Abstract
Type 1 diabetes (T1D) is an autoimmune disease with a prolonged and variable latent period that culminates in the destruction of pancreatic β-cells and the development of hyperglycemia. There is a need for diagnostic biomarkers to detect more accurately detect individuals with prediabetes to expedite targeting for prevention and intervention strategies. To assess the current ability to predict the insidious development of T1D, we conducted a comprehensive systematic review for established and prospective predictive markers of T1D using the Medline, OVID, and EMBASE databases. Resulting citations were screened for relevance to subject. Our research generated five major categories of markers that are either currently used or forthcoming: genetic, autoantibodies, risk score quantification, cellular immunity, and β-cell function. The current standard used to assess T1D onset or predisposition focuses on autoimmune pathology and disease-associated autoantibodies. Research studies in general go beyond autoantibody screening and assess genetic predisposition, and quantitate risk of developing disease based on additional factors. However, there are few currently used techniques that assess the root of T1D: β-cell destruction. Thus, novel techniques are discussed with the potential to gauge degrees of β-cell stress and failure via protein, RNA, and DNA analyses.
The discovery and subsequent development of recombinant human insulin, designer insulin analogs, sophisticated insulin pumps, and sensors as therapies for type 1 diabetes (T1D) represent collectively a remarkable therapeutic achievement. However, insulin is not a cure, and T1D remains an irreversible and progressive disease with life-threatening complications. In light of this, there remains a tremendous need for continued research into primary and secondary prevention strategies as well as improvements in treatment modalities.
One major hindrance in the design of effective prevention studies is the lack of precise biological measures to detect individuals early during their course of β-cell decompensation. The latent period in the progression to T1D can be defined as the period during which indolent β-cell immune destruction has started, yet adequate residual insulin-secreting β-cells remain to prevent overt hyperglycemia. This period is clinically silent and characterized by β-cell stress, β-cell destruction, insulitis, and autoimmunity.1,2 During the preclinical stage of progression to T1D, there is an associated decrease in β-cell function as measured by β-cell glucose sensitivity during oral glucose tolerance testing.3 The length of this “prediabetic” period is unpredictable, sometimes lasting for just a few months and sometimes spanning many years.4
Before the implementation of prevention modalities, especially those that have significant risks, biomarkers and diagnostic tests that can indicate accurately the impending development and progression of T1D need to be established. These tests may also shed light on novel mechanisms leading to the development of T1D. Risk scores have been developed to determine which antibody (Ab)-positive relatives of persons with T1D are most likely to develop T1D during the subsequent 5 years, yet the positive predictive power of these scores before the development of dysglycemia remains insufficient to provide a precise prognosis for a given individual.5 Yet, it is during this time that immunomodulatory therapies targeted to prevent irreversible loss of β-cells may be most efficacious. Clinical intervention trials have been most successful in individuals with greater residual insulin production and those with the least time since disease onset.6–8
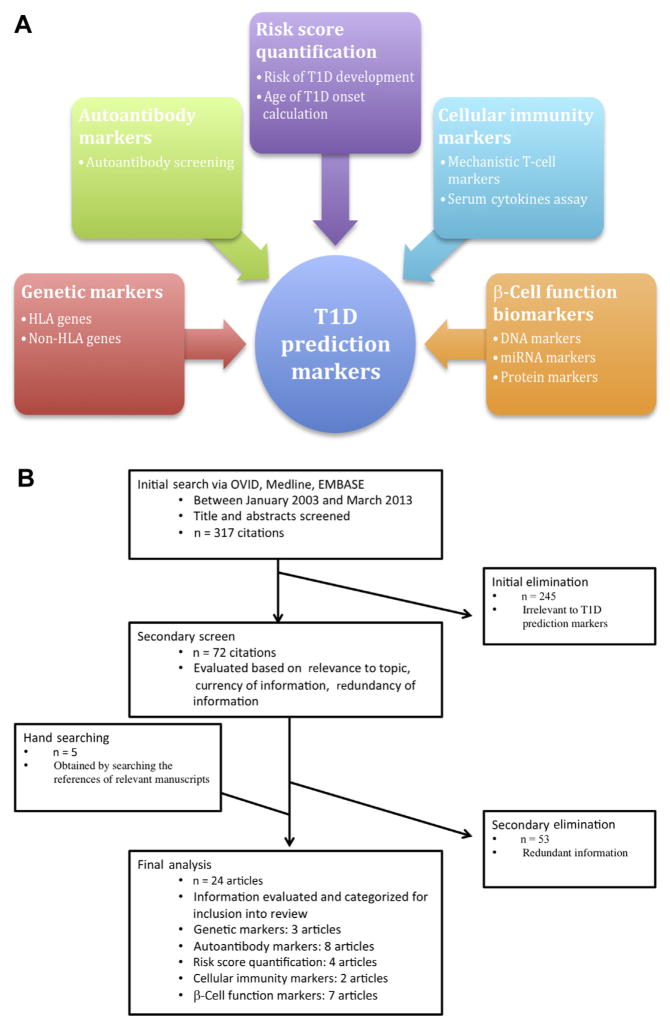
This systematic review aims to highlight established and emerging biomarkers in the detection of incipient T1D. Using a systematic literature review, we identified an array of available or emerging biomarkers to detect β-cell autoimmunity or loss of β-cell mass. These include genetic markers, autoantibodies, cellular immunity markers, risk score quantification techniques, and β-cell stress markers (Fig 1, A).
Fig 1.
(A) Depiction of predictive markers in the type 1 diabetes (T1D) field. (B) Flowchart presentation of the selection method used for identifying articles in the systematic review. HLA, human leukocyte antigen; miRNA, microRNA.
REVIEW METHODS
We searched Medline, OVID, and EMBASE for English-language articles published between January 2003 and March 2013. We used the following phrases: predictive type 1 diabetes markers, detection of β-cell death, biomarkers in type 1 diabetes, and immune auto-antibody markers type 1 diabetes. Two reviewers conducted these searches and results were combined. Titles and abstracts of articles were screened initially to identify those most relevant to the topic area of bio-markers predictive of T1D. Articles were retrieved and then screened secondarily based on topic, information relevance, and redundancy of material with other identified resources.
This review conforms with relevant ethical guidelines pertaining to the use of humans and animals in research.
REVIEW RESULTS
Our initial search yielded 317 citations. Of these, 72 citations were identified that referred to markers in T1D. Nineteen articles were identified as relevant for the purposes of this review. Five additional articles were identified for this review using the references of articles found in the secondary screen (Fig 1, B). Table I provides a list of the articles covered in this review and the category of biomarkers the article covered.
Table I.
Listing of manuscripts covered in review
| Group | Articles in systematic review |
|---|---|
| Genetic markers |
|
| Autoantibody markers |
|
| Risk score quantification |
|
| Cellular immunity markers |
|
| β-Cell function markers |
|
DISCUSSION
Genetic markers in the prediction of T1D
T1D usually arises in individuals who have both a genetic susceptibility and subsequent exposure to elusive environmental factors. Although only 10% of individuals with T1D have a positive family history, this genetic predisposition puts first-degree relatives at a 20-fold increased risk of disease.9–11 Therefore, further refinement of genetic markers that predict susceptibility to or protection against disease may assist in identifying those individuals with the greatest lifetime risk, and may allow therapies to be targeted to those already in the latent period of T1D development.
Human leukocyte antigen (HLA) genes and non-HLA genes play roles in the genesis of T1D. HLA corresponds to the major histocompatibility complex genes in humans. HLA genes are responsible for cellular immune responses and play a key role in autoimmunity.12 Certain HLA-DR and HLA-DQ genetic polymorphisms (in particular, HLA-DR3 and DR4 at the DRB1 locus) are well known to be associated with increased T1D risk.13 Three specific HLA polymorphisms confer the greatest risk for developing T1D (Table II14). The greatest predisposition occurs with commonly abbreviated short serology notation DR3/DR4. Children heterozygous for the high-risk DR3/DR4 genotype have a 1 in 20 chance of developing T1D by the age of 15 years.14 Conversely, HLA-DR2 is associated with protection from T1D development and is linked to the most common DR-DQ haplotype in whites.
Table II.
Odds ratio of developing T1D based on the HLA genotypes with greatest susceptibility and the most common genotype with protective association
| Genotype | Full name | Odds ratio (95% CI) |
|---|---|---|
| DR3/dr4 | T1D DRB1*03:01-DQA1*05:01-DQB1*02:01 and DRB1*04:01/02/04/05/08DQA1*03:01-DQB1*03:02/04 (or DQB1*02) | 16.59 (13.70–20.10) |
| DR3/DR3 | DRB1*03:01-DQA1*05:01-DQB1*02:01 | 6.32 (5.12–7.80) |
| DR4/DR4 | DRB1*04:01/02/04/05/08-DQA1*03:01-DQB1*03:02/04 | 5.68 (3.91–8.23) |
| DR2/DR2 | DRB1*15:01-DQA1*01:02-DQB1*06:02 | 0.03 (0.01–0.07) |
Abbreviations: CI, confidence interval; HLA, human leukocyte antigen; T1D, type 1 diabetes.
Adapted from Noble et al14
More than 40 non-HLA genes are also known to contribute to the risk of T1D, although with much more modest effects than HLA genes.15 Disease-associated single nucleotide polymorphisms (SNPs) have been found in genes including insulin (INS), protein tyrosine phosphatase nonreceptor type 22 (PTPN22), interleukin 2 receptor alpha (IL2RA), SH2B adaptor protein 3 (SH2B3), protein tyrosine phosphatase nonreceptor type 2 (PTPN2), C-type lectin domain family 16 member A (CLEC16 A), ubiquitin associated and the SH3 domain containing A (UBASH3A), and cytotoxic T-lymphocyte-associated protein 4 (CTLA4).15,16 SNPs in these genes have been used to stratify risk further in persons with high-risk HLA genotypes.17 In the Diabetes and Autoimmunity Study in the Young population 1743 non-Hispanic white individuals (861 first-degree relatives of T1D and 882 from the general population) with high-risk HLA genotypes were monitored prospectively for the risk of developing islet autoimmunity and T1D.17,18 Multivariate analyses adjusting for family history of T1D and HLA high-risk genotype were done to find hazard ratios for each of the significant SNPs. Four of 20 examined SNPs had increased hazard ratios for the development of islet autoimmunity, T1D, or both: PTPN22 UBASH3A, PTPN2, and INS (Table III).
Table III.
Hazard risk association of non-HLA genes with islet autoimmunity and T1D in a DAISY non-Hispanic white population using multivariate analyses, adjusted for HLA-DR3/4,DQB1*0302 and family history of T1D as reported by Steck et al17
| Gene | SNP | Risk allele | Islet autoimmunity
|
T1D
|
||
|---|---|---|---|---|---|---|
| Hazard risk (95% CI) | P value | Hazard risk (95% CI) | P value | |||
| INS | rs689 | A | 1.32 (0.94–1.85) | 0.11 | 1.75 (1.08–2.83) | 0.02 |
| PTPN2 | rs1893217 | G | 1.42 (1.02–1.99) | 0.04 | 0.99 (0.60–1.66) | 0.98 |
| PTPN22 | rs2476601 | T | 1.87 (1.31–2.68) | 0.001 | 1.74 (1.04–2.90) | 0.03 |
| UBASH3A | rs11203203 | A | 1.46 (1.11–1.91) | 0.01 | 1.83 (1.28–2.64) | 0.001 |
Abbreviations: CI, confidence interval; DAISY, Diabetes and Autoimmunity Study in the Young; HLA, human leukocyte antigen; SNP, single nucleotide polymorphism; T1D, type 1 diabetes.
Polymorphisms in the PTPN22 and UBASH3A genes are associated with gain-of-function mutations in tyrosine-specific phosphatase signaling, leading to auto-immunity associated with T1D and other autoimmune disorders such as rheumatoid arthritis, systemic lupus erythematosus, and Graves’ disease.15,16 PTPN22 and UBASH3A have been associated both with the onset of islet autoimmunity and T1D development. PTPN2 is another protein tyrosine phosphatase also associated with several autoimmune diseases, including T1D, rheumatoid arthritis, Crohn’s disease, and celiac disease. PTPN2 SNP analysis modestly predicted the presence of islet autoimmunity. SNPs in INS were also associated with T1D development17 (Table III).
Recently, the histone deacetylase SIRT1 gene has been emphasized as a β-cell gene that modulates the development of T1D. SIRT1 is involved in nuclear transcription, DNA replication, and DNA repair. It is expressed predominantly in β cells, where it appears to play key roles in the regulation of insulin secretion, prevention of β-cell apoptosis, and cytokine production.19,20 An autosomal dominant mutation in SIRT1 has been reported in a family with predisposition to early-onset T1D.21 Interestingly, misexpression of this mutant form of SIRT1 in a β-cell line resulted in an increased inflammatory response to cytokine-mediated signaling. These findings describe a novel monogenic form of T1D and indicate a possible role for the modulation of β-cell gene expression in T1D.
With increasing ease of genetic screening, it is likely that a combination of HLA and non-HLA predictive gene polymorphisms will prove useful in refining risk estimations for development of T1D in both relatives of persons with T1D as well as in the general population. Yet, genetic predisposition information alone will likely not indicate precisely when a given individual will develop T1D, given environmental influences on disease. Therefore, there remains a continued need for exploration of nongenetic markers that can work coherently with genetic information to elucidate the timing of the onset of T1D.
Autoantibody markers
Islet cell autoantibodies (ICAs) were first reported approximately 40 years ago using immunofluorescence assays.22 Autoantibodies have been crucial in establishing T1D as an autoimmune disorder. With the reporting of autoantibodies in pancreatic islet cells in 1974 in a subset of patients with diabetes, evidence to support the hypothesis of insulitis in T1D was elucidated. Detection of varying autoantibody titers in patients with diabetes was the first indicator of a humoral component to T1D.22
From then to now, new islet cell-associated Ab targets have been established, and autoantibody panels are used more frequently to identify individuals at risk for the development of T1D and to confirm clinically diagnosed T1D at onset of disease. Commercially available T1D-associated autoantibodies include those detecting antibodies to insulin (insulin autoantibodies [IAAs]), glutamic acid decarboxylase (GAD), the tyrosine phosphataselike insulinoma antigen 2 (IA2) and islet cell antibody 512 (ICA512), the latter with autoreactivity to the predominant C-terminal epitope of IA2. Rates of positivity for islet antigen-specific autoantibodies at onset of T1D for individuals from the general population have been reported23 and are as follows: ICA, 70%–80% of individuals; IAA, 60% of children; IA2, 60% of individuals; and GAD, 70%–80% of individuals.23–25 Secondary screening for antibodies to cytoplasmic ICA in GAD and IA2 Ab-positive first-degree relatives of persons with T1D can detect individuals at greater risk for T1D. Additional positivity for Ab’s to cytoplasmic ICA can further predict a more rapid progression to T1D than Ab’s to GAD and IA2 predictions alone.26
Autoantibodies to zinc transporter 8 (ZnT8) documented in 2007, are a relatively new addition to this armamentarium.25 ZnT8 is a multispanning trans-membrane cation efflux transporter expressed in pancreatic islets.27 ZnT8 autoantibodies can be detected in the serum of individuals with prediabetes and persist in individuals with longstanding T1D. ZnT8 Ab’s have been reported to be present in 63% of individuals with new-onset T1D. As evaluated in 223 individuals with new-onset T1D, the addition of this autoantibody to the biochemical panel reduces the number of autoantibody-negative individuals with new-onset T1D from 5.8% to 1.8%. A panel of 4 auto-antibodies—IAA, IA2, GAD, and ZnT8—is estimated to detect 98% of individuals as autoimmune reactive at the time of onset of T1D.25
The true power of ZnT8 autoantibodies stands in their combined use with the established biochemical autoantibodies to enhance T1D development predictions significantly.25,28 In 2256 first- and second-degree biochemical Ab-positive relatives of T1D subjects screened through the Type 1 Diabetes TrialNet natural history study, adding ZnT8 in a sequential fashion to individuals positive for GAD, IA2, or IAA identified individuals at greater risk of T1D. Seventy-five percent of this cohort was positive initially for only 1 biochemical autoantibody. When ZnT8 was added, 8% of these individuals were also ZnT8 Ab positive. ZnT8 Ab positivity increased the 4-year estimated risk of development of T1D from 7% to 31%.28 Concentrations and prevalence of ZnT8 Ab increase with age.25 In the European Nicotinamide Diabetes Intervention Trial cohort of 526 ICA Ab-positive first-degree relatives of individuals with T1D, including ZnT8 Ab in low-genetic risk individuals and individuals older than 20 years augmented prediction of T1D.29
The ZnT8 example illustrates how the detection and implementation of additional autoantibodies in screening has the potential to hone the prediction of individuals at risk. Additional biomarkers such as proinsulin autoantibodies, carbonic anhydrase autoantibodies, lactoferrin autoantibodies, GM2-1 islet ganglioside autoantibodies and heat shock protein antigens have been identified, but currently these assays need refinement of their sensitivity and/or specificity before being able to be implemented in screening.23 Proinsulin autoantibodies have been reported in 7.4% of first-degree relatives of persons with T1D vs 1.9% of the control population.30 Interestingly, in Japan, autoantibodies to the exocrine pancreas-associated molecules carbonic anhydrase II and lactoferrin have been reported in both autoantibody-positive and -negative subjects with T1D. Both of these autoantibodies have also been reported in other autoimmune conditions.31 GM2-1 islet ganglioside autoantibody has been reported in individuals with new-onset T1D but not in age-matched control subjects. In addition, the presence of this autoantibody at onset correlated significantly with the GAD Ab presence in patients.32
Autoantibodies, although reflecting immune activity, may not always mediate tissue destruction. However, immunoglobulin (Ig) G antibodies, which predominate in T1D, can cause cellular cytotoxicity and are felt to be indicative of greater risk. Some, but not all, studies have indicated that changes in response from IgM to IgG1 and IgG3 antibodies may occur before the onset of T1D.33 This indicates that examination of autoantibody isotypes may be useful in following and understanding the pathogenesis of T1D.
Beyond the presence or absence of specific autoantibodies, the number of autoantibodies and titers of a specific autoantibody are also useful in predicting T1D. This was verified by a prospective analysis done on a total of 29,035 relatives of individuals with T1D screened and enrolled in the Diabetes Prevention Trial 1 (DPT-1).24 The cohort included a DPT-1 screened-only group of 28,507 individuals (97.6% ICA-negative individuals) and a DPT-1 enrolled cohort of 528 individuals (ICA-positive with glucose metabolic impairment). Ab’s to ICA, GAD65, and ICA512 were all similarly significant in predicting T1D when evaluated as a screened, single positive autoantibody and, when added as a autoantibody screened secondarily in persons with 1 prior positive autoantibody, the predictive power for T1D increased significantly. As a single autoantibody screen and when added as a second autoantibody screen, IAA did not have significant predictive ability in predicting T1D onset.
Most studies to date using autoantibodies to predict T1D have been done in relatives of persons with T1D, in whom there is the greatest prior probability of disease (approximately 5%). It is estimated that 90% of persons with new-onset T1D have no prior family history of T1D9; thus, general population screening is needed to identify T1D in persons with no known family history of disease. Because both the presence and the number of autoantibodies are predictive of T1D, some efforts are focused on developing high-throughput screening of serum for autoantibody detection. During a 15-year and 27-year period, the predictive ability of GAD and IA2 Ab’s were evaluated in 2 study populations: first-degree relatives (n = 755 from the Childhood Diabetes in Finland Study)34,35 and the general population (n = 3475 from the Study on Cardiovascular Risk Factors in Young Finns).35,37,38 Testing positive for a single Ab was associated with a greater cumulative disease risk in first-degree relatives than in the general population. However, for those who were positive for 2 autoantibodies, the cumulative disease risk was of the same magnitude (Table IV). Any individuals with double positive autoantibodies are at an extremely high risk of developing T1D. The authors concluded that, in the Finnish population, general childhood screening for GAD and IA2 Ab’s would identify ~60% of those individuals who would develop T1D during the next 27 years.36 Although this study was conducted on the Finnish population, which has a high prevalence of T1D, with the advent of high-throughput autoantibody platforms, multiple autoantibody screenings in populations with lower disease prevalence is becoming increasingly less costly.
Table IV.
Cumulative disease risk in the general population representation group and in first-degree relatives of individuals with T1D monitored for a 15-year period35
| Antibody
|
Cumulative disease risk, % (95% CI)
|
||
|---|---|---|---|
| GAD Ab | IA2 Ab | General population group | First-degree T1D relative group |
| + | − | 24 (9–38) | 61 (48–74) |
| − | + | 32 (12–51) | 74 (61–88) |
| + | + | 86 (60–100) | 83 (69–97) |
Abbreviations: Ab, antibody; CI, confidence interval; GAD, glutamic acid decarboxylase; IA, insulinoma antigen 2; T1D, type 1 diabetes.
Although the identification of detectable autoantibodies has marked a large step forward in predicting T1D, autoantibody assays in differing labs sometimes have widely divergent sensitivity and specificity, and differing absolute values for autoantibody titers.37 This has led to efforts to standardize and harmonize laboratory assays detecting autoantibodies so that results will be consistent and interpretable across sites. This includes the Diabetes Antibody Standardization Program workshops, an international initiative aimed to evaluate laboratory performance, to improve measurement of autoantibodies associated with T1D, and to establish reference ranges for autoantibodies. These workshops have provided evaluation data on autoantibodies to insulin, IA-2, GAD, and ZnT8 that have been instrumental in progressing the effort toward standardized assays.37–40 These efforts have improved the reliability of certain autoantibody assessments.39 Harmonized autoantibody detection is critical before conducting large-scale screening of persons to determine risk of T1D.
The HLA genotype also appears to have an influence on the specific islet autoantibodies expressed during the course of T1D development.33 Associations between HLA-DRQ8 and IA2-A and IAA, HLA-DR4 with IA2-A and IAA, HLA-DR3 and GAD65, and HLA-DR2 and GAD65 have all been reported.41–43 However, the presence of a particular HLA genotype is neither necessary nor sufficient, even in persons with T1D, to result in a particular autoantibody profile.41 The mechanisms of these genetic/humoral interactions still need to be elucidated as well.
Although the detection of autoantibodies has been important in identifying individuals at risk for T1D, there are still many limitations in their overall usage. An autoantibody panel of IAA, IA2, and GAD at the time of onset of T1D will identify approximately 20% of individuals with clinical T1D as autoantibody negative, and thus provide false-negative test results.27 Autoantibodies also do not provide precise determination of the time frame to onset because they can be detected many years before the clinical onset of T1D. In addition, the presence of autoantibodies does not mandate the development of T1D, because only 40% of ICA-positive relatives develop T1D within 10 years.44,45 Furthermore, autoantibody titers in those who will develop T1D do not always increase as disease onset nears, but rather can wax and wane during the prediabetes period.36 Also, although high-risk genetics and the presence of autoantibodies are both related to the diagnosis of T1D, for an individual they are not entirely prognostic, particularly in individuals with high-risk HLA and autoantibodies selected from the general population. In particular, in a Swedish study, adding GAD autoantibody screening to HLA screening in the general population was insufficient to improve T1D risk assessment to a clinically significant degree.41
Interesting new data suggest that differences in metabolomic profiles, particularly lower methionine concentrations, are found in children who develop autoantibodies.46 Whether these profiles persist beyond the development of autoimmunity or are predictive of T1D onset remains to be seen.
Risk score quantification
None of the T1D autoantibodies currently have high enough specificity or are available in high enough throughput methods to be used for routine population screening. Designing “risk scores” based on other clinical criteria brings us 1 step closer to identifying more accurately individuals in the prediabetic phase, thereby supplementing autoantibody screening. Numerous attempts to quantify risk have been made. All calculations use different continuous (eg, age, body mass index [BMI]) or categorical (eg, gender) characteristics with a goal of quantifying the likelihood that an individual is in the latent phase toward the development of T1D. Data from the DPT-1 have been used to develop a DPT-1 risk score (DPTRS) for the prediction of T1D in first- and second-degree ICA-positive relatives of persons with T1D.5 The score uses age, BMI, fasting C-peptide levels, and total glucose, and total C-peptide derived from a 2-hour oral glucose tolerance test. The DPTRS can be converted to a 5-year risk estimation for T1D.5 The DPTRS has been validated in the TrialNet population of relatives of individuals with T1D with at least 1 detectable autoantibody.47 This score can also be used to monitor the progression to disease and to target the greatest risk individuals for intervention studies. However, work has not been conducted to demonstrate the predictive ability in the general population.
Receiver-operating characteristic curves have been used to combine immunologic and metabolic parameters in predicting T1D in DPT-1 study population members with diminished first-phase insulin response and/or abnormal glucose tolerance.48 After testing a variety of variables, including Ab titers for ICA, IAA, ICA512, GAD65, fasting insulin concentrations, fasting glucose levels from oral glucose tolerance tests and IVGTT, first phase insulin response, and peak C-peptide, adding a combination of 2-hour glucose, peak C-peptide, and area-under-the-curve C-peptide improved significantly the prognostic accuracy compared with any single variable. Adding age, sex, and BMI to the model did not improve the prediction accuracy. Interestingly, like the DPTRS model, this model does not incorporate any autoantibody measures (such as number of autoantibodies or Ab titers) except that all individuals were ICA positive. It is possible that autoimmune reactivity may not affect risk greatly after metabolic function has declined significantly.48
Mrena et al49 present a strategy for the identification of siblings of children with new-onset T1D who will possibly progress to T1D. Using Cox regression analysis they identified the following factors as useful in predicting those who will progress to T1D: age, HLA-DR conferred susceptibility, increased number of autoantibodies, decreased first-phase insulin response, and reduced insulin sensitivity relative to insulin secretion. Uniquely, this group went further to develop models to predict age at T1D diagnosis based on subject age, age of sibling T1D diagnosis, IA2 Ab levels, the number of autoantibodies present, HLA-DR conferred susceptibility, and initial first-phase insulin response level. The model predicting age at diagnosis worked well for individuals in the cohort who were true progressors to T1D, but was not adequate in predicting age of onset of T1D for those who did not progress to a diagnosis of T1D during the study period. In the models used, all nonprogressors were falsely predicted as progressors to T1D within the study observation period.
Although diabetes risk scores establish a probability of the eventual clinical onset of T1D, this method of prediction does not assess the degree of ongoing autoimmune disease or β-cell failure. There are proposed biologic markers that can measure directly the level of immune activation. These markers can, potentially, be incorporated into screening Ab’s and risk scores. Combining these predictive indicators has the potential not only to identify prediabetic status, but also to suggest a physiological marker of the level of immune activation.
Cellular immune reaction markers
T1D has a large humoral and cellular inflammatory component. Yet, humoral activation in T1D as evidenced by the magnitude of autoantibody titers does not always correlate with cellular immune system activation.50 Furthermore, Ab titers do not correlate with the overall severity of the autoimmune disease or provide clues about the time to clinical disease onset. Even with the accepted autoimmune nature of T1D, a case of development of T1D in a patient with X-linked gammaglobulinemia who was B-cell deficient with normal T-cell function highlights a role of the cellular immune system in the development and progression of T1D.51 There are currently no available clinical measurements of the degree of cellular immune system reactivity specific to the T1D immune activated state. Yet, there is the potential for exploring cellular immune system activation in T1D as a biomarker.
Mechanistic T-cell activation markers
T1D is mediated by T-cell activation leading to β-cell destruction. In the past, efforts to develop T-cell reactivity assays focused on quantitating CD4+ T-cell subsets in T1D. However, CD8+ T cells are now proposed to be an important mediator in the pathway to T1D development, because insulitis has been shown to be driven by CD8+ T cells in nonobese diabetic (NOD) mice.52,53 CD8+ T-cell populations have been studied using immunoblots, enzyme-linked immunospots (elispots), and tetramers as a means of differentiating between individuals with new-onset T1D and healthy subjects.54 Islet-specific interferon-gamma CD8+ T-cell responses, ISL8Spot, have the potential to serve as a new immune marker of T1D.54 An assay using a panel of HLA-A2-restricted β-cell epitopes derived from 15 epitopes including preproinsulin, GAD, and islet glucose-6-phosphatase catalytic subunit-related protein run on unfractionated peripheral blood mononuclear cells without preliminary expansion differentiated patients with new-onset T1D effectively from healthy control subjects (sensitivity, 86%; specificity, 91%). Interestingly, in the healthy cohort the T-cell reactivity assay identified positively an individual who was clinically healthy but contained the T1D predisposing HLA-DR3 genotype and positive titers for IAA.
When combined with autoantibody assays, T-cell responses using immunoblot and T-cell proliferation assays in 1 study showed 75% sensitivity and 100% specificity in differentiating persons with T1D from control subjects.55 This highlights the potential for these sorts of T-cell assays also to differentiate individuals in the prediabetic stage of T1D; however, further validation of these assays is needed.
Serum cytokine assays
Cytokines have also been explored as a marker of T1D in persons 1–40 years after diagnosis.56 One example is chemokine ligand 1 (CXCL1) measured in sera using solid-phase enzyme-linked immunosorbent assays. CXCL1 is released by monocytes, dendritic cells, and pancreatic β cells and induces T-cell chemotaxis. In persons with T1D, CXCL1 concentrations are increased relative to individuals with type 2 diabetes (T2D). Elevations of CXCL1 have also been reported in other autoimmune conditions. CXCL1 has potential as a marker of incipient T1D, but first needs to be validated in healthy control subjects and populations with prediabetes.
β-cell function markers
Ultimately, T1D is characterized by β-cell stress and eventual apoptosis.57 At the time of clinical onset of T1D, approximately less than 20%–30% of functional β-cell mass remains. Even with genetic screening, autoantibodies, cellular immune system activation assessments, and risk score quantification, the crucial problem of irreversible β-cell death has only recently been considered as a potential biomarker. In light of the current inability to detect insidious β-cell death before the onset of overt hyperglycemia, markers of β-cell stress and destruction may be useful in identifying individuals with prediabetes. Ideally, these markers could be among the earliest indicators to predict onset and the degree or severity of β-cell loss of homeostasis in the progression to the development of T1D. There are several assays with the potential for detecting β-cell destruction at the levels of DNA, RNA, and protein.
β-cell specific DNA
Quantitative polymerase chain reaction techniques can be used for systemic detection of demethylated insulin DNA as a marker of β-cell death. Increased β-cell-derived DNA has been observed in streptozotocin β-cell toxin-induced diabetic and NOD mice before hyperglycemia. In patients with new-onset T1D, increases in demethylated insulin DNA have been observed compared with age-matched control subjects.58,59 This technique was implemented recently to quantitate β-cell death using serum samples obtained during an intervention trial with teplizumab. Improvements in C-peptide responses with immune drug intervention were associated with decreased demethylated insulin DNA.59 Further development of this methodology is still needed, because it has not yet been evaluated in individuals who are in the latent/prediabetic phase of disease as a predictive marker.
β-cell specific RNA
In T1D, the activation of specific β-cell health maintenance pathways such as oxidative stress or endoplasmic reticulum (ER) stress during the course of T1D can lead to increased expression of specific microRNAs (miRNAs). miRNAs are known to play a central role in posttranscriptional gene regulation, cellular differentiation, proliferation, and apoptosis. However, the mechanism of their upregulation is not fully known.60 Specific miRNA expression has been explored as a marker for T1D glycemic control and residual β-cell function in the T1D population.61 In individuals with new-onset T1D 1 month after diagnosis relative to age-matched healthy control subjects, 12 miRNAs were upregulated, several of which were linked to apoptosis and β-cell gene regulatory networks.62,63 Of significance, miR25 levels present soon after diagnosis were associated with β-cell function as measured by improvements in stimulated C-peptide and with hemoglobin A1C levels 3 months after diagnosis.
The possibility that miR-375, an islet-cell-specific miRNA,64 might be used to detect prediabetes has been explored.65 Similar to demethylated DNA, it is proposed miR-375 is released from β cells during apoptosis. Studies using streptozotocin-treated and NOD mice have shown increased levels of miR-375 before hyperglycemia. These studies must be verified in human subjects. However, these studies indicate a specific miRNA may have potential as a biomarker of β-cell function before the onset of T1D and in very early T1D to identify individuals for β-cell sparing or regenerative therapies.
These studies allude to the biomarker potential of miRNAs in T1D as a means to quantify β-cell function. As biomarkers, miRNAs show great potential. They are stable under harsh conditions and can be detected easily in bodily fluids such as plasma and serum. In addition, quantification of miRNAs using quantitative real-time polymerase chain reaction techniques may offer sensitive and specific assays.60 One potential limitation to the usage of miRNA as a biomarker is the uncertainty of the source of detected miRNA, because it is difficult to show isolated release from islet β cells. There is still a significant amount research needed before miRNA can be used as a clinical marker of T1D onset and progression, but it holds tremendous potential.
β-cell-specific proteins
β-cell stress was thought originally to be a phenomenon primarily of persons with T2D, but has since been investigated in T1D.66,67 Proinflammatory cytokines are proposed to be responsible for ER stress observed in T1D whereas glucolipotoxicity is proposed as a causative factor in T2D ER stress. In human pancreatic islet autopsy specimens, the ER stress markers C/EBP homologous protein and immunoglobulin heavy chain have been detected in insulitis-positive islets. There is potential for using ER stress markers as a detection tool or marker of the level of intrinsic β-cell function in persons with T1D. Secreted molecules from ER-stressed β cells may be used as early markers of preclinical T1D.67,68
Tersey et al67 validated the theory that β-cell ER stress precedes the onset of hyperglycemia in the NOD mouse model, with both morphologic changes and secretory functional changes in the ER. In NOD mice during the prediabetic phase, there is ER loss of homeostatic function accompanied by increases in serum proinsulin and decreases in insulin production. Increased serum levels of proinsulin compared with serum levels of the fully processed insulin molecule (assessed by measuring C-peptide), the proinsulin-to-C-peptide (PI:C) ratio has potential as a diagnostic marker of individuals during the latent period of β-cell failure with escalating amounts of ER stress. As a biomarker, this will be able uniquely to measure directly the functional ability of the pancreatic β cells, and there is potential to quantify the severity of β-cell dysfunction during the preclinical stages of T1D development.
An increased ratio in the random serum PI:C ratio has been shown to precede the onset of T1D.69 Truyen et al70 reported that random PI:C measurements complement autoantibody status in first-degree relatives of individuals with T1D in assessing the risk of T1D development. The predictive power of PI:C to assess β-cell function is evident in findings of similar glycemic control between the autoantibody-positive relatives cohort and the autoantibody-negative relatives cohort; but, significant elevation of PI:C was detected in the autoantibody-positive cohort. The PI:C levels in the autoantibody-negative cohort were not distinguishable from the general population cohort. Of note, the elevation of PI:C correlated significantly with the number of autoantibodies present and was also elevated more strikingly in individuals with prediabetes who were sampled within 40 months of diagnosis.
CONCLUSION
This review has explored a variety of biomarkers in the field of T1D. We discussed genetic markers, autoantibody markers, risk score quantification, cellular immune system markers, and indicators of β-cell stress/death. The number of potential biomarkers discussed here and the varying pathophysiological basis of their use in the analysis of T1D pathogenesis and progression highlight recent progress in the field of T1D research. However, at the level of the individual subject, where prognostication may matter most, the predictive value of any individual biomarker “signal” is generally limited. Currently, there remains no single major test or combination of tests that can detect individuals definitively during the latent phase as they progress toward the development of T1D, and it is evident that continued research is still warranted.
Although these numerous techniques may, individually, hold great potential in diagnosing T1D before the clinical onset of disease, one must pause to consider the obstacles or hindrances that broadly limit these methods of testing. Perhaps, instead of looking to develop a single marker to detect preclinical T1D accurately and universally, efforts should be focused on the combined usage of multiple biomarkers in validated risk algorithms. Also, longitudinal data will be necessary to assess how panels of biomarkers change over time throughout the progression of T1D.
Acknowledgments
Work on this manuscript was supported by the following grants: JDRF 47-2012-744 and JDRF-Helmsley Charitable Trust 47-2013-253, National Institutes for Health National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award UL1 TR 000006 U01, RO1 AI079065 (to JSB), R01 DK093954 (to CEM), and VA Merit Award 1I01BX001733 (to CEM); foundation support from the Sigma Beta Sorority (to CEM); and funding from the Herman B. Wells Center for Pediatric Research at Riley Hospital for Children at IU Health (to LAD).
Abbreviations
- Ab
antibody
- BMI
body mass index
- CXCL1
chemokine ligand 1
- DPT-1
Diabetes Prevention Trial 1
- DPTRS
DPT-1 risk score
- ER
endoplasmic reticulum
- GAD
glutamic acid decarboxylase
- HLA
human leukocyte antigen
- IA2
insulinoma antigen 2
- IAA
insulin autoantibody
- ICA
islet cell autoantibody
- Ig
immunoglobulin
- miRNA
micro-RNA
- NOD
nonobese diabetic
- PI:C
proinsulin-to-C-peptide ratio
- SNP
single nucleotide polymorphism
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- ZnT8
zinc transporter 8
Footnotes
Conflicts of Interest: All authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare.
References
- 1.Akirav E, Kushner JA, Herold KC. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes. 2008;57:2883–8. doi: 10.2337/db07-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherry NA, Tsai EB, Herold KC. Natural history of beta-cell function in type 1 diabetes. Diabetes. 2005;54:S32–9. doi: 10.2337/diabetes.54.suppl_2.s32. [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS. Progression to diabetes in relatives of type 1 patients with diabetes: mechanisms and mode of onset. Diabetes. 2010;59:679–85. doi: 10.2337/db09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner SG, Gale EA, Williams AJ, et al. Progression to diabetes in relatives with islet autoantibodies: is it inevitable? Diabetes Care. 1999;22:2049–54. doi: 10.2337/diacare.22.12.2049. [DOI] [PubMed] [Google Scholar]
- 5.Sosenko JM, Krischer JP, Palmer JP, et al. A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care. 2008;31:528–33. doi: 10.2337/dc07-1459. [DOI] [PubMed] [Google Scholar]
- 6.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 7.Bougneres PF, Carel JC, Castano L, et al. Factors associated with early remission of type I diabetes in children treated with cyclosporine. N Engl J Med. 1988;318:663–70. doi: 10.1056/NEJM198803173181103. [DOI] [PubMed] [Google Scholar]
- 8.Herold KC, Gitelman SE, Willi SM, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391–400. doi: 10.1007/s00125-012-2753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simell O, Winter WE, Schatz D. Enhancing the understanding of pre-type 1 diabetes in the general population. Diabetes Care. 2010;33:1403–5. doi: 10.2337/dc10-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillil H, Kobberling J. Age-corrected empirical genetic risk estimates for first-degree relatives of IDDM patients. Diabetes. 1987;36:93–9. doi: 10.2337/diab.36.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Bonifacio E, Ziegler AG. Advances in the prediction and natural history of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:513–25. doi: 10.1016/j.ecl.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Horton R, Wilming L, Rand V, et al. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889–99. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 13.Baisch JM, Weeks T, Giles R, Hoover M, Stastny P, Capra JD. Analysis of HLA-DQ genotypes and susceptibility in insulin-dependent diabetes mellitus. N Engl J Med. 1990;322:1836–41. doi: 10.1056/NEJM199006283222602. [DOI] [PubMed] [Google Scholar]
- 14.Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Current Diabetes Rep. 2011;11:533–42. doi: 10.1007/s11892-011-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet. 2011;12:781–92. doi: 10.1038/nrg3069. [DOI] [PubMed] [Google Scholar]
- 16.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med. 2009;360:1646–54. doi: 10.1056/NEJMra0808284. [DOI] [PubMed] [Google Scholar]
- 17.Steck AK, Wong R, Wagner B, et al. Effects of non-HLA gene polymorphisms on development of islet autoimmunity and type 1 diabetes in a population with high-risk HLA-DR,DQ genotypes. Diabetes. 2012;61:753–8. doi: 10.2337/db11-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rewers M, Bugawan T, Norris J, et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY) Diabetologia. 1996;39:807–12. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 19.Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–17. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Tang M-M, Zhu Q-E, Fan W-Z, et al. Intra-arterial targeted islet-specific expression of Sirt1 protects β cells from streptozotocin-induced apoptosis in mice. Mol Ther. 2010;19:60–6. doi: 10.1038/mt.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biason-Lauber A, Böni-Schnetzler M, Hubbard BP, et al. Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab. 2013;17:448–55. doi: 10.1016/j.cmet.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;ii:1279–83. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 23.Winter WE, Schatz DA. Autoimmune markers in diabetes. Clin Chem. 2011;57:168–75. doi: 10.1373/clinchem.2010.148205. [DOI] [PubMed] [Google Scholar]
- 24.Orban T, Sosenko JM, Cuthbertson D, et al. Pancreatic islet auto-antibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32:2269–74. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–5. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietropaolo M, Yu S, Libman IM, et al. Cytoplasmic islet cell antibodies remain valuable in defining risk of progression to type 1 diabetes in subjects with other islet autoantibodies. Pediatr Diabetes. 2005;6:184–92. doi: 10.1111/j.1399-543X.2005.00127.x. [DOI] [PubMed] [Google Scholar]
- 27.Wenzlau JM, Frisch LM, Hutton JC, Davidson HW. Mapping of conformational autoantibody epitopes in ZNT8. Diabetes Metab Res Rev. 2011;27:883–6. doi: 10.1002/dmrr.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L, Boulware DC, Beam CA, et al. Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care. 2012;35:1213–8. doi: 10.2337/dc11-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long AE, Gooneratne AT, Rokni S, Williams AJ, Bingley PJ. The role of autoantibodies to zinc transporter 8 in prediction of type 1 diabetes in relatives: lessons from the European Nicotinamide Diabetes Intervention Trial (ENDIT) cohort. J Clin Endocrinol Metab. 2012;97:632–7. doi: 10.1210/jc.2011-1952. [DOI] [PubMed] [Google Scholar]
- 30.Kuglin B, Halder B, Bertrams J, Grüneklee D, Gries FA, Kolb H. Proinsulin autoantibodies: association with type I diabetes but not with islet cell antibodies, insulin autoantibodies or HLA-DR type. J Autoimmun. 1990;3:573–7. doi: 10.1016/s0896-8411(05)80023-x. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi T, Okazaki K, Okamoto M, et al. High prevalence of autoantibodies against carbonic anhydrase II and lactoferrin in type 1 diabetes: concept of autoimmune exocrinopathy and endocrinopathy of the pancreas. Pancreas. 2003;27:26–30. doi: 10.1097/00006676-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Dotta F, Falorni A, Tiberti C, et al. Autoantibodies to the GM2-1 islet ganglioside and to GAD-65 at type 1 diabetes onset. J Autoimmun. 1997;10:585–8. doi: 10.1006/jaut.1997.0166. [DOI] [PubMed] [Google Scholar]
- 33.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54:S52–61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 34.Kulmala P, Savola K, Petersen JS, et al. Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes: a population-based study: the Childhood Diabetes in Finland Study Group. J Clin Invest. 1998;101:327. doi: 10.1172/JCI119879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siljander HT, Veijola R, Reunanen A, Virtanen SM, Akerblom HK, Knip M. Prediction of type 1 diabetes among siblings of affected children and in the general population. Diabetologia. 2007;50:2272–5. doi: 10.1007/s00125-007-0799-5. [DOI] [PubMed] [Google Scholar]
- 36.Knip M, Korhonen S, Kulmala P, et al. Prediction of type 1 diabetes in the general population. Diabetes Care. 2010;33:1206–12. doi: 10.2337/dc09-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bingley PJ, Williams AJ, Colman PG, et al. Measurement of islet cell antibodies in the Type 1 Diabetes Genetics Consortium: efforts to harmonize procedures among the laboratories. Clin Trials. 2010;7:S56–64. doi: 10.1177/1740774510373496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ. Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia. 2008;51:846–52. doi: 10.1007/s00125-008-0967-2. [DOI] [PubMed] [Google Scholar]
- 39.Schlosser M, Mueller PW, Achenbach P, Lampasona V, Bingley PJ. Diabetes Antibody Standardization Program: first evaluation of assays for autoantibodies to IA-2beta. Diabetes Care. 2011;34:2410–2. doi: 10.2337/dc11-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlosser M, Mueller PW, Torn C, Bonifacio E, Bingley PJ. Diabetes Antibody Standardization Program: evaluation of assays for insulin autoantibodies. Diabetologia. 2010;53:2611–20. doi: 10.1007/s00125-010-1915-5. [DOI] [PubMed] [Google Scholar]
- 41.Hagopian WA, Sanjeevi CB, Kockum I, et al. Glutamate decarboxylase-, insulin-, and islet cell-antibodies and HLA typing to detect diabetes in a general population-based study of Swedish children. J Clin Invest. 1995;95:1505–11. doi: 10.1172/JCI117822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham J, Hagopian WA, Kockum I, et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51:1346–55. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler R, Alper CA, Awdeh ZL, et al. Specific association of HLA-DR4 with increased prevalence and level of insulin autoantibodies in first-degree relatives of patients with type I diabetes. Diabetes. 1991;40:709–14. doi: 10.2337/diab.40.6.709. [DOI] [PubMed] [Google Scholar]
- 44.Bingley PJ, Christie MR, Bonifacio E, et al. Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes. 1994;43:1304–10. doi: 10.2337/diab.43.11.1304. [DOI] [PubMed] [Google Scholar]
- 45.Bonifacio E, Bingley PJ, Shattock M, et al. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet. 1990;335:147–9. doi: 10.1016/0140-6736(90)90013-u. [DOI] [PubMed] [Google Scholar]
- 46.Pflueger M, Seppanen-Laakso T, Suortti T, et al. Age- and islet autoimmunity-associated differences in amino acid and lipid metabolites in children at risk for type 1 diabetes. Diabetes. 2011;60:2740–7. doi: 10.2337/db10-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sosenko JM, Skyler JS, Mahon J, et al. Validation of the Diabetes Prevention Trial-Type 1 risk score in the TrialNet Natural History Study. Diabetes Care. 2011;34:1785–7. doi: 10.2337/dc11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu P, Beam CA, Cuthbertson D, Sosenko JM, Skyler JS, Krischer JP. Prognostic accuracy of immunologic and metabolic markers for type 1 diabetes in a high-risk population: receiver operating characteristic analysis. Diabetes Care. 2012;35:1975–80. doi: 10.2337/dc12-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mrena S, Virtanen SM, Laippala P, et al. Models for predicting type 1 diabetes in siblings of affected children. Diabetes Care. 2006;29:662–7. doi: 10.2337/diacare.29.03.06.dc05-0774. [DOI] [PubMed] [Google Scholar]
- 50.Hummel M, Durinovic-Bello I, Ziegler AG. Relation between cellular and humoral immunity to islet cell antigens in type 1 diabetes. J Autoimmun. 1996;9:427–30. doi: 10.1006/jaut.1996.0059. [DOI] [PubMed] [Google Scholar]
- 51.Martin S, Wolf-Eichbaum D, Duinkerken G, et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N Engl J Med. 2001;345:1036–40. doi: 10.1056/NEJMoa010465. [DOI] [PubMed] [Google Scholar]
- 52.DiLorenzo TP, Serreze DV. The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev. 2005;204:250–63. doi: 10.1111/j.0105-2896.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 53.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2m null mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–9. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 54.Mallone R, Martinuzzi E, Blancou P, et al. CD8+T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613–21. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 55.Seyfert-Margolis V, Gisler TD, Asare AL, et al. Analysis of T-cell assays to measure autoimmune responses in subjects with type 1 diabetes: results of a blinded controlled study. Diabetes. 2006;55:2588–94. doi: 10.2337/db05-1378. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi K, Ohara M, Sasai T, et al. Serum CXCL1 concentrations are elevated in type 1 diabetes mellitus, possibly reflecting activity of anti-islet autoimmune activity. Diabetes Metab Res Rev. 2011;27:830–3. doi: 10.1002/dmrr.1257. [DOI] [PubMed] [Google Scholar]
- 57.Papa FR. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harbor Perspect Med. 2012;2:a007666. doi: 10.1101/cshperspect.a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akirav EM, Lebastchi J, Galvan EM, et al. Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci U S A. 2011;108:19018–23. doi: 10.1073/pnas.1111008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lebastchi J, Deng S, Lebastchi AH, et al. Immune therapy and beta-cell death in type 1 diabetes. Diabetes. 2013 doi: 10.2337/db12-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bushati N, Cohen SM. MicroRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 61.Nielsen LB, Wang C, Sorensen K, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res. 2012;2012:896362. doi: 10.1155/2012/896362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin W, Shi Y, Zhao B, et al. miR-24 regulates apoptosis by targeting the open reading frame (ORF) region of FAF1 in cancer cells. PLoS One. 2010;5:e9429. doi: 10.1371/journal.pone.0009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Razumilava N, Bronk SF, Smoot RL, et al. miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology. 2012;55:465–75. doi: 10.1002/hep.24698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–30. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 65.Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ. Circulating miR-375 as a biomarker of beta-cell death and diabetes in mice. Endocrinology. 2013;154:603–8. doi: 10.1210/en.2012-1744. [DOI] [PubMed] [Google Scholar]
- 66.Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55:2417–20. doi: 10.1007/s00125-012-2604-3. [DOI] [PubMed] [Google Scholar]
- 67.Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the non-obese diabetic mouse model. Diabetes. 2012;61:818–27. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Sullivan-Murphy B, Urano F. ER stress as a trigger for beta-cell dysfunction and autoimmunity in type 1 diabetes. Diabetes. 2012;61:780–1. doi: 10.2337/db12-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Røder M, Knip M, Hartling S, Karjalainen J, Akerblom H, Binder C. Disproportionately elevated proinsulin levels precede the onset of insulin-dependent diabetes mellitus in siblings with low first phase insulin responses: the Childhood Diabetes in Finland Study Group. J Clin Endocrinol Metab. 1994;79:1570–5. doi: 10.1210/jcem.79.6.7989457. [DOI] [PubMed] [Google Scholar]
- 70.Truyen I, De Pauw P, Jørgensen P, et al. Proinsulin levels and the proinsulin: C-peptide ratio complement autoantibody measurement for predicting type 1 diabetes. Diabetologia. 2005;48:2322–9. doi: 10.1007/s00125-005-1959-0. [DOI] [PubMed] [Google Scholar]