion at a rate of 2 ml/min. Clamping currents were recorded on LabChart Pro software (Colorado Springs, CO), which was interfaced to the oocyte voltage-clamp apparatus via a PowerLab 4/30 data acquisition system (AD Instruments, Colorado Springs, CO).
Glycine Concentration–Response Curves
Glycine concentration–response curves were generated for oocytes expressing α2- and α3-GlyRs. For wild type (WT) receptors, a series of glycine concentrations (10 μM to 100 mM) were tested. Each respective concentration of glycine was applied for ~30 seconds and a 7-minute washout period followed each application. The concentration of glycine that elicited the largest response was determined to be maximal (Imax), and the effects of the remaining glycine concentrations were calculated and recorded as a percent of Imax. To determine the effects of zinc chelation on sensitivity to glycine, the same protocol was repeated in a buffer solution containing 10 mM tricine. Similarly, to determine the effects of added zinc on glycine responses, the same glycine response curve protocol was again repeated, but in the presence of 1 μM zinc, which produces a near maximal zinc enhancement of glycine-activated currents (Bloomenthal et al., 1994), added to the buffer solution. Because α3P185L-GlyRs are known to confer very high sensitivity to glycine (Legendre et al., 2009), this difference in glycine sensitivity between WT α3- and α3P185L-GlyRs was confirmed by generating concentration–response curves as described above. However, a wider range of glycine concentrations was tested (100 nM to 100 mM) due to published reports of edited α3-GlyRs conferring significantly higher affinity for glycine than WT channels (Legendre et al., 2009; Meier et al., 2005).
EtOH Sensitivity in the Absence and Presence of Zinc
Oocytes were first perfused with two ~15-second applications of 10 mM glycine each followed by a 7-minute washout period. The peak current elicited by the second application of glycine was considered to be Imax and was used to determine a concentration of glycine that produced ~5 to 10% (EC5-10) of the maximal glycinergic effect (α2: 110.8 ± 15 μM; α3: 182.2 ± 14.3 μM). This experimentally derived EC5-10 concentration of glycine was then applied, and following a 7-minute washout period, EtOH was first applied alone for 1 minute, and then, it was applied concurrently with the experimentally derived EC5-10 concentration of glycine for ~45 seconds. The EC5-10 concentration of glycine was again applied alone for 45 seconds following a 7-minute washout. This procedure was used to test the effects of a series of EtOH concentrations (20, 50, 100, and 200 mM). To determine the effects of chelating contaminating zinc on EtOH modulation of α2- and α3-GlyRs, we repeated the same protocol in the presence of 10 mM tricine, which was added to the buffer solution (as previously described in McCracken et al., 2010b). In addition, to test the hypothesis that added zinc would produce the opposite effect of a zinc chelator, we repeated the procedure for testing EtOH sensitivity a third time in a buffer solution containing 500 nM added zinc. In all 3 conditions, the effects of EtOH were determined as percent potentiation of the glycine EC5-10 response.
EtOH Sensitivity in Edited α3-GlyRs
To screen for potential differences in EtOH sensitivity between edited and unedited α3-GlyRs, the above protocol was used to test the effects of 20, 50, and 200 mM EtOH on WT α3- and mutant α3P185L-GlyRs in standard MBS and in MBS containing 500 nM added zinc.
Homology Modeling
A homology model of an α3-GlyR was built by threading 5 replicates of the primary sequence onto the X-ray structure template of the glutamate receptor chloride channel (GluCl; PDB ID: 3RHW) (Hibbs and Gouaux, 2011). The sequence of the intracellular loop (TM3-TM4) of the GlyR α3-subunit was edited to match that of the GluCl template. Then, 20 homology models were built with the Modeler module of Discovery Studio 3.1 (Accelrys Inc., San Diego, CA). We selected the “best” model based on total energy with the CHARMm force field. This pentameric model was refined with an extensive search for side chain rotamers with the Modeler module. The resulting model was assigned a mild harmonic restraint of 10 kcal/(mol × Å2) on only the backbone atoms and then was minimized to a gradient of 0.001 kcal/(mol × Å). The model was relaxed with 1,000,000 one fs steps of molecular dynamics at 300 K using the CHARMm force field and the same restraints. Next, this model was saved and a copy was “mutated” to have the P185L mutation in only a single GlyR α3-subunit. To investigate the local effect of the mutation at position 185, all atoms of both models were fixed in position except for residues 180 to 190 (loop 9), which were unrestrained. Both models were then examined with an additional 1,000,000 one fs steps of molecular dynamics as described above. Steps in the trajectory were saved every 10,000 steps to make an animation of the resulting changes. The final structures after both simulations were then minimized to a gradient of 0.001 kcal/(mol × Å) and were superimposed.
Data Analysis
Nonlinear regression analyses, using the following 4-parameter logistic equation for sigmoidal dose–response curves (with variable slopes), were performed to calculate glycine EC50 values and Hill coefficients for glycine concentration–response curves, and then, either 1-way analyses of variance (ANOVAs) (followed by Tukey’s post hoc analyses) or t-tests were used to determine significant differences in glycine EC50 and Hill slopes among the conditions tested.
Two-way ANOVAs followed by Bonferroni post hoc analyses were used to determine differences in EtOH sensitivity. Overall, statistical differences were determined at p < 0.05, and all analyses were conducted using GraphPad Prism software (San Diego, CA).
Results
Two-Electrode Voltage-Clamp Electrophysiology
For both WT α2- and α3-GlyRs, adding 1 μM zinc, which is an approximately maximally enhancing concentration (Bloomenthal et al., 1994), resulted in leftward shifts in the glycine concentration–response curves, whereas chelating nanomolar levels of contaminating zinc with tricine produced a rightward shift in the glycine concentration–response curve for α3-GlyRs only (Fig. 1). More specifically, the glycine EC50 in the presence of added zinc was significantly reduced compared with both the MBS and tricine conditions for α2-GlyRs, F(2, 15) = 15.54, p = 0.002, and α3-GlyRs, F(2, 18) = 656.0, p = 0.0001; however, the glycine EC50 was significantly increased in the presence of tricine for α3-GlyRs only, F(2, 18) = 656.0, p < 0.0001. The Hill slopes were not significantly different among any of the conditions for α2-GlyRs, F(2, 15) = 2.56, p = 0.11; however, significant differences were detected between the MBS and tricine conditions for α3-GlyRs, F(2, 18) = 5.26, p = 0.02. Neither adding nor removing zinc from our MBS buffers changed the maximal glycine-activated currents for either α2-GlyRs, F(2, 21) = 0.24, p = 0.79, or α3-GlyRs, F(2, 32) = 0.59, p = 0.56. All glycine EC50, Hill slope, and Imax values are presented in Tables 1 and 2.
Figure 1.
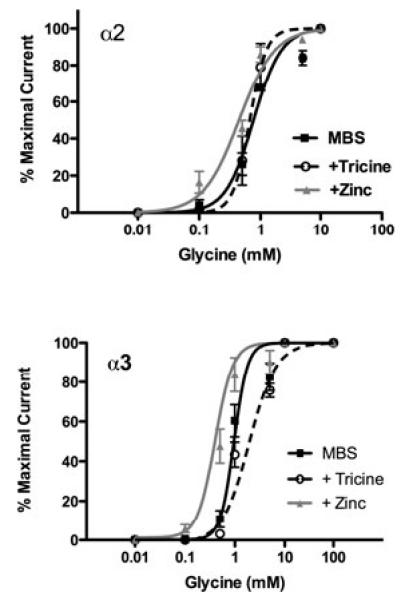
Glycine concentration–response curves were generated for α2- and α3-GlyRs. (A) The responses of α2-GlyRs to a series of glycine concentrations (10 μM to 10 mM) in standard modified Barth’s solution (MBS), MBS with 10 mM tricine, and MBS with 1 μM added zinc. In the added zinc condition, the glycine EC50 was significantly reduced compared with both the MBS and tricine conditions, F(2, 15) = 15.54, p = 0.002, indicating a leftward shift in the curve; however, no differences in glycine EC50 were detected between the MBS and tricine conditions, F(2, 15) = 15.54, Tukey’s post hoc: p > 0.05. The Hill slopes were not significantly different among any of the 3 conditions, F(2, 15) = 2.56, p = 0.111. Mean ± SEM represent data from 6 oocytes. (B) The responses of α3-GlyRs to glycine (10 μM to 100 mM) in MBS, MBS with 10 mM tricine, and MBS with 1 μM added zinc. Significant differences were detected among all 3 conditions such that adding zinc significantly reduced the glycine EC50, and tricine significantly increased the glycine EC50 indicating that added zinc resulted in a leftward shift in the responses to submaximal concentrations of glycine, whereas chelating low nanomolar contaminating zinc resulted in decreased sensitivity to submaximal concentrations of glycine, F(2, 18) = 656.0, Tukey’s post hoc: p < 0.0001 for both zinc and tricine. In addition, significant differences in the Hill slopes were detected between the MBS and tricine conditions, F(2, 18) = 5.26, Tukey’s post hoc: p = 0.016. Mean ± SEM represent data from 7 oocytes.
Table 1.
Glycine EC50, Hill Slopes, and Maximal Currents for α2-GlyRs
| α2 | MBS | +Tricine | +Zinc |
|---|---|---|---|
| EC50 (μM) | 769 ± 34 (n = 6) |
669 ± 39 (n = 6) |
446 ± 52*** (n = 6) |
| Hill slope | 1.91 ± 0.37 (n = 6) |
3.20 ± 0.85 (n = 6) |
1.49 ± 0.27 (n = 6) |
| Imax (nA) | 11,100 ± 1,420 (n = 8) |
9,910 ± 1,030 (n = 8) |
10,200 ± 1,540 (n = 8) |
Glycine concentration–response curves were generated for homomeric α2-GlyRs in each of 3 conditions: modified Barth’s solution (MBS), MBS with 10 mM tricine, and MBS with 1 μM zinc. The addition of zinc produced a leftward shift in the concentration–response curve significantly reducing the glycine EC50; however, the addition of the zinc chelator tricine did not produce a rightward shift in the curve and had no significant effect on the glycine EC50, F(2, 15) = 15.54, Tukey’s post hoc: p < 0.001 for zinc, p > 0.05 for tricine. There was no effect of adding or chelating zinc on either the Hill slopes, F(2, 15) = 2.55, p = 0.111, or on the maximal glycine-activated currents (Imax), F(2, 21) = 0.24, p = 0.785.
p < 0.001.
Table 2.
Glycine EC50, Hill Slopes, and Maximal Currents for α3-GlyRs
| α3 | MBS | +Tricine | +Zinc |
|---|---|---|---|
| EC50 (μM) | 962 ± 17 (n = 7) |
1,940 ± 39*** (n = 7) |
402 ± 31*** (n = 7) |
| Hill slope | 3.20 ± 0.48 (n = 7) |
1.59 ± 0.13* (n = 7) |
2.44 ± 0.35 (n = 7) |
| Imax (nA) | 8,152 ± 1,100 (n = 13) |
8,957 ± 970 (n = 19) |
7,354 ± 930 (n = 13) |
Glycine concentration–response curves were generated for homomeric α3-GlyRs in each of 3 conditions: modified Barth’s solution (MBS), MBS with 10 mM tricine, and MBS with 1 μM zinc. Adding zinc produced a leftward shift in the concentration–response curve significantly reducing the glycine EC50, whereas chelating low nanomolar zinc contamination produced a rightward shift significantly increasing the glycine EC50, F(2, 18) = 656.0, Tukey’s post hoc: p < 0.0001 for both zinc and tricine. In addition, tricine significantly decreased the Hill slope, F(2, 18) = 5.26, Tukey’s post hoc: p = 0.016. There was no effect of either adding or chelating zinc on the maximal glycine-activated currents (Imax), F(2, 32) = 0.59; p = 0.562.
p < 0.001,
p < 0.02.
We sought to test the hypothesis that zinc is critical in determining the magnitude of EtOH’s effects on α2- and α3-GlyRs, as we previously have shown for α1-GlyRs (McCracken et al., 2010b, 2013). First, we tested the potentiation of glycine EC5-10 responses by EtOH (20, 50, 100, and 200 mM) for α2-GlyRs in standard MBS and then subsequently in the presence of the zinc chelating agent tricine, as well as in the presence of 500 nM added zinc. Two-way ANOVA detected a significant interaction between zinc and EtOH concentration, F(6, 45) = 3.76, p = 0.0041, a significant main effect of zinc, F(2, 45) = 27.8, p < 0.0001, and a significant main effect of EtOH concentration, F(3, 45) = 12.1, p < 0.0001. Figure 2 shows that overall, the magnitude of EtOH enhancement of GlyR function was greatest in the presence of 500 nM added zinc, whereas it was least in the presence of the zinc chelating agent tricine. More specifically, Bonferroni post hoc analyses revealed that the effects of 50, 100, and 200 mM EtOH were significantly greater in the added zinc (+500 nM) condition versus no zinc (+tricine) condition (p < 0.05 for 50 mM; p < 0.0001 for 100 mM; p < 0.01 for 200 mM). In addition, comparisons of the MBS and added zinc conditions revealed that the addition of 500 nM zinc significantly enhanced potentiation of GlyR function by 100 mM EtOH (p < 0.001). Although chelation of contaminating zinc with tricine appears to decrease the magnitude of EtOH’s effects at α2-GlyRs, comparisons of the MBS and tricine conditions revealed no statistically significant differences.
Figure 2.
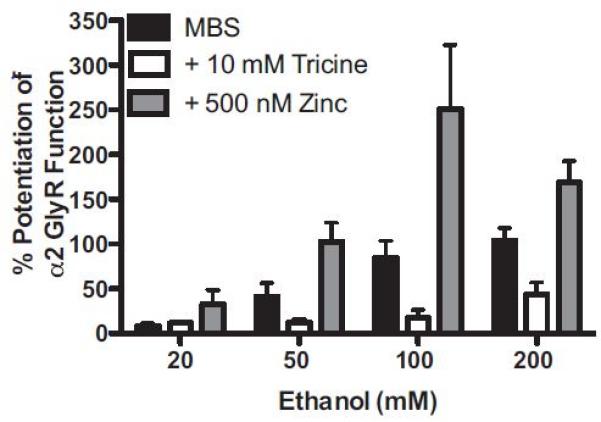
Potentiation of glycine EC5-10 responses by a series of ethanol (EtOH) concentrations (20, 50, 100, and 200 mM) was tested in standard modified Barth’s solution (MBS) (containing contaminating levels of nanomolar zinc), in MBS containing the zinc chelator tricine (no free zinc), and in MBS with 500 nM added zinc to determine whether modulation of α2-GlyRs by EtOH is zinc dependent. The magnitude of EtOH enhancement of GlyR function was greatest in the presence of 500 nM added zinc, whereas it was least in the presence of the zinc chelating agent tricine. Two-way ANOVA followed by Bonferroni post hoc analyses revealed a significant interaction between zinc and EtOH concentration, F(6, 45) = 3.76, p = 0.0041, a significant main effect of zinc, F(2, 45) = 27.8, p < 0.0001, and a significant main effect of EtOH concentration, F(3, 45) = 12.1, p < 0.0001. The effects of 50, 100, and 200 mM EtOH were significantly greater in the added zinc (+500 nM) condition versus no zinc (+tricine) condition (p < 0.05 for 50 mM; p < 0.0001 for 100 mM; p < 0.01 for 200 mM). Comparisons of the MBS and added zinc conditions revealed that the addition of 500 nM zinc significantly enhanced potentiation of GlyR function by 100 mM EtOH (p < 0.001) only. Although chelation of contaminating zinc with tricine appears to decrease the magnitude of EtOH’s effects at α2-GlyRs, comparisons of the MBS and tricine conditions revealed no statistically significant differences. Mean ± SEM represent data from 4 to 6 oocytes.
EtOH modulation of α3-GlyRs was previously unreported, so we first tested the hypothesis that the α3-GlyR subunit, like the α1- and α2-subunits, is sensitive to EtOH. Figure 3 shows that EtOH sensitivity was confirmed in α3-GlyRs. Potentiation of submaximal glycine responses (EC5-10) by EtOH (20, 50, 100, 200 mM) was measured in standard MBS (containing nanomolar zinc contamination), in MBS containing the zinc chelator tricine (no free zinc), and in MBS containing 500 nM added zinc to investigate whether or not the effects of EtOH are modulated by zinc as they are in α1- and α2-GlyRs. Two-way ANOVA detected a significant interaction between zinc and EtOH concentration, F(6, 33) = 4.51, p = 0.0019, a significant main effect of zinc, F(2, 33) = 83.9, p < 0.01, and a significant main effect of EtOH, F(3, 33) = 33.3, p < 0.0001. Overall, EtOH potentiation of α3-GlyR function was greatest in the presence of 500 nM added zinc, whereas it was smallest in the presence of the zinc chelating agent tricine. More specifically, Bonferroni post hoc analyses revealed that the effects of all 4 concentrations of EtOH were significantly greater in the zinc (+500 nM) versus no zinc (+tricine) condition (p < 0.05 for 20 mM; p < 0.0001 for 50 mM; p < 0.0001 for 100 mM; p < 0.0001 for 200 mM). In addition, comparisons of the MBS and added zinc conditions reveal that the addition of 500 nM zinc significantly enhanced potentiation of GlyR function by 50 (p < 0.0001), 100 (p < 0.001), and 200 (p < 0.001) mM EtOH. Although the zinc chelator tricine appears to reduce the magnitude of EtOH’s effects at α3-GlyRs, EtOH potentiation was only significantly reduced by tricine at 200 mM (p < 0.0001).
Figure 3.
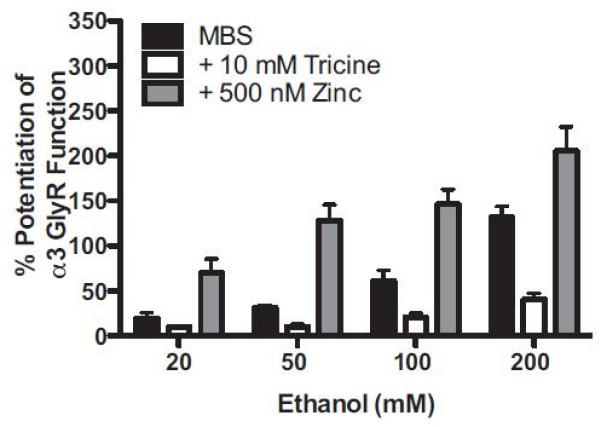
Ethanol (EtOH) sensitivity was confirmed in α3-GlyRs, and then the potentiation of submaximal glycine responses (EC5-10) by EtOH (20, 50, 100, 200 mM) was measured in standard modified Barth’s solution (MBS) (containing nanomolar zinc contamination), in MBS containing the zinc chelator tricine (no free zinc), and in MBS containing 500 nM added zinc to determine whether EtOH modulation of α3-GlyRs is zinc dependent. EtOH potentiation of GlyR function was greatest in the presence of 500 nM added zinc, whereas it was smallest in the presence of the zinc chelating agent tricine. Two-way ANOVA and Bonferroni post hoc analyses revealed a significant interaction between zinc and EtOH concentration, F(6, 33) = 4.51, p = 0.0019, a significant main effect of zinc, F(2, 33) = 83.9, p < 0.01, and a significant main effect of EtOH concentration, F(3, 33) = 33.3, p < 0.0001. The effects of all 4 concentrations of EtOH were significantly greater in the zinc (+500 nM) versus no zinc (+tricine) condition (p < 0.05 for 20 mM; p < 0.0001 for 50 mM; p < 0.0001 for 100 mM; p < 0.0001 for 200 mM). Comparisons of the MBS and added zinc conditions revealed that the addition of 500 nM zinc significantly enhanced potentiation of GlyR function by 50 (p < 0.0001), 100 (p < 0.001), and 200 (p < 0.001) mM EtOH. Although chelation of low nanomolar contaminating zinc with tricine appears to reduce the magnitude of EtOH’s effects at α3-GlyRs, a significant reduction in EtOH potentiation between the MBS and tricine conditions was detected only at the highest concentration tested, 200 mM (p < 0.0001). Mean ± SEM represent data from 4 to 6 oocytes.
Some α3-GlyRs undergo RNA editing resulting in α3P185L-GlyRs with high agonist sensitivity (Legendre et al., 2009; Meier et al., 2005). Our goal was to test for differences in EtOH sensitivity between WT and mutant α3P185L-GlyRs. First, we generated glycine concentration–response curves for both WT and mutant α3-GlyRs to confirm that our mutant contained high glycine sensitivity consistent with previous findings. Figure 4A shows the P185L mutation indeed resulted in GlyRs with leftward shifted glycine concentration–response curves and a significantly reduced glycine EC50, t(11) = 14.29, p < 0.0001 (Table 3). In addition, introduction of the mutation significantly reduced the Hill slope of the glycine concentration–response curve, t(11) = 3.86, p = 0.003. However, the maximal glycine-activated currents were not significantly different between WT and mutant α3-GlyRs, t(11) = 0.98, p = 0.35. Next, we tested for differences in EtOH sensitivity between WT and mutant α3-GlyRs (Fig. 4B). Two-way ANOVA detected a significant interaction between the mutation and EtOH concentration, F(2, 24) = 12.46, p < 0.0001. Post hoc analyses revealed that the effects of 200 mM EtOH were significantly reduced in mutant α3P185L-GlyRs (p < 0.001); however the effects of 20 and 50 mM were not significantly different from WT (p > 0.05; for 20 and 50 mM EtOH). The addition of 500 nM zinc resulted in a significant increase in the effects of 200 mM EtOH on α3P185L-GlyRs (p < 0.01); however, it had no effect on the other EtOH concentrations tested.
Figure 4.
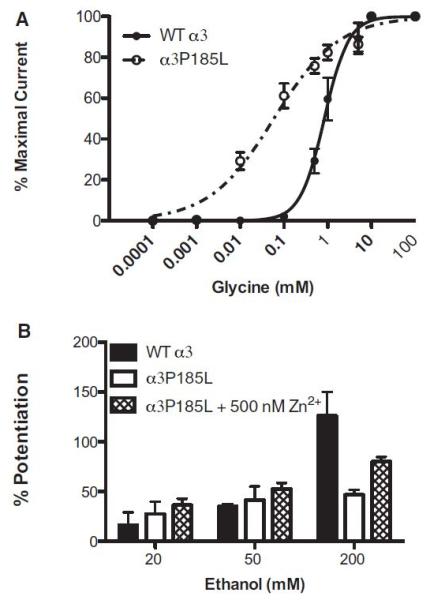
Glycine and ethanol (EtOH) sensitivity in wild type (WT) versus mutant α3P185L-GlyRs. (A) Glycine concentration–response curves were generated for mutant α3P185L- and WT α3-GlyRs. Responses to a series of glycine concentrations (100 nM to 100 mM) were tested. Mutation of the P185 position resulted in a leftward shift in the glycine concentration–response curve. Both the glycine EC50, t(11) = 14.29, p < 0.0001, and Hill slopes, t(11) = 3.86, p = 0.003, were significantly reduced in mutant α3-GlyRs. Mean ± SEM represent data from 6 to 7 oocytes. (B) Potentiation of submaximal glycine responses (EC5-10) by EtOH (20, 50, and 200 mM) was measured in WT and α3P185L-GlyRs. Two-way ANOVA detected a significant interaction between the mutation and EtOH concentration, F(2, 24) = 12.46, p < 0.0001. Post hoc analyses revealed that the effects of 200 mM EtOH were significantly reduced in mutant α3P185L-GlyRs (p < 0.001); however, the effects of 20 and 50 mM were not significantly different from WT (p > 0.05; for 20 and 50 mM EtOH). The addition of 500 nM zinc resulted in a significant increase in the effects of 200 mM EtOH on α3P185L-GlyRs (p < 0.01); however, it had no effect on the lower EtOH concentrations tested (for 20 and 50 mM: p > 0.05).
Table 3.
Glycine EC50, Hill Slopes, and Maximal Currents for α3P185L-GlyRs
| Subunit | EC50 | Hill slope | Imax (nA) |
|---|---|---|---|
| α3 (n = 6) | 837.1 ± 40 | 1.57 ± 0.27 | 7,715 ± 460 |
| α3P185L (n = 7) | 59.03 ± 7*** | 0.59 ± 0.47** | 6,814 ± 750 |
Glycine concentration–response curves were generated for α3-WT and mutant P185L GlyRs. Introduction of the P185L mutation, which corresponds to RNA editing that occurs in vivo, produced a leftward shift in the concentration–response curve significantly reducing the glycine EC50, t(8) = 14.29, p < 0.0001, and the Hill slope, t(11) = 3.86, p = 0.003. However, the mutation had no significant effect on the maximal glycine-activated currents, t(11) = 0.98, p = 0.4.
p < 0.0001,
p < 0.01.
To test for differences in EtOH sensitivity between α2- and α3-GlyRs, we measured the percent potentiation of glycine EC5-10 responses by a range of EtOH concentrations (20, 50, 100, and 200 mM) in the presence of tricine. The effects of EtOH were not significantly different for the 2 subunits in the absence of zinc (Fig. S1).
Homology Modeling
The GluCl X-ray structure served as a suitable template for a homology model of the GlyR α3-subunit as these proteins share considerable sequence identity and many key amino acid residues in GlyR are conserved in GluCl, including the signature Cys-loop cysteines. In our model (Fig. 5), we highlighted sites previously shown to mediate potentiation or inhibition of GlyR function by zinc ions (D80, H109, T112, T133, T151, E192, D194, H215), and as a control, we also identified only 2 residues, E192 and H215, shown to mediate inhibition of GlyRs by copper ions (Ruthstein et al., 2010). In addition to the amino acid residues important for the modulation of GlyRs by cations, we also labeled the proline residue at position 185, which resides among a segment of residues (180 to 190) located between beta strands 8 and 9. According to the nomenclature of the AChBP X-ray crystal structure, this region is designated as loop 9 (Brejc et al., 2001) and was of particular interest in this study because some α3-GlyRs undergo RNA editing at this site in vivo to produce α3P185L-GlyRs. Therefore, we also introduced the P185L mutation into our model, which resulted in small changes to the structure of loop 9. Displacement of residues is denoted as changes in color coding in the side chains in Fig. 5B,C.
Figure 5.
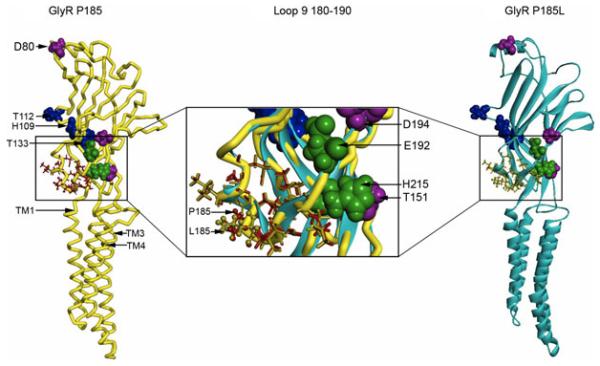
Homology models of wild type (WT) α3- and α3P185L-GlyR subunits were constructed with the X-ray structure of GluCl as a template. The backbone of the WT model is rendered as a yellow tube, whereas the P185L model is rendered as a flat cyan ribbon. (A) A model of a WT α3-GlyR subunit viewed from the plane of the membrane. The extracellular ligand-binding domain is above, and the transmembrane domain is below. The pore-lining alpha helix, TM2, is on the side away from the viewer. Metal-binding residues are rendered with a space-filling surface; zinc-potentiating sites are shown in pink, zinc inhibitory sites are shown in blue, and the sites inhibited by copper are shown green. All side chain residues of loop 9 (residues 180 to 190) are rendered as red sticks, except P185, which is a ball and stick. (B) The WT and P185L models were superimposed, and the regions shown in the boxes were expanded to reveal the changes in the final position of the residues in loop 9 after introducing the proline to leucine substitution at position 185. (C) A model of α3 P185L showing the relative changes in loop 9 after the molecular dynamics simulations.
Discussion
Zinc ions, which exist endogenously in the CNS at basal nanomolar concentrations (Frederickson et al., 2006), biphasically modulate GlyR function (Bloomenthal et al., 1994; Harvey et al., 1999; Laube et al., 2000). In this study, adding enhancing concentrations of zinc to our buffer solutions resulted in changes in the effects of submaximal but not maximal concentrations of glycine on α2- and α3-GlyRs, whereas chelating zinc with tricine only produced effects on the glycine responses of α3-GlyRs. This is consistent with previous findings that α2-GlyRs are less sensitive to nanomolar zinc enhancement than α1- or α3-GlyRs (Miller et al., 2005b).
We tested the hypothesis that the magnitude of EtOH’s effects on α2- and α3-GlyRs is facilitated by physiological zinc. Overall, EtOH potentiation of both α2- and α3-GlyR function was greater in the presence than in the absence of 500 nM added zinc. However, chelating contaminating zinc in our buffers, which we previously tested and reported to be less than 50 nM (McCracken et al., 2010b), resulted in significantly reduced effects of EtOH at α3-GlyRs, but only at the highest concentration tested, and had no significant effect on EtOH modulation of α2-GlyRs. This result is partially consistent with our previous findings in α1-GlyRs (McCracken et al., 2010a,b, 2013), but suggests that the low nanomolar concentrations of zinc that are suffice for enhancing EtOH modulation of α1-GlyRs are not adequate for α2- or α3-GlyRs as these particular GlyRs require higher nanomolar concentrations of zinc to augment EtOH’s effects.
The mechanisms for zinc modulation of GlyR function are not completely understood, but several amino acid residues located in the GlyR N-terminal domain are key for the effects of zinc on GlyR function (Table 4). The potentiating effects of zinc require high-affinity binding to amino acids distinct from those required for zinc inihibiton (Laube et al., 2000; Miller et al., 2005a, b). Alignment of the amino acid sequences for the GlyR α1-, α2-, and α3-subunits reveals conservation of most, but not all positions that are important for zinc modulation (Table 4). For example, H107 in the α1-subunit is not conserved at the homologous positions in α2 and α3, which are N114 and N107, respectively. The significance of this is that α1-GlyRs are more sensitive to zinc inhibition than α2- or α3-GlyRs (Miller et al., 2005a), and therefore, in the current study, we were able to measure the effects of higher nanomolar enhancing concentrations of zinc on EtOH modulation without the onset of zinc inhibition occluding our results. In addition, D194 in α1 and α3 is not conserved at the homologous position in α2, which is E201. The importance of this site is that α2-GlyRs are less sensitive to the potentiating effects of zinc than α1- or α3-GlyRs (Miller et al., 2005b), which is consistent with our finding that tricine did not right shift the glycine concentration–response curve of α2-GlyRs. Despite these differences in zinc modulation of GlyR alpha subunits, it is important to note that nanomolar concentrations of zinc, albeit higher for α2 and α3 than α1, enhance EtOH’s effects at all 3 alpha subunits expressed in brain.
Table 4.
Amino Acid Residues Important for Zinc Modulation of GlyR Function
| GlyR | Potentiating sites | Inhibitory sites | |||||||
|---|---|---|---|---|---|---|---|---|---|
| α1 | D80 | T151 | E192 | D194 | H215 | H107* | H109 | T112 | T133 |
| α2 | D87 | T158 | E199 | E201* | H222 | N114 | H116 | T119 | T140 |
| α3 | D80 | T151 | E192 | D194 | H215 | N107 | H109 | T112 | T133 |
Summary of the amino acid residues important for zinc action at GlyRs. Alignment of the amino acid sequences for the α1-, α2-, and α3-GlyR subunits reveals that of the known putative zinc-binding sites at the GlyR, all are conserved in these 3 subunits except for 2 positions (*), H107 in α1 and E201 in α2. Each of the amino acid residues listed has been shown to be important either for high-affinity zinc binding and enhancement of GlyR function or for lower affinity zinc binding and inhibition of GlyR function (Laube et al., 2000; Miller et al., 2005a,b).
Findings from a series of studies in the α1-GlyR subunit suggest an alcohol and anesthetic binding pocket within the transmembrane domain of each subunit (Lobo et al., 2004, 2006, 2008; Mascia et al., 2000; McCracken et al., 2010a; Mihic et al., 1997). Additional sites important for alcohol action on the α1-GlyR have also been suggested. These include residues in loop 2 of the N-terminal domain (Crawford et al., 2008; Davies et al., 2004; Mascia et al., 1996; Perkins et al., 2009, 2012), as well as residues of the large intracellular loop linking TM3 and TM4 (Yevenes et al., 2008).
In this study, we demonstrated that α3-GlyRs are sensitive to EtOH and that this effect is zinc dependent. We also tested α3-GlyRs for differences in EtOH sensitivity between unedited and RNA-edited receptors using the α3P185L-mutant GlyR that corresponds to RNA-edited receptors occurring in vivo (Meier et al., 2005). Although there were no differences in the effects of low concentrations of EtOH (20 and 50 mM), the effects of 200 mM EtOH were reduced in mutant α3P185L-GlyRs. In addition, added zinc had no effect on the effects of 20 or 50 mM EtOH, but did enhance the effects of 200 mM EtOH on P185L GlyRs. Previous findings and ours show that the P185L receptors have higher affinity for glycine than unmodified α3-GlyRs (Eichler et al., 2009; Legendre et al., 2009; Meier et al., 2005). Alignment of the GlyR α3-subunit amino acid sequence with other members of the Cys-loop family reveals that the only other subunit in this family that endogenously contains a leucine at the position homologous to 185 in GlyR α3 is the GABAA receptor α6-subunit. However, there is functional homology at this position with the GABAA receptor α4- and δ-subunits, which also contain aliphatic residues, valine and alanine, respectively, at this position. These GABAA receptor subunits have high agonist sensitivity, mediate extrasynaptic tonic inhibition (Belelli et al., 2009), and may contain increased sensitivities to low concentrations of EtOH relative to other GABAA receptors (Borghese et al., 2006; Wallner et al., 2003, 2006).
In our homology model, we constructed a representative α3-GlyR subunit using the GluCl X-ray crystal structure as a template (Fig. 5). First, we highlighted several amino acid residues (D80, H109, T112, T133, T151, E192, D194, H215) that others have shown to be important for the modulation of GlyR function by divalent cations, specifically zinc and copper (Laube et al., 2000; Miller et al., 2005a,b; Ruthstein et al., 2010). Interestingly, 2 of these highlighted sites, E192 and H215, have been implicated in both the actions of zinc and copper ions at GlyRs, are proximal, and in an ideal position to chelate a metal ion. Second, we introduced the α3P185L-mutation into our model, which corresponds to RNA-edited α3-GlyRs in vivo. Residue P185 is located near the apex of loop 9 and is positioned to interact with other loops at the interface between the ligand-binding and transmembrane domains including the TM2-3 linker (Kash et al., 2003). Overall, the P185L mutation only modestly perturbed the structure of loop 9, and this is consistent with the effect of other mutations at the ligand-binding domain and transmembrane interface (Kash et al., 2003; Perkins et al., 2012).
Our findings, which demonstrate zinc-dependent modulation of α2- and α3-GlyRs, in combination with evidence from studies of GlyR gene and receptor membrane expression support future investigations of the sites and mechanisms of action of EtOH to include α2- and α3-GlyRs. Of the GlyR alpha subunits present in brain, the α2-subunit predominates in limbic and reward centers, such as the nucleus accumbens and amygdala where α3-GlyRs are also expressed (Jonsson et al., 2009, 2012). In addition, membrane expression of α2-subunit protein is the highest of the GlyR alpha subunits expressed in the amygdala, suggesting that there is a greater abundance of receptors not just higher mRNA levels (Delaney et al., 2010).
In conclusion, we demonstrate that like α1- and α2-GlyRs, α3-GlyRs are modulated by EtOH and show that the effects of EtOH on α2- and α3-GlyRs are zinc dependent. Our findings suggest that accounting for the effects of zinc ions on EtOH action at GlyRs is important for understanding the sites and mechanisms of alcohol action as this may ultimately help contribute to improved treatments for alcoholism by specifically targeting α2- and α3-GlyRs.
Supplementary Material
Supplemental Figure 1. Comparison of ethanol sensitivity between homomeric α2 and α3 GlyRs Potentiation of glycine EC5-10 responses by a series of ethanol concentrations (20, 50, 100, and 200 mM) was tested in the presence of the zinc chelator tricine in order to eliminate zinc sensitivity as a confounding variable. There were no significant differences in ethanol modulation between α2 and α3 GlyRs [for 20 mM: t(6)= 0.86; p= 0.42; for 50 mM: t(7)= 0.42; p= 0.69; for 100 mM: t(5)= 0.31, p= 0.77; for 200 mM: t(9)= 0.25, p= 0.80]. Mean± SEM represents data from 4-6 oocytes.
Acknowledgments
This work was funded by 5R01AA006399 (RAH), 5R01AA013378 (JRT), and 1F31AA019852 (LMM).
References
- 1.Adermark L, Clarke RB, Ericson M, Soderpalm B. Subregion-specific modulation of excitatory input and dopaminergic output in the striatum by tonically activated glycine and GABA(A) receptors. Front Syst Neurosci. 2011;5:85. doi: 10.3389/fnsys.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baer K, Waldvogel HJ, Faull RL, Rees MI. Localization of glycine receptors in the human forebrain, brainstem, and cervical spinal cord: an immunohistochemical review. Front Mol Neurosci. 2009;2:25. doi: 10.3389/neuro.02.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckstead MJ, Phelan R, Trudell JR, Bianchini MJ, Mihic SJ. Anesthetic and ethanol effects on spontaneously opening glycine receptor channels. J Neurochem. 2002;82:1343–1351. doi: 10.1046/j.1471-4159.2002.01086.x. [DOI] [PubMed] [Google Scholar]
- 4.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloomenthal AB, Goldwater E, Pritchett DB, Harrison NL. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+ Mol Pharmacol. 1994;46:1156–1159. [PubMed] [Google Scholar]
- 6.Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- 7.Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 8.Chen RQ, Wang SH, Yao W, Wang JJ, Ji F, Yan JZ, Ren SQ, Chen Z, Liu SY, Lu W. Role of glycine receptors in glycine-induced LTD in hippocampal CA1 pyramidal neurons. Neuropsychopharmacology. 2011;36:1948–1958. doi: 10.1038/npp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford DK, Perkins DI, Trudell JR, Bertaccini EJ, Davies DL, Alkana RL. Roles for loop 2 residues of alpha1 glycine receptors in agonist activation. J Biol Chem. 2008;283:27698–27706. doi: 10.1074/jbc.M802384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies DL, Crawford DK, Trudell JR, Mihic SJ, Alkana RL. Multiple sites of ethanol action in alpha1 and alpha2 glycine receptors suggested by sensitivity to pressure antagonism. J Neurochem. 2004;89:1175–1185. doi: 10.1111/j.1471-4159.2004.02390.x. [DOI] [PubMed] [Google Scholar]
- 11.Delaney AJ, Esmaeili A, Sedlak PL, Lynch JW, Sah P. Differential expression of glycine receptor subunits in the rat basolateral and central amygdala. Neurosci Lett. 2010;469:237–242. doi: 10.1016/j.neulet.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Eichler SA, Forstera B, Smolinsky B, Juttner R, Lehmann TN, Fahling M, Schwarz G, Legendre P, Meier JC. Splice-specific roles of glycine receptor alpha3 in the hippocampus. Eur J Neurosci. 2009;30:1077–1091. doi: 10.1111/j.1460-9568.2009.06903.x. [DOI] [PubMed] [Google Scholar]
- 13.Eichler SA, Kirischuk S, Juttner R, Schafermeier PK, Legendre P, Lehmann TN, Gloveli T, Grantyn R, Meier JC. Glycinergic tonic inhibition of hippocampal neurons with depolarizing GABAergic transmission elicits histopathological signs of temporal lobe epilepsy. J Cell Mol Med. 2008;12:2848–2866. doi: 10.1111/j.1582-4934.2008.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Muelle RN, Zeng Y, Balaji RV, Masalha R, Thompson RB, Fierke CA, Sarvey JM, de Valdenebro M, Prough DS, Zornow MH. Concentrations of extracellular free zinc (pZn)(e) in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol. 2006;198:285–293. doi: 10.1016/j.expneurol.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Harris RA, Trudell JR, Mihic SJ. Ethanol’s molecular targets. Sci Signal. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor alpha1 subunit. J Physiol. 1999;520:53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonsson S, Kerekes N, Hyytia P, Ericson M, Soderpalm B. Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res. 2009;1305(Suppl):S27–S36. doi: 10.1016/j.brainres.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson S, Morud J, Pickering C, Adermark L, Ericson M, Soderpalm B. Changes in glycine receptor subunit expression in forebrain regions of the Wistar rat over development. Brain Res. 2012;1446:12–21. doi: 10.1016/j.brainres.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 20.Kash TL, Jenkins A, Kelley JC, Trudell JR, Harrison NL. Coupling of agonist binding to channel gating in the GABA(A) receptor. Nature. 2003;421:272–275. doi: 10.1038/nature01280. [DOI] [PubMed] [Google Scholar]
- 21.Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol. 2000;522:215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58:760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legendre P, Forstera B, Juttner R, Meier JC. Glycine receptors caught between genome and proteome—functional implications of RNA editing and splicing. Front Mol Neurosci. 2009;2:23. doi: 10.3389/neuro.02.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobo IA, Harris RA, Trudell JR. Cross-linking of sites involved with alcohol action between transmembrane segments 1 and 3 of the glycine receptor following activation. J Neurochem. 2008;104:1649–1662. doi: 10.1111/j.1471-4159.2007.05090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobo IA, Mascia MP, Trudell JR, Harris RA. Channel gating of the glycine receptor changes accessibility to residues implicated in receptor potentiation by alcohols and anesthetics. J Biol Chem. 2004;279:33919–33927. doi: 10.1074/jbc.M313941200. [DOI] [PubMed] [Google Scholar]
- 26.Lobo IA, Trudell JR, Harris RA. Accessibility to residues in transmembrane segment four of the glycine receptor. Neuropharmacology. 2006;50:174–181. doi: 10.1016/j.neuropharm.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 28.Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996;50:402–406. [PubMed] [Google Scholar]
- 29.Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci U S A. 2000;97:9305–9310. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCracken LM, Blednov YA, Trudell JR, Benavidez JM, Betz H, Harris RA. Mutation of a zinc-binding residue in the glycine receptor alpha1 subunit changes ethanol sensitivity in vitro and alcohol consumption in vivo. J Pharmacol Exp Ther. 2013;344:489–500. doi: 10.1124/jpet.112.197707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCracken LM, McCracken ML, Gong DH, Trudell JR, Harris RA. Linking of glycine receptor transmembrane segments three and four allows assignment of intrasubunit-facing residues. ACS Chem Neurosci. 2010a;1:482. doi: 10.1021/cn100019g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCracken LM, Trudell JR, Goldstein BE, Harris RA, Mihic SJ. Zinc enhances ethanol modulation of the alpha1 glycine receptor. Neuropharmacology. 2010b;58:676–681. doi: 10.1016/j.neuropharm.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier JC, Henneberger C, Melnick I, Racca C, Harvey RJ, Heinemann U, Schmieden V, Grantyn R. RNA editing produces glycine receptor alpha3(P185L), resulting in high agonist potency. Nat Neurosci. 2005;8:736–744. doi: 10.1038/nn1467. [DOI] [PubMed] [Google Scholar]
- 34.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 35.Miller PS, Beato M, Harvey RJ, Smart TG. Molecular determinants of glycine receptor alphabeta subunit sensitivities to Zn2+-mediated inhibition. J Physiol. 2005a;566(Pt 3):657–670. doi: 10.1113/jphysiol.2005.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller PS, Da Silva HM, Smart TG. Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J Biol Chem. 2005b;280:37877–37884. doi: 10.1074/jbc.M508303200. [DOI] [PubMed] [Google Scholar]
- 37.Muller E, Le-Corronc H, Legendre P. Extrasynaptic and postsynaptic receptors in glycinergic and GABAergic neurotransmission: a division of labor? Front Mol Neurosci. 2008;1:3. doi: 10.3389/neuro.02.003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perkins DI, Trudell JR, Asatryan L, Davies DL, Alkana RL. Charge and geometry of residues in the loop 2 beta hairpin differentially affect agonist and ethanol sensitivity in glycine receptors. J Pharmacol Exp Ther. 2012;341:543–551. doi: 10.1124/jpet.111.190942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkins DI, Trudell JR, Crawford DK, Asatryan L, Alkana RL, Davies DL. Loop 2 structure in glycine and GABA(A) receptors plays a key role in determining ethanol sensitivity. J Biol Chem. 2009;284:27304–27314. doi: 10.1074/jbc.M109.023598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruthstein S, Stone KM, Cunningham TF, Ji M, Cascio M, Saxena S. Pulsed electron spin resonance resolves the coordination site of Cu(2)(+) ions in alpha1-glycine receptor. Biophys J. 2010;99:2497–2506. doi: 10.1016/j.bpj.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on alpha4beta3delta GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci U S A. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weltzien F, Puller C, O’Sullivan GA, Paarmann I, Betz H. Distribution of the glycine receptor beta-subunit in the mouse CNS as revealed by a novel monoclonal antibody. J Comp Neurol. 2012;520:3962–3981. doi: 10.1002/cne.23139. [DOI] [PubMed] [Google Scholar]
- 44.Yevenes GE, Moraga-Cid G, Peoples RW, Schmalzing G, Aguayo LG. A selective G betagamma-linked intracellular mechanism for modulation of a ligand-gated ion channel by ethanol. Proc Natl Acad Sci U S A. 2008;105:20523–20528. doi: 10.1073/pnas.0806257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Comparison of ethanol sensitivity between homomeric α2 and α3 GlyRs Potentiation of glycine EC5-10 responses by a series of ethanol concentrations (20, 50, 100, and 200 mM) was tested in the presence of the zinc chelator tricine in order to eliminate zinc sensitivity as a confounding variable. There were no significant differences in ethanol modulation between α2 and α3 GlyRs [for 20 mM: t(6)= 0.86; p= 0.42; for 50 mM: t(7)= 0.42; p= 0.69; for 100 mM: t(5)= 0.31, p= 0.77; for 200 mM: t(9)= 0.25, p= 0.80]. Mean± SEM represents data from 4-6 oocytes.


