Abstract
Purpose
Esophageal cancer (EC) is an aggressive malignancy and often resistant to therapy. Overexpression of EGFR has been associated with poor prognosis of EC patients. However, clinical trials using EGFR inhibitors have not provided benefit for EC patients. Failure of EGFR inhibition may be due to crosstalk with other oncogenic pathways.
Experimental Design
In this study, expression of YAP1 and EGFR were examined in EAC resistant tumor tissues vs sensitive tissues by immunohistochemistry. Western blot, immunofluorescence, real-time PCR, promoter analysis, site-directed mutagenesis and in vitro and in vivo functional assays were performed to elucidate the YAP1 mediate EGFR expression and transcription and the relationship with chemoresistance in esophageal cancer.
Results
We demonstrate that Hippo pathway coactivator YAP1 can induce EGFR expression and transcription in multiple cell systems. Both YAP1 and EGFR are overexpressed in resistant EC tissues compared to sensitive EC tissues. Further, we found that YAP1 increases EGFR expression at the level of transcription requiring an intact TEAD binding site in the EGFR promoter. Most importantly, exogenous induction of YAP1 induces resistance to 5-FU and docetaxcel, while knockdown of YAP sensitizes EC cells to these cytotoxics. Verteporfin, a YAP1 inhibitor, effectively inhibits both YAP1 and EGFR expression and sensitizes cells to cytotoxics.
Conclusions
Our data provide evidence that YAP1 up-regulation of EGFR plays an important role in conferring therapy resistance in EC cells. Targeting YAP1-EGFR axis may be more efficacious than targeting EGFR alone in EC.
Keywords: Hippo, YAP, EGFR, chemo-resistance and esophageal cancer
Introduction
Esophageal cancer (EC) is a lethal illness with high incidence globally and significantly increased incidence in USA with an estimated 18,170 new cases and an estimated death of 15,450 cases in 2014(1) and the 5-year survival rate for patients with advanced EC is <10%(2). EC is inherently resistant to therapy. Even localized EC is frequently resistant (3). Considerable lack of understanding of molecular underpinnings of EC has been an ongoing barrier for the development of effective strategies. Epidermal growth factor (EGFR) and the Hippo pathway coactivator YAP play important role in control of cell growth. Deregulation of these pathways may represent key elements for resistance in EC.
EGFR is a transmembrane protein with intrinsic kinase activity (4). Activation of EGFR is due to the binding of its specific ligands such as EGF, TGF-α and amphiregulin and, abundance in EGFR protein levels per se results in dimerization of EGFR and activation of downstream signal cascades that regulate cell proliferation, invasion, and survival. EGFR overexpression or amplification has been reported in several human tumors including those of head and neck, breast, colon, lung, stomach and esophagus (5-8). Increased EGFR expression has been associated with advanced stage, higher metastatic potential and shorter survival of patients with breast, colon, lung, and EC (8, 9). However, several clinical trials targeting EGFR either by antibodies (10-13) or kinase inhibitors (14, 15) have been disappointing in patients with gastroesophageal cancer. Therefore, inhibition of EGFR alone does not seem sufficient and it may be that EGFR is activated through other oncogenic signaling and targeting those pathways may be advantageous.(16, 17).
The Hippo signaling pathway regulates organ size and cell proliferation. YAP1 is a key downstream effector of the Hippo signaling pathway and is tightly regulated by a number of upstream kinases and their adaptors such as Mst1/2, Sav1 and Lats1/2 which are tumor suppressors in several tumor types (18). Conditional deletion of these molecules in mice led to a dramatic increase in organ size and tumor formation that are largely dependent on YAP1(18). In transgenic mice, tissue specific expression of YAP1 results in tissue overgrowth and tumor formation (19). EGFR activation occurs frequently in Hippo pathway defective mouse liver tumors (20). Therefore, we hypothesized that YAP1 may regulate the sustained EGFR overexpression and activation accounting for therapy resistance.
In this study, we provide novel information that YAP1 up-regulates EGFR expression at the level of transcription through a TEAD binding site in the EGFR promoter. YAP1 mediates sustained EGFR up-regulation, increases cell proliferation and confers therapy resistance. A YAP1 inhibitor verteporfin sensitizes cells to cytotoxics. Our data demonstrate that targeting YAP1 may be an alternative and promising strategy for overcoming resistance in EC.
Materials and Methods
Cells and reagents
The human Barrett's cell lines CPA and CPC and EC cell lines Flo-1, SKGT-4, BE3, OE33, JHESO, OACP, YES-6 and KATO-TN have been previously described (21-23). Fetal Liver cell line B299 and tumor cell lines from tumor tissues of Mst1/2-/- were generated by published methods. All human cell lines were tested and authenticated in the characterized cell line core facility of U.T.M D Anderson Cancer Center. Verteporfin was purchased from United States Pharmacopeia (Rockville, Maryland). Doxycycline hyclate was obtained from Sigma-Aldrich (St.Louis, MO). Antibody against YAP and phospho-EGFR, phospho-AKT (473), MCL-1 was purchased from Cell Signaling Technology (Beverly, MA). EGFR antibody was obtained from Santa Cruz Biotechnology. Antibodies against SOX-9 and Hes-1 were purchased from Chemicon (Billerica, MS). DNA plasmids that encode wild type human YAP1 (hYAP, CMV-YAP) or a mutant protein that can no longer be phosphorylated at Ser127 (24)(hYAP1 S127A, CMV-S127A-YAP) and Tead2 cDNA vector (pcDNA2-TEAD2) were obtained from addgene. Doxycycline inducible YAP1 lentivirus expression plasmid (PIN20YAP) and Lentiviral shRNA plasmids for knockdown YAP1 were previously described (25).
Primary mouse esophageal epithelial cells isolation and culture
Mouse primary esophageal cells were isolated according to published methods as described previously (26-28).
Protein extraction and Western blot analysis
Protein isolation and Western blot analyses were performed as previously described and immunoreactive bands were visualized by chemiluminescence detection (29).
Luciferase reporter assays and Transient transfection
The EGFR promoter (around 2.3k) containing an intact TEAD binding site (TCATTCCT) was amplified using high fidelity PCR with primers (EGFRp23k.F5.Kpn and EGFRp.R1b.Xho) from genomic DNA extracted from SKGT-4 cells with the following sequences: EGFRp23k.F5.Kpn 5′ aaaGGTACCgttgctggacaagaggggta 3′;EGFRp.R1b.Xho 5′ aaaCTCGAGggggctagctcgggactc 3′ The native fragment of EGFR promoter (-2286bp to +102bp) was digested with KpnI and XhoI and then cloned into pGL4.22 (Promega, Madison, WI) at the site of KpnI and XhoI.
The EGFR promoter-luciferase constructs with two mutant Tead binding sites TCATTCCT on the EGFR 2.3k promoter (-2178bp to -2170bp) were generated according to the site-directed mutagenesis kit (Stratagene). Mutant Mt1 replaced 3 bp from Tead binding site TCATTCCT to TCTCGCCT, while the mutant Mt2 deleted internal 6 bp from Tead binding site TCATTCCT. The fragments were verified by sequencing before cloning into pGL4.22 vector. The primers for Mutant1 and Mutant 2 EGFR promoters as followings: EGFR-p2.3k-Mt1.F 5′ agcaactgggccactattgTCTCGCCtgtggtggtggcacacacacccag 3′ EGFR-p2.3k-Mt1.R 5′ctgggtgtgtgtgccaccaccacAGGCGAGAcaatagtggcccagttgct 3′; EGFR-p2.3k-Mt2.F 5′ agcaactgggccactattgTTgtggtggtggcacacacacccag 3′ EGFR-p2.3k-Mt2.R 5′ ctgggtgtgtgtgccaccaccacAAcaatagtggcccagttgct 3′ Transient co-transfection with EGFR luciferase reporter either wide type or mutants and Renilla vector were performed as previously described (30).
Immunohistochemistry
Immunohistochemical staining for YAP1 and EGFR were performed on tissue microarray slides consisting of 113 EA and non-neoplastic esophageal tissue samples from patients who underwent esophagogastrectomy without neoadjuvant therapy and has been described previously (9, 30). In addition, 10 cases of pretreatment biopsies with complete response tissues term as pCR or P0 or partial response (P1) and 10 cases of pre or post resistant tumors term as P2 using antibodies against YAP1 (1:100) and EGFR as described previously(30). The staining results were evaluated by two immunohistochemistry experts from the service laboratory (N.K.; C Q) and a scientist (S.S) from the research laboratory at the same time to score the percentage of tumor cell nuclei stained (0, no staining; 1, ≤10%; 2, 10-50% and 3, >50%) and the staining intensity (0-negative, 1-weak, 2-moderate and 3- strong).
Indirect immunofluorescence
Indirect immunofluorescence staining was performed as described (29). Expression and localization of the proteins were observed under a confocal microscope system (FluoView FV500; Olympus, Melville, NY) and analyzed by CellQuest PRO software (BD Biosciences).
Tumor sphere formation assay
Sphere culture was performed as previously described (30). Briefly, single cell suspension of KATO-TN cells with (DOX+) or without (DOX-) YAP induction and JHESO cells with or without knock down YAP were seeded in triplicate onto a 6-well ultra-low attachment plate (2500 cells/well) (Corning) in serum-free DMEM/F-12 supplemented with 20 ng/ml epidermal growth factor, 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 2% B27 supplement w/o vitamin A and 1% N2 Supplement (Invitrogen). After 10-20 days of culture, the number of tumor spheres formed (diameter >100 µm) was counted under microscope.
Establishment of 5 FU-resistant subclones from EC cells
To establish 5FU-resistant subclones, Flo-1 parent EC cells were cultured with various concentrations of 5FU for 3-5 weeks, and the surviving cells were collected. This collection procedure was repeated four times. The establishment of these 5FU-resistant subclones took 3-6 months and newly derived 5FU-resistant clones, designated as Flo-1RF.
In vivo xenograft mouse model
JHESO cells were subcutaneously injected with 1×106 cells in nude mice. n=5 each group. After around 10 days invocation, VP was applied by intra-peritoneal (IP), 50mg/kg/mouse, 5-FU was applied by intra-peritoneal (IP), 30mg/kg/mouse and their combination, three times a week for total three weeks. Control group was applied same volume of PBS (100ul/mouse). The tumor size and volume were measured as previously (28). All the measurements were compared using unpaired Student's t test.
Statistical Analysis
Data were analyzed using the student t test and Fisher's exact test (for IHC); A P value of <0.05 was required for statistical significance, and all tests were two-sided as previously described.
Results
YAP1 and EGFR are overexpressed in EC tumor tissues and are associated with therapy resistance
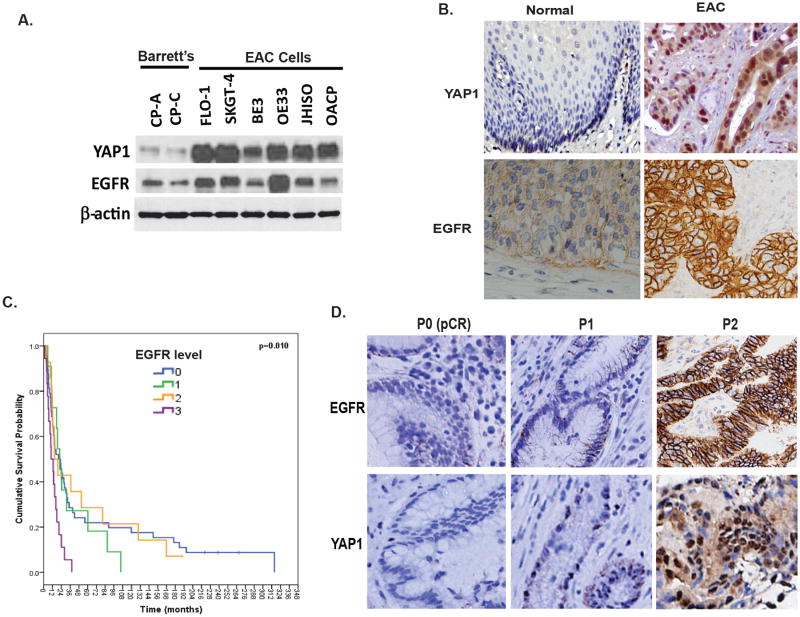
Both EGFR and YAP1 play important role in control growth and tumor maintenance. Previously, we have shown that EGFR is up-regulated in both EAC and ESCC and increased EGFR expression correlates with a shorter survival (9). To determine if both YAP1 and EGFR expressions are associated in EAC, immunoblotting was performed in two benign Barrett's cell lines CPA and CP-C and six EAC cell lines. Results in Figure 1A showed that expression of both YAP1 and EGFR are increased in EAC tumor cell lines compared to Barrett's cell lines. Immunohistochemistry was performed on a tissue microarray containing 113 cases of EAC together with normal controls using specific YAP1 and EGFR antibodies. As shown in Figure 1B, nuclear staining of YAP1 and membrane staining of EGFR are weak in normal squamous epithelium. However, strong nuclear staining of YAP1 was present in 56% of EAC tumor tissues; while membrane expression of EGFR was found in 32% of tumor tissues (Figure 1B) and was correlated with a shorter overall survival in univariate analysis (p=0.01; Figure 1C). To explore if both YAP1 and EGFR are associated with therapy resistance, we measured the expression of both YAP1 and EGFR in resistant tumors (P2) compared with the sensitive tumors (pretreatment biopsies tissues (P0/P1) and found that expression of both YAP1 and EGFR in resistant tumor tissues (P2) is correlated and much higher than in sensitive tumors (P0 or P1) (Figure 1D). 50% of resistant tissues (P2) has strong staining (3+) for both EGFR and YAP1, while only 20% of sensitive tumors (P0/P2) has weak staining (1+) for both EGFR and YAP1. These data support the notion that both YAP1 and EGFR are involved in EC tumor progression as well as therapy resistance.
Figure 1. YAP and EGFR are overexpressed in EC tumor tissues and associated with therapy resistance.
A. Expression of YAP and EGFR were determined by immunoblotting in Barrett's and EAC cell lines as described in Materials & Methods. B. Expression of YAP and EGFR were determined by immunohistochemistry using antibodies against YAP1 and EGFR in EAC TMA tissues. Representative YAP1 and EGFR staining are shown in normal and EAC tissues. C. Expression of YAP and EGFR were determined by immunohistochemistry in sensitive EAC tumor tissues (P0/pCR), relative sensitive tumor tissues (P1) and resistant tumor tissues (P2). Both EGFR and YAP highly expressed in majority of P2 tissues. D. Expression of EGFR significantly correlated the poor survival of EAC patients. Cox Regression for OS analysis; p<0.01.
YAP1 induces EGFR overexpression in EC tumor Cells
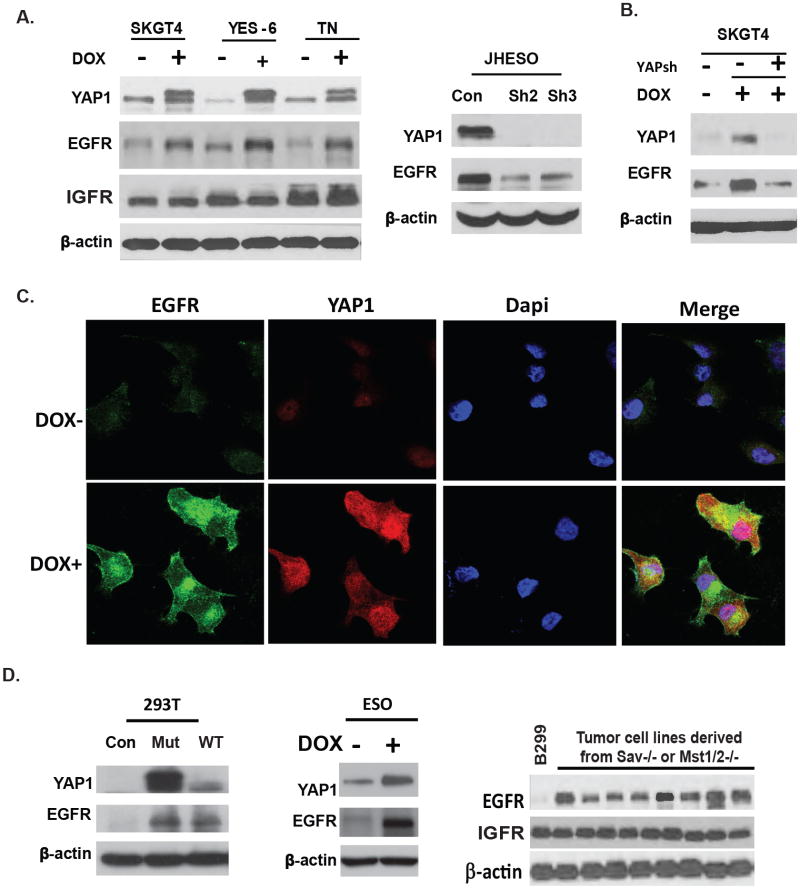
EGFR is overexpressed in many tumor types; and tumor cells utilize EGFR signaling to maintain their growth advantage, however, how EGFR is up-regulated is not well defined. We have previously demonstrated that conditional deletion of the core Hippo signaling components Sav1, Mst1/2 result in tumors of the mouse liver through deregulation of YAP1 (18). A transposon mutagenesis screen in a Sav1 mutant background revealed activation of EGFR is a frequent co-occuring event found in 50-60% of tumors. This observation led to the hypothesis that YAP1 might further activates EGFR signaling by increasing EGFR expression. To determine this possibility and to gain further insight into the relationship between YAP1 and EGFR expression, we first transduced the EC cells SKGT-4, YES-6 and KATO-TN cells with a doxycycline-inducible human flag-tagged YAP1S127A cDNA (PIN20 YAPS127A). Successful YAP1 induction in SKGT-4, YES-6 and KATO-TN cells by doxycycline at 1μg/ml increased expression of EGFR in concert with increased YAP1 (Figure 2A, left panel); while expression of IGFR was not affected (Figure 2A). In contrast, shRNA-mediated knockdown of YAP1 in JHESO cells greatly reduced EGFR protein levels (Figure 2A, right panel). Moreover, in SKGT-4 (PIN20YAP) cells, YAP1 induced EGFR expression was diminished by knockdown of YAP1 in doxycycline induced SKGT-4 cells (Figure 2B) confirming the direct regulation of EGFR expression by YAP1. Furthermore, immunofluorescence demonstrates that induction of YAP1 by doxycycline at 1μg/ml increases EGFR expression in SKGT-4 cells (Figure 2C). Similar findings were also seen in KATO-TN cells. These data indicate YAP1 up-regulates EGFR expression in EC cells.
Figure 2. YAP UP-Regulates EGFR Expression in both Normal and Malignant EC Cells.
A. SKGT-4, YES-6 and KATO-TN cells were transduced with lentiviral plasmid containing inducible YAP1 cDNA (PIN20YAP1). YAP expression was induced by doxycycline to the culture medium at 1μg/ml. Immunoblotting using antibodies against YAP, EGFR and IGFR were performed (left). Immunoblotting of YAP, EGFR was performed in JHESO cells with two independent YAP shRNAs (YAP sh2 and YAPsh3) clones (right). B. Immunoblotting of YAP, EGFR was performed in SKGT-4 (PIN20YAP) cells with (DOX+) or without (DOX-) YAP induction and knockdown YAP in SKGT-4 (PIN20YAP) DOX+ induced cells. C. Immunofluorescent staining of YAP and EGFR in SKGT-4 cells that were transduced with inducible YAP (PIN20YAP) with or without doxycycline induction at 1μg/ml. D. YAP and EGFR was detected by immunoblotting in 293T cells transfected with either mutant YAPS127A or wt YAP expression vectors (left). YAP and EGFR was detected by immunoblotting in primary murine esophageal cells transduced with lentiviral plasmid containing inducible YAP1 cDNA (PIN20YAP1) (middle). EGFR expression was examined by immunoblotting in B299 cells and tumor cell lines isolated from Mst1/2-/- mouse tumor tissues (right).
To determine if EGFR expression is regulated by YAP1 in primary esophageal cells and in other cell types, transfection of human embryonic kidney (HEK293T) cells with constitutively active mutant YAPS127A cDNA or with wild-type YAP1 induced EGFR expression (Figure 2D,left). In addition, primary murine Esophageal cells that expressed YAP1S127A (DOX+) demonstrated higher EGFR expression than cells without YAP1 induction (Figure 2D, middle). In addition, western blots analysis in hippo deregulated mice tumor cells lines isolated from hippo mutant (Sav-/- and Mst1/2-/-) mice tumor tissues demonstrated elevated EGFR expression compared to the immortalized liver B299 cells (Figure 2D, right), while there is no change in the level of IGFR. Hence, EGFR can be elevated in multiple cell types by expression of a constitutively active mutant form of YAP1 or by activation of endogenous YAP1 protein that occurs following deletion of Hippo pathway signaling components.
YAP1 Induced EGFR Transcription via TEAD binding site in the promoter of EGFR
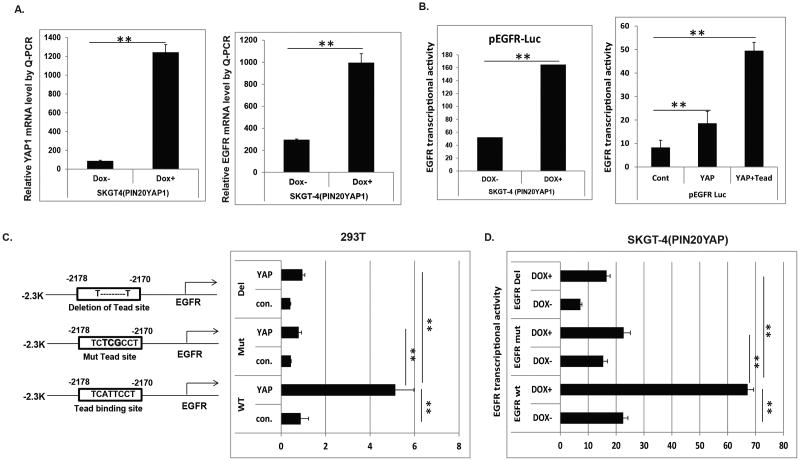
Having established that YAP1 regulates EGFR expression in multiple cellular contexts, we next examined this regulation occurs at the transcriptional or posttranslational level. First, EGFR mRNA level was up-regulated in EC cells stably transfected with YAP1 by Q-PCR as shown in Figure 3A (right panel) which is in concert with increased YAP1 mRNA level (left panel). Analysis of the human EGFR proximal promoter region reveals an intact TEAD (CATTCC) binding site located around -2178 to -2170 of base pairs upstream of the transcription start site. The 2.3k EGFR promoter containing this TEAD binding from the transcription start site was cloned and fused to a luciferase cDNA and cloned to the pGL4.22 vector and then were transfected into SKGT4 EC cells contain a stably integrated doxycycline-inducible YAP1S127A cDNA. Upon YAP1S127A induction by doxycycline (DOX+) administration, more than threefold induction of EGFR luciferase activity was observed (Figure 3B, left). As YAP1 is known to bind to TEAD transcription factors, we investigated whether YAP and TEADs can transactivate EGFR promoter-luciferase construct in EC cells. Hence, the 2.3 k EGFR promoter was co-transfected with either YAP1 or YAP1 and TEAD into 293T cells, EGFR Luciferase activities were increased more than two fold by YAP1, while co-transfected both YAP and TEAD, the EGFR transcriptional activity was increased by 5 fold (Figure 3, right). This indicates that YAP1 and TEAD cooperate to induce EGFR transcription. To determine if the TEAD binding site in the EGFR promoter is crucial for induction of EGFR by YAP1, either a mutation (ATT-TCG) or deletion of the TEAD binding site was generated in the EGFR promoter using site-directed mutagenesis as depicted in Figure 3C (left panel). Induction of EGFR transcriptional activity by YAP1 was greatly diminished, when mutation or deletion of the TEAD binding site in the EGFR promoter were introduced into 293T cells (Figure 3C, right panel). Similarly, in SKGT4 EC cells, induction of EGFR transcriptional activity by YAP1 induction upon doxycycline treatment was significantly reduced, when mutation or deletion of the TEAD binding site in the EGFR promoter was introduced (Figure 3D). These data indicate that YAP1 induces EGFR transcription requires an intact TEAD binding site in the EGFR promoter.
Figure 3. YAP increases EGFR transcription through an intact Tead binding site.
A. mRNA levels of YAP and EGFR were determined by Q-PCR in SKGT-4 cells transduced with lentiviral plasmid containing inducible YAP1 cDNA (PIN20YAP1) and induce YAP with or without doxycycline at 1 μg/ml. B. Transient transfection of EGFR luciferase promoter reporter into SKGT-4 (PIN20YAP) cells with or without induced YAP by doxycycline at 1 μg/ml;. EGFR luciferase reporter activity was measured after 48 hours (left panel). Co-transfection of EGFR luciferase promoter reporter with YAP or YAP plus TEAD into 293T cells; EGFR luciferase reporter activity was detected after 48 hours (right panel). C. Wide type Tead binding site and a mutation (ATT-TCG) or deletion of the TEAD binding sites were depicted (left panel). Co-transfection of EGFR luciferase promoters (wide type or mutated or deleted in the Tead binding site) with YAP or control vector into 293T cells; EGFR luciferase reporter activity was detected after 48 hours (right panel). D. Co-transfection of EGFR luciferase promoters (wide type or mutated or deleted in the Tead binding site) with YAP or control vector into SKGT-4(PIN20YAP) with (DOX+) or without (DOX-) YAP induction; EGFR luciferase reporter activity was detected after 48 hours. For all experiments, values shown represent the mean and SD of at least triplicate assays (**p<0.01).
YAP1 activates EGFR signaling and mediates cell survival and CSCs properties
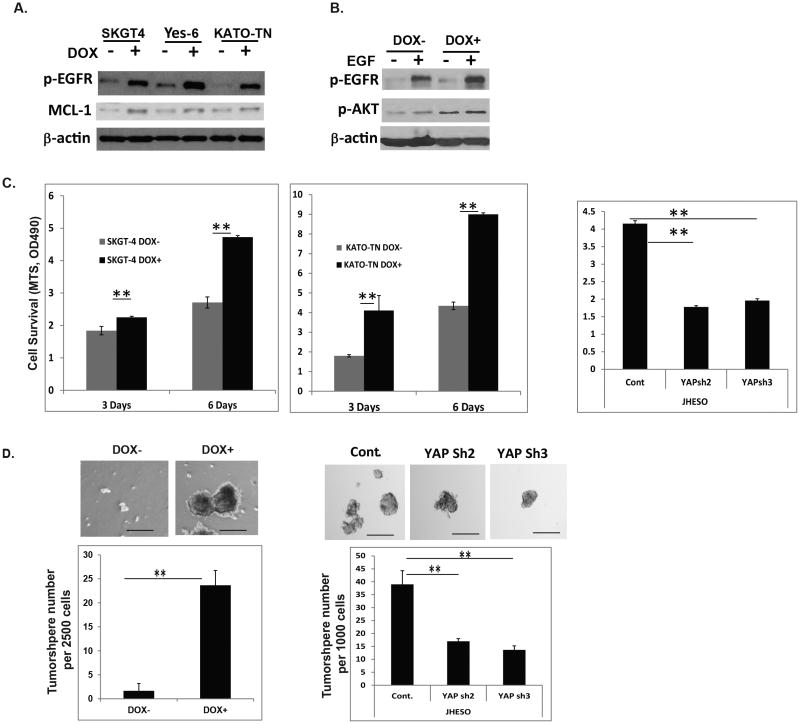
Activation of EGFR downstream signaling depends on both increased abundance of EGFR level as well as its phosphorylation. Having shown that YAP1 increases EGFR expression, next we sought to determine if YAP1 also increases its phosphorylation. Increased expression of YAP1 by doxycycline induction in three EC cell lines-SKGT-4, YES-6 and KATO-TN significantly induced phosphorylation of EGFR at pY1068 in concert with the increase in anti-apoptotic protein MCL-1 although not so dramatic change in its protein level (Figure 4A). Further, YAP increased and sustained EGF induced phospho-EGFR at pY1068 and its downstream AKT phosphorylation (Figure 4B). To determine the functionality of YAP1 induction in EC cells, we employed several assays and found that YAP1 induction in SKGT-4 and KATO-TN cells increased EC cell proliferation (Figure 4C,left and middle), and tumor sphere forming capacity (Figure 4D, left). In contrast, down-regulation of YAP by lentivirus shRNA in JHESO cells decreased cell viability (Figure 4C, right) and greatly reduced tumor sphere forming capacities (Figure 4D, right). These indicate that YAP1 is required for tumor cell survival and maintenance which probably involves activation of EGFR signaling.
Figure 4. YAP activates EGFR signaling and mediates cell survival and CSCs properties.
A. Phospho-EGFR and MCL-1 were detected by immunoblotting in SKGT-4, YES-6 and KATO-TN cells transduced with YAP1S127 cDNA (PIN20YAP1) with (DOX+) or without (DOX-) doxycycline induction; B. Phospho-EGFR and phosphor-AKT were detected by immunoblotting in SKGT-4 cells transduced with YAP1S127 cDNA (PIN20YAP1) with (DOX+) or without (DOX-) doxycycline induction and treated with EGF at 50ng/ml. C. Cell growth of SKGT-4 (PIN20YAP) and KATO-TN (PIN20YAP) with (DOX+) or without (DOX-) YAP induction were determined using MTS as described in Materials & Methods to determine the rate of proliferation at three and six days. **P<0.05 (left and middle). Cell growth of JHESO control and YAP knockdown cells (YAP sh2 and sh3) were determined using MTS to determine the rate of proliferation at three days. **P<0.05 (right panel). D. Representative images of spheres in KATO-TN (PIN20YAP) cells with (DOX+) or without YAP1 induction (DOX-) (top); Representative bar graph demonstrating the sphere numbers in KATO-TN (PIN20YAP) cells with (DOX+) or without YAP1 induction (DOX-) (low) (left panel). Representative images of spheres in JHESO cells with control and its knockdown (YAPsh) cells (top); Representative bar graph demonstrating the sphere numbers in JHESO cells with control and its knockdown (YAPsh) cells (low) (right panel). Data are represented as mean and SD from three experiments. ***p<0.001.
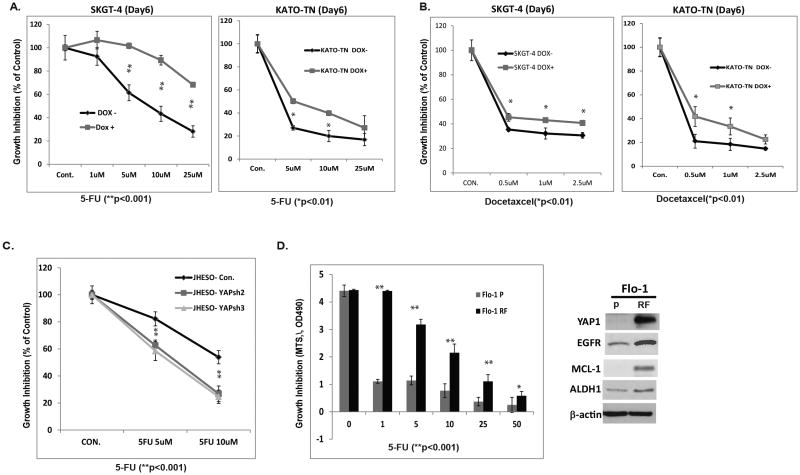
YAP1 mediates constitutive and acquired therapy resistance in EC cells
Expression of YAP1 and EGFR are increased in residual resistant tumor tissues in most post treated tumor tissues as shown in Figure 1C; we next sought to determine whether YAP1 mediated EGFR is responsible for constitutive or acquired chemoresistance in both EAC and ESCC cells. SKGT-4 and KATO-TN have constitutively high or low YAP and EGFR expression; and SKGT-4 cells with high YAP1 and EGFR expression have more invasive capacity than KATO-TN cells with low YAP and EGFR (Supplemental Figure 1A). When treating with 5-FU in these cells, SKGT-4 demonstrated more resistance than KATO-TN cell 5-FU treatment in different dosages (Supplemental Figure 1B, 1C). To further confirm the direct relationship between YAP1 and chemoresistance, induction of YAP1 in both EAC cell line SKGT-4 and ESCC cell line KATO-TN by doxycycline demonstrated more resistant to either 5-FU (Figure 5A) or docetaxel (Figure 5B) than EC cells without YAP1 induction (DOX-). In addition, down-regulation of YAP1 in JHESO cells in two individual clones greatly increased cell sensitivities to 5-FU than its parental cells JHESO (Figure 5C). Furthermore, in the established chemoresistant EC cells Flo-1RF, there is high expression of YAP1 and EGFR compared to their parental cells that is in concert with significant resistance to 5-FU treatment (Figure 5D). Moreover, as shown in Supplemental Figure 2, when we knocked down EGFR in YAP1 induced EC cells (SKGT-4 DOX+) using Lenti-Crisp system, EC cells become more sensitive to 5-FU treatment which phenocopys the effects by knocking down YAP1 as shown in Figure 5C. These data suggest that YAP1 induction of EGFR is associated with constitutive and acquired chemoresistance in EC cells.
Figure 5. YAP mediates chemoresistance in EC cells.
A&B. SKGT-4 (PIN20YAP) EAC cells and KATO-TN (PIN20YAP) ESCC cells with (DOX+) or without (DOX-) YAP induction and treated with 5-FU(A) or Docetaxcel (B) at the dosage indicated for six days then cell growth was determined using MTS as indicated in Materials and Methods. C. JHESO cells and its YAP knockdown cells (YAPsh2, YAPsh3) were treated with 5-FU at the dosage indicated for five days; cell growth was determined using MTS. D. Cell growth was determined using MTS in Flo-1 and its 5-FU resistant clone (Flo-1 RF) under the treatment of 5-FU at different dosage(left panel). Immunoblotting for YAP1, EGFR, ALDH1 and MCL-1 was performed in Flo-1 and its 5-FU resistant clone (Flo-1 RF) as indicated in Materials and Methods (right panel).
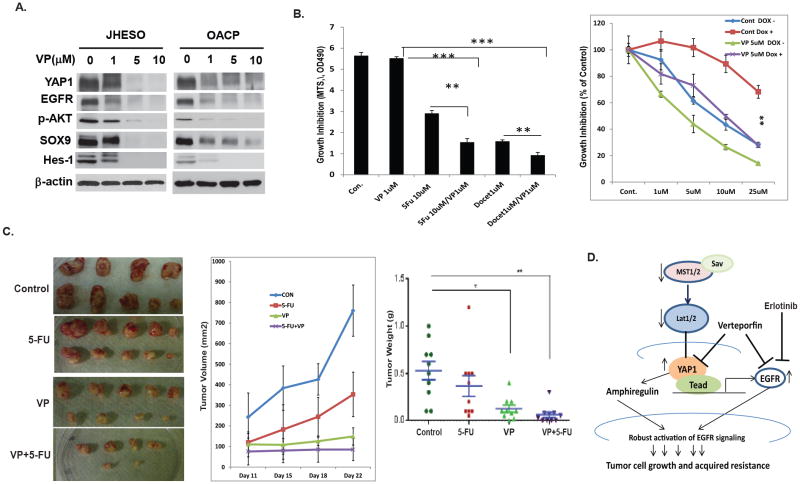
YAP1 inhibitor inhibits YAP1 and EGFR expression and sensitizes cytotoxic drugs in vitro and in vivo
Increased EGFR expression is associated with poor clinical outcome for EC patients and clinical trials based on EGFR inhibition have not been successful so far which may be due in part to the sustained increased EGFR expression and activation by YAP1. Therefore, alternative means of inhibition of EGFR signaling should be highly considered. We have demonstrated that YAP1 is responsible for sustained EGFR overexpression and activation, and hence targeting YAP1 may be an effective means to utmost inhibit EGFR signaling. Recently, Verteporfin has been identified as a small molecule inhibitor of YAP1 and TEAD association and a means of inhibiting YAP1's oncogenic activity (31). As demonstrated in Figure 6A, expression of EGFR and YAP1 were dramatically reduced by VP in a dose dependent manner in both JHESO and OACP cells. In addition, phosphorylation of AKT and other stem cell markers such as SOX9 and Hes-1 are reduced as well. Importantly, VP treatment alone at nontoxic concentration (1μM) has minimal inhibition on JHESO cells, while in combination with 5-FU or docetaxcel, the inhibitory effects on EC cells are dramatically increased (Figure 6B,left). This indicates that VP sensitizes the toxicity of 5-FU or docetaxcel on EC cells. In addition, to test if the combination of VP and the EGFR inhibitor, erlotinib, synergistically inhibits EC cell growth, low dosage of VP (0.5μM) or ErL (1μM) or 5-FU (5μM) either alone or in combination was applied in JHESO cells. Inexpertly, the combination of a low dosage of VP (0.5μ M) and Erlotinib (1μM) has minimal inhibition on JHESO cells, however, the triple combination of VP (0.5μ M) and Erlotinib (1μM) and 5-FU has best effects to inhibit EC cell survival (Supplemental Figure 3). To further determine if the combination of VP and 5-FU depends on the inhibition of YAP1, SKGT-4 stably YAP1 expression cells with (DOX+) or without (DOX-) YAP induction were treated with 5-FU and VP. The result in Figure 6B (right panel) demonstrated that YAP induction (DOX+) made SKGT-4 cells more resistant to 5-FU, however, VP in combination of 5-FU dramatically increases the response of SKGT-4 cells on 5-FU especially in inducible YAP1 expression SKGT-4 (DOX+) cells. Results from in vivo xenograft model further confirmed that mice treated with VP greatly reduced tumor growth in vivo, while the mice treated with VP in combination with 5-FU, the significant reduction of tumor weights and tumor volumes were observed compared with 5-FU alone (Figure 6C). In addition, the level of YAP1, EGFR and proliferation marker KI67 in mice tumors was dramatically diminished by the combination treatment of VP and 5-FU (Supplemental Figure 4). Thus, VP through inhibition of both EGFR and YAP1 can overcome the acquired chemo-resistance and sensitize 5-FU effects on EC tumors.
Figure 6. Pharmacological Inhibition of YAP1 and EGFR expression and sensitizes cytotoxics drugs in vitro and in vivo.
A. Effect of VP at different dosage on YAP1 and EGFR and other oncogenic and stem cell signaling gene expression in JHESO and OACP cells were determined using immunoblotting. B. JHESO cells were treated with VP, 5-FU, Docetaxcel and their combination as indicated; cell growth was determined using MTS. Data are represented as mean and SD from three experiments. **p<0.01; ***p<0.001 (left panel). SKGT-4 stably transduced inducible YAP cDNA(PIN20YAP) cells with (DOX+) or without (DOX-) YAP induction using doxycycline and treated with 5-FU and VP at the dosage indicated for six days, cell growth was determined using MTS. Data are represented as mean and SD from three experiments. **p<0.01 (right panel). C. JHESO cells (1.5×106) were injected subcutaneously in nude mice, 5 mice/group. Representative tumors after 5 weeks are shown in different group indicated (left). Tumor volumes and tumor weights and SD from different group are shown (middle). D. Proposed model and mechanisms by which YAP regulates sustained EGFR overexpression, activate EGFR signaling and mediates chemoresistance in esophageal cancer.
Discussion
In this study, we demonstrated for the first time that YAP1 up-regulates EGFR expression at the level of transcription through the intact TEAD binding site. YAP1 mediates sustained EGFR overexpression; increases cell proliferation and confers therapy resistance. Both YAP and EGFR are overexpressed in resistant EC tumor tissues and associated with acquired chemo-resistance in tumor tissues and in cell lines. VP, a novel YAP1 inhibitor, effectively inhibits both YAP1 and EGFR expression and sensitizes the cytotoxic effects of 5-FU or docetaxcel on EC cells. Our data demonstrated that YAP1-targeted modalities could be a promising strategy when in combination with cytotoxics to achieve maximal therapeutic effects.
The Hippo signaling pathway is gaining recognition as an important player in both organ size control and tumorigenesis, since the disruption of several important components (Mst1/2, Sav1 and Lats1/2 and YAP1) in this pathway can lead to tumorigenesis (18, 19, 32). YAP1, an effector of the Hippo signaling pathway, has been reported as an oncogene in several tumor types such as HCC and breast cancer and ESCC(33-35). EGFR overexpression or amplification has been reported in many human tumors and increased EGFR expression has been associated with advanced disease, development of metastasis and poor clinical prognosis in a subset of tumors including esophageal ESCC and EAC. Both EGFR and YAP1 play important role in cell proliferation, survival and tumor maintenance, perhaps chemoresistance. The cross-talk between these two pathways is merging. Zhang J et al first identified that EGFR ligand-amphiregulin (AREG) is a transcriptional target of YAP1, whose induction contributes to YAP-mediated cell proliferation and migration (17). Anterior Gradient Homolog 2 (AGR2) induction of EGFR ligand, AREG is mediated by activation of the Hippo signaling pathway co-activator, YAP1 (36). Similarly, TAZ, a paralog of YAP also induces AREG production and activation of EGFR signaling (37). Vice versa, EGFR ligands HB-EGF, AR and EGFR transactivator TGF-β stimulate expression of YAP1 target CTGF in hepatocellular carcinoma cell lines through up-regulation of YAP (38). A recent study from Reddy B et al demonstrated that the EGFR-RAS-MAPK branch of EGFR signaling activate YAP1 via promoting phosphorylation of Ajuba family protein WTIP and enhancing WTIP binding to Lats1/2(39). Hong X et al found that transforming activity of oncogenic RasV12 depends on its ability to downregulate SOCS-box proteins and thereby stabilize YAP1. Thus, the transforming potential of the Ras pathway appears to be mediated in part at the level of YAP1 protein turnover (40). In our study, we identified that YAP1 up-regulates EGFR protein expression at the level of transcription. Mutation or deletion of the TEAD binding site in the EGFR promoter diminished EGFR transcriptional induction by YAP1 indicating that an intact TEAD binding site is necessary for YAP1's induction of EGFR. Thus, Hippo signaling influences EGFR signaling by distinct mechanism by directly up-regulation of EGFR expression and up-regulation of its ligand amphiregulin identified previously (17). Both mechanisms can sustain robust activation of EGFR signaling, thereby increasing tumor cell survival and therapy-resistance (Figure 6D).
Although the current literature suggests that EGFR is overexpressed and activated in tumor cells and tissues, few reports discuss how EGFR is up-regulated or activated in tumor tissues. Our study sheds more light on one mechanism by which EGFR is deregulated in tumor tissues and indicates that YAP1 is responsible for the EGFR sustained overexpression in tumor cells.
EC is a lethal illness and an inherently resistant to therapy even if it is diagnosed as localized cancer. Following preoperative chemoradiation, residual cancer is noted in 70% of surgical specimens (41). However, the tumor resistant mechanisms are still unclear. We have previously reported that hedgehog (Hh) pathway is often upregulated in EC and mediates therapy resistance (42). mTOR activates Hh signaling in EAC by phosphorylation of S6K and the crosstalk between mTOR and Hh signaling confers tumor cell growth advantage and resistant to therapy(23). Our current data suggest that YAP1 mediated EGFR up-regulation in EC cells might be the driver for constitutive or acquired resistance. First, EC Cells with high YAP1 and EGFR are more invasive and more resistant to cytotoxic drugs. Second, both YAP1 and EGFR are up-regulated in post-treatment resistant tumor tissues (P2) compared to sensitive tissues (P0 or P1); and 5-FU resistant EC cell lines have more YAP1 and EGFR expression compared to their parental cells. Furthermore, the direct evidence for YAP1 mediated resistance is seen by introducing YAP1 into EC cells by doxycycline induction and cells with YAP1 induction have more growth advantage when treated with cytotoxic drugs. In contrast, knock down of YAP1 by lentivirus shRNA in EC cells decrease the tumor cell growth advantage and increase 5-FU sensitivity in EC cells which consistent with the recent finding of Huang J et al that knockdown of YAP1 sensitizes ovarian cancer cells to cisplatin and survivin inhibitors (43). This indicates that YAP1 confers the tumor cell growth advantage and mediates chemo resistance. Targeting YAP1 may be a means to overcome chemoresistance.
Although EGFR is overexpressed and amplified in EC, inhibition in the clinic has failed (11, 14). YAP1 mediated sustained EGFR up regulation and activation may be one explanation for this phenomenon. Although inhibition of EGFR signaling at the baseline by EGFR inhibitor or its monoclonal antibody, YAP1 keeps activation and up-regulation of EGFR that seems to confer therapy resistance as we have demonstrated in this report. Therefore, inhibition of YAP1 or in combination of EGFR and plus cytotoxics may be the best way to gain advantage. In fact, our data demonstrates that the YAP1 inhibitor VP effectively inhibits both YAP1 and EGFR protein levels and its downstream signaling and synergistically inhibit tumor cell growth when in combination with a cytotoxic in vitro and in vivo.
In conclusion, we have identified that YAP1 positively regulates EGFR expression at the level of transcription through an intact TEAD binding site at the EGFR promoter. Expression of both YAP1 and EGFR was increased in resistant EC tumors (P2) compared to sensitive tumors (P0/P1) and mediated therapy-resistance. Thus, the YAP1-EGFR axis could be an important therapeutic target in EC (Figure 6D). Future preclinical and clinical studies targeting YAP1-EGFR axis and in combination of chemotoxics are warrantied.
Supplementary Material
Translational Relevance.
Esophageal cancer (EC) is aggressive and therapy resistant malignancy. Overexpression of EGFR is poor prognosticator but in clinical trials, inhibition of EGFR has not provided benefit for EC patients. The reason for the lack of benefit is unclear. In this study, we document novel finding that the Hippo pathway coactivator YAP1 positively regulates sustained EGFR expression at the level of transcription through a TEAD binding site. YAP1 up-regulation of EGFR plays an important role in conferring therapy resistance in EC cells. Verteporfin, a small molecular inhibitor of YAP1, effectively diminishes both YAP and EGFR expression and sensitizes cells to cytotoxics. Therefore, targeting YAP-EGFR axis may be more promising than targeting EGFR alone in EC.
Acknowledgments
Grant Support: American Gastroenterological Association Research Scholar Award (Song S), and Public Health Service Grant DF56338 which supports the Texas Medical Center Digestive Diseases Center (Song S); UTMDACC IRG (3-0026317, Song S); NIH, CA129906, CA138671, and CA172741 (JAA).
Footnotes
There is no conflict interest in this study for all authors
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Paulson TG, Reid BJ. Focus on Barrett's esophagus and esophageal adenocarcinoma. Cancer Cell. 2004;6:11–6. doi: 10.1016/j.ccr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Wang X, Song S, Suzuki A, Taketa T, Sudo K, et al. ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Mol Oncol. 2014;8:142–9. doi: 10.1016/j.molonc.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–12. [PubMed] [Google Scholar]
- 5.Ishitoya J, Toriyama M, Oguchi N, Kitamura K, Ohshima M, Asano K, et al. Gene amplification and overexpression of EGF receptor in squamous cell carcinomas of the head and neck. Br J Cancer. 1989;59:559–62. doi: 10.1038/bjc.1989.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas ME, Leung DH, et al. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol. 2001;158:1245–51. doi: 10.1016/S0002-9440(10)64075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veale D, Kerr N, Gibson GJ, Kelly PJ, Harris AL. The relationship of quantitative epidermal growth factor receptor expression in non-small cell lung cancer to long term survival. Br J Cancer. 1993;68:162–5. doi: 10.1038/bjc.1993.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itakura Y, Sasano H, Shiga C, Furukawa Y, Shiga K, Mori S, et al. Epidermal growth factor receptor overexpression in esophageal carcinoma. An immunohistochemical study correlated with clinicopathologic findings and DNA amplification. Cancer. 1994;74:795–804. doi: 10.1002/1097-0142(19940801)74:3<795::aid-cncr2820740303>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Wang KL, Wu TT, Choi IS, Wang H, Resetkova E, Correa AM, et al. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer. 2007;109:658–67. doi: 10.1002/cncr.22445. [DOI] [PubMed] [Google Scholar]
- 10.Richards D, Kocs DM, Spira AI, David McCollum A, Diab S, Hecker LI, et al. Results of docetaxel plus oxaliplatin (DOCOX) +/- cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: results of a randomised Phase 2 study. Eur J Cancer. 2013;49:2823–31. doi: 10.1016/j.ejca.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627–37. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee MS, Mamon HJ, Hong TS, Choi NC, Fidias PM, Kwak EL, et al. Preoperative cetuximab, irinotecan, cisplatin, and radiation therapy for patients with locally advanced esophageal cancer. Oncologist. 2013;18:281–7. doi: 10.1634/theoncologist.2012-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan JA, Blaszkowsky LS, Enzinger PC, Ryan DP, Abrams TA, Zhu AX, et al. A multicenter phase II trial of single-agent cetuximab in advanced esophageal and gastric adenocarcinoma. Ann Oncol. 2011;22:1367–73. doi: 10.1093/annonc/mdq604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendell JC, Meluch A, Peyton J, Rubin M, Waterhouse D, Webb C, et al. A phase II trial of preoperative concurrent chemotherapy/radiation therapy plus bevacizumab/erlotinib in the treatment of localized esophageal cancer. Clin Adv Hematol Oncol. 2012;10:430–7. [PubMed] [Google Scholar]
- 15.Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922–7. doi: 10.1200/JCO.2006.07.1316. [DOI] [PubMed] [Google Scholar]
- 16.Al-Aidaroos AQ, Yuen HF, Guo K, Zhang SD, Chung TH, Chng WJ, et al. Metastasis-associated PRL-3 induces EGFR activation and addiction in cancer cells. J Clin Invest. 2013;123:3459–71. doi: 10.1172/JCI66824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–50. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–42. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Bard-Chapeau EA, Nguyen AT, Rust AG, Sayadi A, Lee P, Chua BQ, et al. Transposon mutagenesis identifies genes driving hepatocellular carcinoma in a chronic hepatitis B mouse model. Nat Genet. 2014;46:24–32. doi: 10.1038/ng.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raju U, Ariga H, Koto M, Lu X, Pickett J, Valdecanas D, et al. Improvement of esophageal adenocarcinoma cell and xenograft responses to radiation by targeting cyclin-dependent kinases. Radiother Oncol. 2006;80:185–91. doi: 10.1016/j.radonc.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Soldes OS, Kuick RD, Thompson IA, 2nd, Hughes SJ, Orringer MB, Iannettoni MD, et al. Differential expression of Hsp27 in normal oesophagus, Barrett's metaplasia and oesophageal adenocarcinomas. Br J Cancer. 1999;79:595–603. doi: 10.1038/sj.bjc.6690094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21:374–87. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–50. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–7. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalabis J, Wong GS, Vega ME, Natsuizaka M, Robertson ES, Herlyn M, et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc. 2012;7:235–46. doi: 10.1038/nprot.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalabis J, Oyama K, Okawa T, Nakagawa H, Michaylira CZ, Stairs DB, et al. A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. J Clin Invest. 2008;118:3860–9. doi: 10.1172/JCI35012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song S, Ajani JA, Honjo S, Maru DM, Chen Q, Scott AW, et al. Hippo coactivator YAP1 upregulates SOX9 and endows stem-like properties to esophageal cancer cells. Cancer Res. 2014;74:4170–82. doi: 10.1158/0008-5472.CAN-13-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, et al. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69:1343–9. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song S, Maru DM, Ajani JA, Chan CH, Honjo S, Lin HK, et al. Loss of TGF-beta adaptor beta2SP activates notch signaling and SOX9 expression in esophageal adenocarcinoma. Cancer Res. 2013;73:2159–69. doi: 10.1158/0008-5472.CAN-12-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–5. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St John MA, Tao W, Fei X, Fukumoto R, Carcangiu ML, Brownstein DG, et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21:182–6. doi: 10.1038/5965. [DOI] [PubMed] [Google Scholar]
- 33.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–10. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muramatsu T, Imoto I, Matsui T, Kozaki K, Haruki S, Sudol M, et al. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32:389–98. doi: 10.1093/carcin/bgq254. [DOI] [PubMed] [Google Scholar]
- 36.Dong A, Gupta A, Pai RK, Tun M, Lowe AW. The human adenocarcinoma-associated gene, AGR2, induces expression of amphiregulin through Hippo pathway co-activator YAP1 activation. J Biol Chem. 2011;286:18301–10. doi: 10.1074/jbc.M110.215707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang N, Morrison CD, Liu P, Miecznikowski J, Bshara W, et al. TAZ induces growth factor-independent proliferation through activation of EGFR ligand amphiregulin. Cell Cycle. 2012;11:2922–30. doi: 10.4161/cc.21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urtasun R, Latasa MU, Demartis MI, Balzani S, Goni S, Garcia-Irigoyen O, et al. Connective tissue growth factor autocriny in human hepatocellular carcinoma: oncogenic role and regulation by epidermal growth factor receptor/yes-associated protein-mediated activation. Hepatology. 2011;54:2149–58. doi: 10.1002/hep.24587. [DOI] [PubMed] [Google Scholar]
- 39.Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell. 2013;24:459–71. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong X, Nguyen HT, Chen Q, Zhang R, Hagman Z, Voorhoeve PM, et al. Opposing activities of the Ras and Hippo pathways converge on regulation of YAP protein turnover. The EMBO journal. 2014;33:2447–57. doi: 10.15252/embj.201489385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honjo S, Ajani JA, Scott AW, Chen Q, Skinner HD, Stroehlein J, et al. Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. Int J Oncol. 2014;45:567–74. doi: 10.3892/ijo.2014.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sims-Mourtada J, Izzo JG, Ajani J, Chao KS. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene. 2007;26:5674–9. doi: 10.1038/sj.onc.1210356. [DOI] [PubMed] [Google Scholar]
- 43.Huang JM, Nagatomo I, Suzuki E, Mizuno T, Kumagai T, Berezov A, et al. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32:2220–9. doi: 10.1038/onc.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.