Abstract
Background
Cyclosporine A (CsA) is an immunosuppressive drug which has been widely used to prevent rejection following organ transplantation. However, its therapeutic use is limited by nephrotoxicity, in part mediated by oxidative stress. The present study aims to investigate the protective effects of Dimethyl Fumarate (DMF) on CsA-induced nephrotoxicity by enhancing the antioxidant defense system.
Methods
Male Sprague-Dawley rats were treated with CsA (n=8, 20 mg/kg/day i.p.) orCsA + DMF (n=7, 50 mg/kg/day p.o.) for 28 days. Renal function, histopathology, malondialdehyde (MDA), myeloperoxidase (MPO) levels and anti-oxidant enzyme expression were determined.
Results
DMF co-treatment ameliorated CsA-induced renal dysfunction as evidenced by significant decrease in serum creatinine (CsA 0.79 ± 0.02 mg/dl vs. CsA + DMF 0.62 ± 0.04 mg/dl, P=0.001) and urea (CsA 66.9 ± 0.4 mg/dl vs. CsA + DMF 53.3 ± 2.6 mg/dl, P<0.0001) levels, as well as improvement of creatinine clearance. DMF also significantly decreased serum MDA and renal tissue MDA and MPO contents. The protein expression of NQO-1, a major cellular anti-oxidant and detoxifying enzyme was significantly enhanced by DMF administration in kidney.
Conclusions
Administration of DMF has a protective potential against CsA nephrotoxicity. The protection afforded by DMF is mediated in part through inhibiting oxidative stress and inflammation and enhancing the antioxidant capacity.
Introduction
Cyclosporin A (CsA) is an important immunosuppressive drug that has been widely used for organ transplantation and for treatment of autoimmune diseases (1). Immunosuppressive therapy with CsA is often limited by serious nephrotoxicity (2). Renal dysfunction occurs in up to 30% of patients (3). The mechanisms by which CsA causes acute reversible and chronic irreversible nephrotoxicity are not well understood but are thought to be, in part, due to oxidative stress and depressed endothelium- derived nitric oxide production (4). An increased production of free radical species and lipid peroxidation products have been demonstrated in kidney tissue under CsA treatment (5). The involvement of oxidative stress was further supported by the finding that many antioxidants and free radical scavengers were capable of reducing experimental renal injury caused by CsA(6, 7).
Dimethyl Fumarate (DMF) was recently approved by FDA for use in the treatment of patients with multiple sclerosis. Although the mechanism of action of DMF is not clearly understood, DMF has been shown to inhibit pro-inflammatory cytokine production and NF-κB signaling via inhibition of its nuclear translocation (8). DMF carries, as well, a unique antioxidant profile (9-14).
In the present study, we sought to investigate whether DMF would have a protective effect against CsA nephrotoxicity.
Results
Effects of CsA and DMF on body weight change and urine output
The CsA-treated group had a non-significant weight loss compared to the control group (P=0.12). However, CsA+DMF group had significantly lower body weight increase compared to control group (P=0.003). Urine output was significantly increased in the CsA group (P=0.03, vs. Normal) and decreased by DMF co-treatment (P=0.01, vs. CsA group). There was no significant difference between the control group and CsA + DMF group (P=0.21). The similar tendency was observed in urine output divided by body weight (Fig.1; A-C)
Figure 1. Effects of CsA and DMF on body weight, urine volume and renal function.
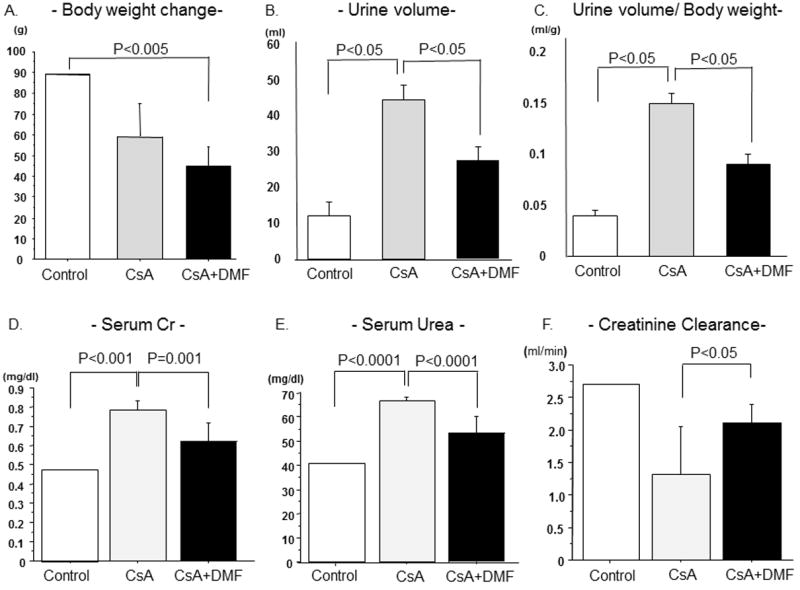
Effects of CsA and DMF on body weight (A), urine volume (B), urine/weight(C), serum creatinine (D), serum urea (E), and Creatinine Clearance (F). Data represent Mean±S.D.
Effects of CsA and DMF on renal function
The effects of CsA and DMF on renal function are shown in Figure 1; D-F. Serum creatinine (Cr) and urea were significantly increased (P < 0.001, P<0.0001, respectively) and creatinine clearance was significantly reduced in the CsA-treated group. DMF Treatment significantly reduced serum Cr and urea (P=0.001, P<0.0001 respectively) concentrations and significantly improved creatinine clearance in DMF + CsA group compared to CsA group (P=0.01).
Histo-pathological changes
The photomicrographs of kidney in experimental groups are depicted in Figure 2, and the histopathological scores are summarized in Figure 3. There is no histological change by DMF administration without CsA. Histological injury including tubular cell vacuolization, tubular atrophy, interstitial fibrosis, arteriolar hyalinosis and glomerular mesangial matrix expansion were observed in CsA group (Fig.2; e-h). These structural alterations were attenuated by DMF administration (Fig. 2; i-l). There was a significant difference between the control and CsA groups in tubular atrophy, interstitial fibrosis, glomerular injury and arteriolar hyalinosis (Fig. 3: A-D). Tubular injury score was significantly reduced by DMF administration (Fig. 3A, P=0.02, vs. CsA group). In addition insignificant trends for improvements in glomerular injury and arteriolar hyalinosis scores were observed in the DMF group. (Fig. 3B and C, P=0.12, P=0.10 respectively vs. CsA group).
Figure 2. Histopathological changes.
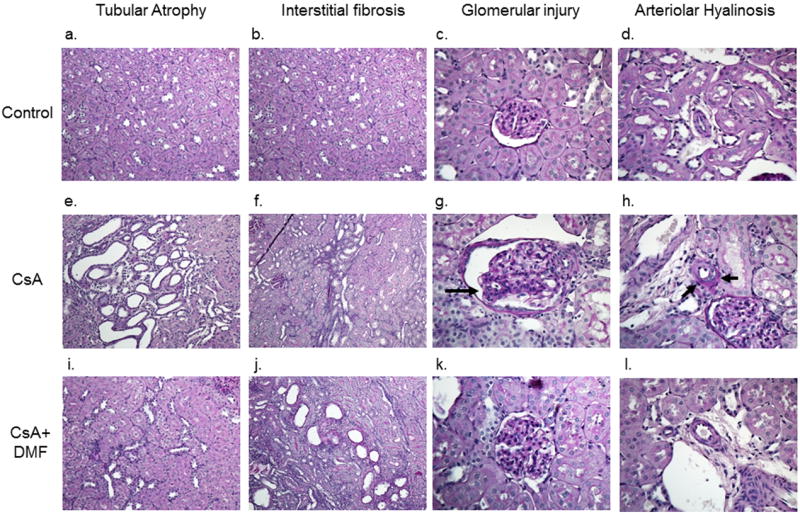
Kidney sections from Normal (a-d), CsA (e-h), CsA+DMF (i-l) groups. CsA treated group showed histological injury included tubular cell vacuolization, tubular atrophy, interstitial fibrosis, glomerular mesangial matrix expansion and arteriolar hyalinosis. These structural alterations were attenuated when CsA treatment was accompanied by DMF.
Figure 3. Histopathological scores.
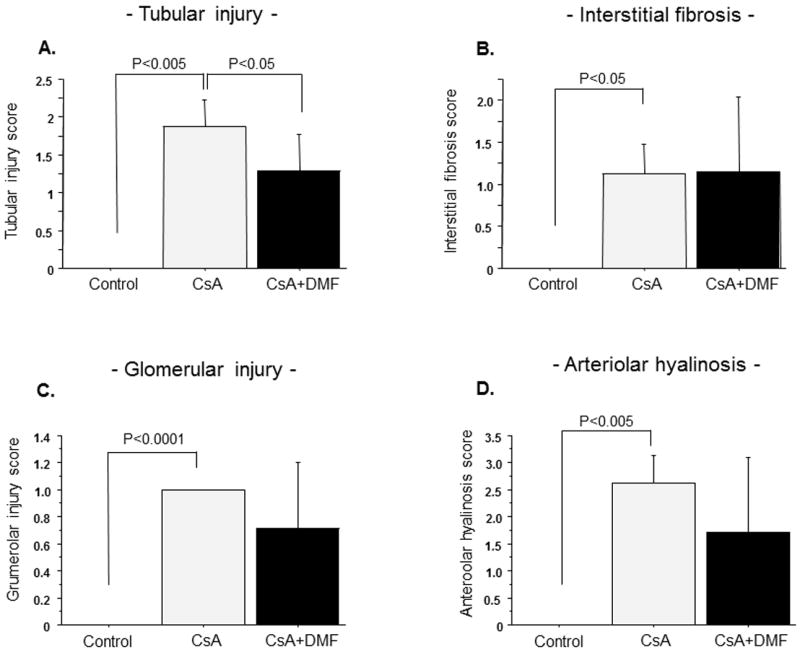
Effects of CsA and DMF on tubular injury (A), interstitial fibrosis (B), glomerular injury (C) and arteriolar hyalinosis (D). Data represent Mean±S.D.
Effects of CsA and DMF on serum and renal MDA levels
As shown in Figure 4A &B, there was a significant increase in the serum MDA level in the CsA treatment group (P=0.01). Co-administration of DMF reduced serum MDA to the level found in the control group (P=0.0003 vs. CsA, P=0.74 vs. normal). CsA administration also resulted in modest increases in the renal tissue MDA level (P=0.26), which was markedly ameliorated by DMF administration (P=0.001 vs. CsA).
Figure 4. Effects of CsA and DMF on serum and tissue MDA, MPO activity.
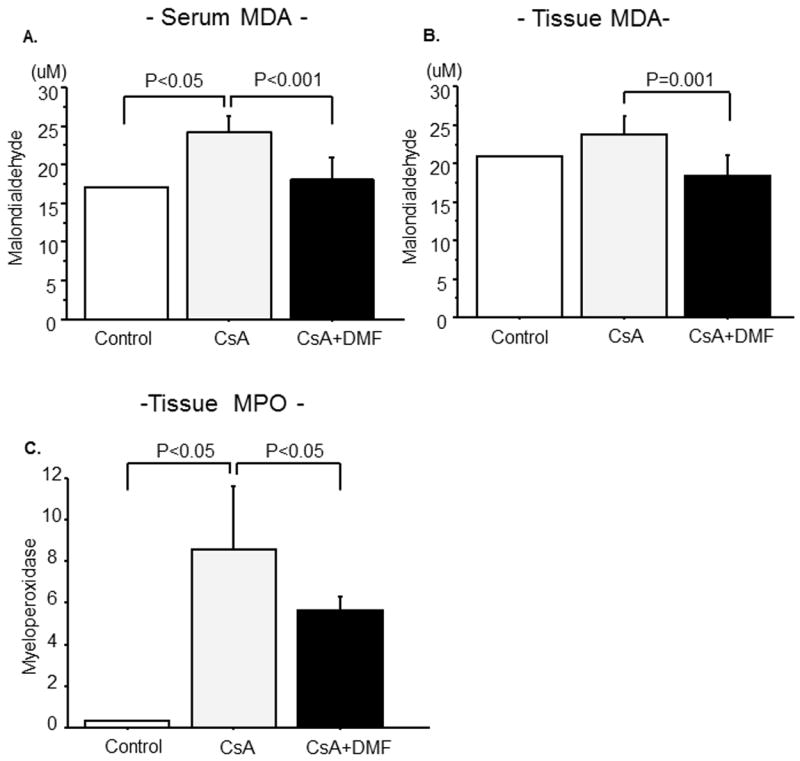
Effects of CsA and DMF on serum (A) and kidney (B) MDA (C) MPO activity. Data represent Mean±S.D.
Effects of CsA and DMF on renal MPO activity
As shown in Figure 4C, there was a significant increase in the renal MPO level in the CsA treatment group (P=0.03). Co-administration of DMF significantly decreased the CsA-induced elevation of the renal tissue MPO level (P=0.03).
Effect of DMF on nuclear Nrf2 protein expression in renal tissue
Renal tissue was evaluated via immunoblotting for protein expression of Nrf2 in nuclear fractions (i.e., activated Nrf2) after 15 or 30 mg/kg administration of DMF. DMF significantly increased the nuclear Nrf2 content in a dose-dependent manner (Figure 5A).
Figure 5. (A) Effect of DMF on nuclear Nrf2 expression in kidney.
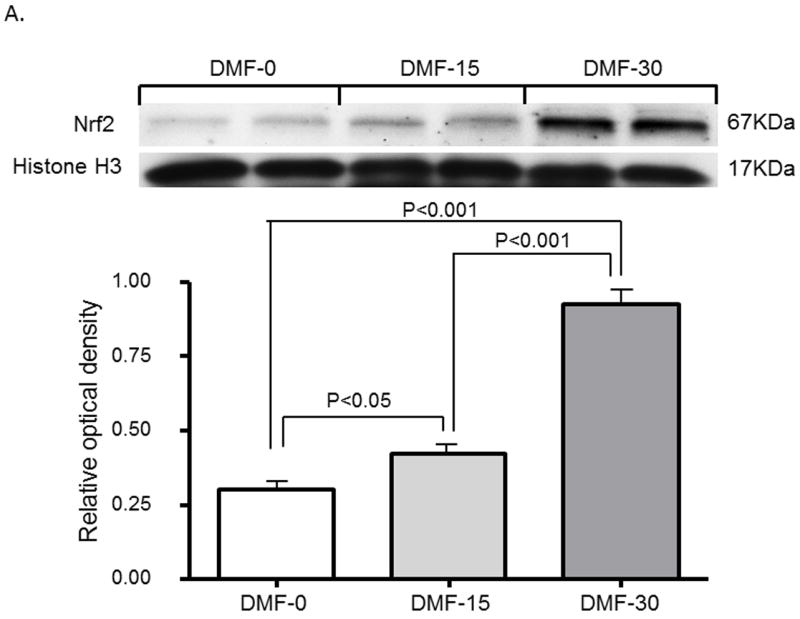
Representative western blots data are presented, depicting protein abundance of Nrf2 in the kidneys of SD rats treated with vehicle, 15 or 30mg/kg of DMF for 1 day. Histone H1 served as the loading control for Nrf2. Histone H1 served as the loading control for Nrf2 depicting protein abundance of Nrf2 in nucleus.
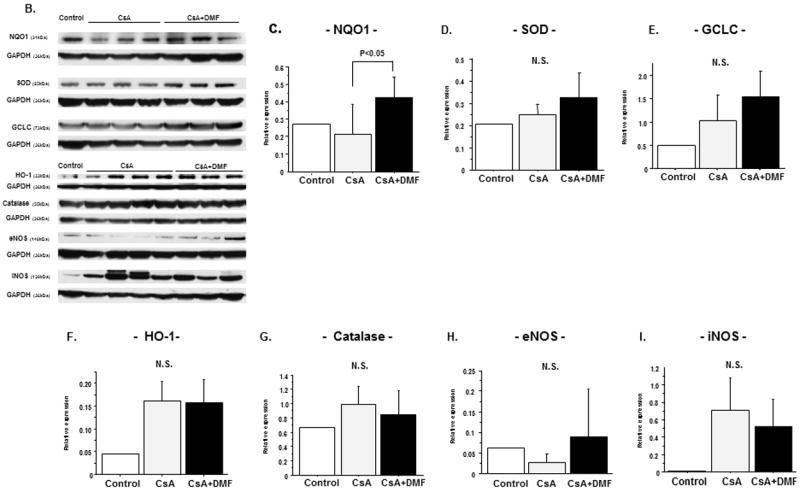
(B) Effects of CsA and DMF on anti-oxidant enzyme expressions in kidney
(B) Representative western blots of NQO-1, SOD, GCLC, HO-1, Catalase, iNOS and eNOS. The bar graph summarizing the western blot data of (C) NQO-1 (D) SOD (E) GCLC (F) HO-1 (G) Catalase (H) iNOS and (I) eNOS. Data represent Mean±S.D.
Effect of CsA and DMF on the activities of anti-oxidant enzymes and nitric oxide system in renal tissue
Figure 5B shows the protein expression of anti-oxidant enzymes and nitric oxide synthase isoforms in the renal tissue. CsA treatment resulted in decrease in the expression of NQO-1 which was improved in CsA+DMF group (P=0.03 vs. CsA). The protein expressions of SOD, catalase, and GCLC were not significantly different among the three groups.
CsA treatment, resulted in no difference in eNOS expression of (P=0.16), and increase in iNOS expression (P=0.12). Administration of DMF increased eNOS expression (P=0.18) and decreased iNOS expression (P=0.15).
Discussion
In the present study, we have demonstrated that DMF may have a protective effect against CsA nephrotoxicity. We found that DMF maintained renal function and conferred protection against CsA nephrotoxicity. This was associated with and possibly, in part, mediated by attenuating oxidative stress, (as evidenced by the reduction of lipid peroxidation product, MDA, and maintenance of the antioxidant enzymes) and inflammation (as evidenced by reduction of tissue MPO level and inflammatory cell infiltration).
Significant increases in serum Cr and urea and decreases in creatinine clearances were found in CsA treated rats. These changes were associated with a remarkable rise in urine flow, pointing to CsA-induced renal tubular dysfunction which was confirmed by histological evidence of tubular injury, interstitial fibrosis, glomerular injury and arteriolar hyalinosis. These observations are consistent with earlier reports (15, 16), which demonstrated significant changes in these renal functional parameters in both patients and experimental animals following CsA administration. DMF administration reversed CSA-induced polyuria suggesting its potential protective effect against tubular injury.
The development of CsA nephrotoxicity in the present study was accompanied by the rise in renal and serum MDA, a byproduct of lipid peroxidation, suggesting excessive generation of reactive oxygen species (ROS) and cellular damage. The increased levels of free radicals, lipid peroxidation products, and reduction of endogenous antioxidants following CsA administration have also been demonstrated in previous reports (6, 17, 18). Thus, we provided further support for the role of oxidative stress as a pathogenic factor in nephrotoxicity induced by CsA. Amelioration of renal dysfunction with DMF administration was accompanied by the reduction in serum and kidney tissue MDA levels which suggests that the salutary effect of DMF may be related to its antioxidant properties. This possibility has been substantiated by numerous reports demonstrating that DMF can activate the Nuclear factor erythroid2-related factor1 (Nrf2)-Kelch-like ECH Associated protein1 (Keap1) pathway (10, 11, 14) and increase cellular levels of anti-oxidant enzymes such as NQO1, HO-1, and GCLC. Nrf2-keap1signaling pathway plays a significant role in protecting the cells against various stresses including endogenous and exogenous oxidants, inflammatory stresses, and chronic exposures to cigarette smoke and other carcinogens (19-25). The cytoprotective effects of Nrf2 are mediated by transcriptional up-regulation of genes encoding numerous antioxidant, detoxifying and cytoprotective enzymes and related molecules.
In the present study, there were no significant changes in anti-oxidant enzyme expressions by CsA administration. Other studies indicate that HO-1 induction exerts renal protective effects in animal models including CsA-induced nephropathy, ischemia-induced acute kidney injury, radiation-induced nephropathy, and cisplatin nephrotoxicity (26, 27). Therefore this increase tendency may be explained by a self-defense system against CsA. DMF administration significantly enhanced the NQO-1 protein expression. This was associated with the reduction of oxidative stress as evidenced by the fall in the serum and renal tissue MDA levels.
Via inhibition of nuclear translocation of NFkB which regulates expression of numerous genes encoding pro-inflammatory cytokines and chemokines, DMF has been shown to exert potent anti-inflammatory actions (28). Recent studies have also demonstrated that NF-κB is involved in the regulation of iNOS expression (29). The induction of iNOS is also involved in CsA-induced renal damage (30). CsA has been shown to induce the expression of certain chemokines and adhesion molecules, such as monocyte chemoattractant protein-1, intercellular molecule-1, and vascular cell adhesion molecule-1, which activate or recruit the transmigration of inflammatory cells into the site of renal injury (31), It is, therefore, conceivable that the protection afforded by DMF may be, in part, mediated through inhibition of NF-κB activation and the associated inflammation. In fact, the renal tissue MPO activity (an indicator of neutrophil infiltration) which was significantly increased in CSA-treated rats was reduced by DMF administration pointing to its anti-inflammatory properties. Chronic CsA administration differentially affects nitric oxide synthase isoforms and NO production (32). CsA causes renal arteriolar vasoconstriction and increase renal vascular resistance by decreasing eNOS-mediated NO production (33). On the other hand, via induction of iNOS and consequent oxidative and nitrosative stress CsA promotes renal damage (30). iNOS produces NO which reacts with superoxide generated by NADPH oxidases (NOX) to form peroxynitrite. Previous reports showed CsA-induced significant decreases in eNOS expression while inducing significant increases in iNOS expression. We observed the same trend in the CsA-treated animals which was reversed with DMF. Our findings also suggest that the ROS/NO systems play a role in the pathogenesis of CsA-induced renal injury, and that DMF favorably influences these abnormalities.
Although antioxidant and anti-inflammatory activities may be the principal mechanism for the protection afforded by DMF, other unidentified actions cannot be excluded. Future works on this aspect are warranted.
DMF has been widely used in Europe for the treatment of psoriasis vulgaris and psoriatic arthritis which are chronic inflammatory disorders, for over 20 years. DMF has recently been investigated clinically in the United States for its neuroprotective effects. In a phase 3 trial for relapsing–remitting multiple sclerosis (MS), Oral DMF significantly reduced the proportion of patients who had a relapse, the rate of disease progression, the annual relapse rate, and the number of gadolinium enhancing lesions and new or enlarging T2 weighted hyperintense lesions on MRI (34).
The most common adverse reactions included flushing and GI events (nausea, diarrhea), which were classified as mild to moderate in nature. Most events occurred at the start of therapy and usually decreased over time. In clinical trials 40% of patients experienced flushing. However, only 3% discontinued the drug because of adverse side effects. The most severe adverse reaction reported was a decrease in lymphocyte count. In clinical trials, mean lymphocyte count decreased by approximately 30% during the first year and then remained stable. Six percent of patient experienced lymphocyte counts less than the lower limit of normal. However the incidence and severity of infections was similar in the treatment and placebo groups. An increased incidence of elevated hepatic transaminases was observed in patients treated with Tecfidera (DMF clinical grade) compared to placebo, primarily in the first 6 months of treatment and most had levels that were less than 3 times the upper limit of normal. Discontinuation of the drug due to elevated hepatic enzymes was < 1% and was similar for both the treatment and placebo patients. According to clinical trial for patients with MS, there is no report regarding the abnormal renal function. In this ongoing trial, patients have been taking more than 5 years.
We also checked islet cells, pancreas, liver and intestine in rat for other projects after DMF treatment, showing no abnormality.
Furthermore, CSA and tacrolimus are calcineurin inhibitors and can both cause nephrotoxicity. It is therefore reasonable to assume that the mechanism by which they cause nepephrotoxicity must be similar and that interventions that attenuate the adverse effects of one may be equally effective for the other. However, since the present study involved CSA alone we cannot comment on the efficacy of DMF in prevention of tacrolimus nephrotoxicity. Future studies are needed to address this important question.
In conclusion, the present study demonstrated that DMF treatment confers renal protection against CsA nephrotoxicity at least, in part, by attenuating oxidative stress and inflammation and enhancing or maintaining the antioxidant defense system. Our findings suggest that DMF may be a promising treatment option for limiting CsA-associated nephrotoxicity.
Methods
Animals
Sixteen pathogen free Sprague-Dawley male rats, weighing 230-250 g were purchased from Charles River (Wilmington, MA). The rats were housed under standard conditions (room temperature 22°C, humidity 50 ± 5%, 12:12 hour light/dark cycle). The study was approved by Institutional Animal Care and Use Committee of University of California, Irvine.
Experimental Design
Sixteen rats were randomly divided into 3 experimental groups: (1) Control: given a vehicle. (2) CsA group (n=8): were treated with CsA (Novartis Pharma AG, Switzerland) i.p. at a dose of 20 mg/kg for 28 days. This dose was chosen based on earlier reports, which have been shown to successfully and consistently produce nephrotoxicity (35, 36) (3) CsA+DMF group (n=7): In addition to CsA, DMF (25 mg / kg, twice / day) was orally administered via gavage for 28 days. Experimental rats were given oral DMF (25mg/kg, twice/day) dissolved in methyl cellulose (SIGMA, MO). After the last dose of CsA, rats were kept individually in metabolic cages for 24 h with free access to food and water to collect urine for the estimation of renal function. Blood samples were taken at the end of urine collection by cardiac puncture under anesthesia. The kidneys were immediately removed for evaluation.
Histo-pathologic Analysis
Hematoxylin and eosin (H.E.) and periodic acid-Schiff (PAS) staining was performed on, kidney tissue samples fixed in 10% buffered formaldehyde and embedded in paraffin. Evaluation of kidney histology was carried out in a blinded manner after a semiquantitative scoring system by two pathologists. Histologies were graded in regards to their tubular epithelial aspects, glomerular and vascular alterations according to modified Banff classification criteria (37). Each kidney sample had 20 randomly selected, non-overlapping fields analyzed for H.E. and PAS stains. Tubular injury (TI) was graded (0 to 3) based on the presence of tubular atrophy (=interstitial widening) and presence/degree of isometric tubular vacuolization: 0 = no changes present, grade 1= ≤25%; grade 2 = 26 to 50 % and grade 3 = > 50% TI involvement. Interstitial fibrosis (IF) was scored as a sign of architectural destruction: 0 = no changes present, grade 1- 25%; grade 2 = 26 % to 50 % and grade 3 = > 50% TI involvement. Glomerular injury (GI) was graded 0-3 for sclerosis and mesangial matrix expansion as a marker for glomerular ischemia and damage. Renal arterioli were evaluated with respect to the presence of hyalinosis or sclerosis. Grade 0 = no arteriolar changes; mild-moderate (grade 1) = 1 arteriole affected; moderate-severe (grade 2) = 1-2 arterioles affected; severe (grade 3) = more than 2 arterioles affected.
Assessment of renal function
Blood and urine samples were analyzed for urea and creatinine using a commercially available kit (BioAssay Systems, CA). Creatinine clearance was calculated using standard formula.
Measurement of serum and renal MDA
Serum and renal tissue malondialdehyde (MDA) was measured using the TBARS assay kit (Cayman, USA) according to the manufacturer’s instructions.
Myeloperoxidase Assay
The presence of myeloperoxidase (MPO) was used as an index of neutrophil accumulation in the kidney using the MPO colorimetric Assay kit (BioVision, Milpitas, CA) according to the manufacturer’s instructions.
Protein Extraction and Western Blots
All steps for protein isolation were conducted at 4°C. Kidney samples were homogenized in Tissue Protein Extraction Reagent (RIPA, Thermo Scientific, NJ) with Protease Cocktail Inhibitor (Sigma, MO). The resulting homogenate was centrifuged at 1600 × g for 10 minutes. The supernatant total protein content was estimated using by the Bio Rad Assay kit (Bio-Rad, CA). Equal 50 μg protein amounts were loaded into 4-12% BIS TRIS (2-[Bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)-1,3-propanediol) (Life Technologies, CA) and transferred to nitrocellulose membrane (GE Healthcare Biosciences, MA), which had been blocked in 5% nonfat dry milk in Tris-buffered saline with Tween- (TBST) for 90 minutes. The membranes were then probed with rabbit polyclonal antibody to inducible nitric oxide synthase (iNOS) (1:400, Santa Cruz, CA), endothelial nitric oxide synthase (eNOS) (1:400, Santa Cruz, CA), glutamate-cysteine ligase catalytic (GCLC) (1:1000, Abcam, CA), superoxide dismutase (SOD) (1:200, Santa Cruz, CA), heme oxygenase-1 (HO-1) (1:1000, Abcam, CA), and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5000, Cell Signaling, MA). Rabbit monoclonal antibody to Catalase (1:5000, Rockland Immunochemicals, PA) and mouse monoclonal antibody to NAD(P)H quinone oxidoreductase-1 (NQO-1) (1:1000, Abcam, CA) followed by secondary anti-rabbit or mouse immunoglobulin G (1:3000, Cell Signaling, MA) were also accessed. The membranes were developed with an enhanced chemoluminescence detection kit (Bio-Rad, CA) and exposed to X-ray film (Kodak, Rochester, NY). GAPDH was the internal reference for quantification. Immunoblots scanned by the densitometer were subjected to grey analysis with Image Quant TL 7.0 (GE healthcare Life Sciences, PA).
Western Blot for nuclear Nrf2
DMF dissolved in methyl cellulose was orally administered via gavage for twice / day. SD rats were treated with vehicle, 15 mg/kg or 30mg/kg. 100 mg of kidney cortex was homogenized on ice in lysis buffer containing 10 mM HEPES, pH7.9, 1.5mM MgCl2, 10mM KCL, 1mM DTT and Protease Inhibitor Cocktail (Sigma–Aldrich, St. Louis, MO). Cellytic TM NuCLEAR™ Extraction Kit (Sigma–Aldrich, St. Louis, MO) was used for isolation of nuclear proteins. Protein concentration in the tissue homogenates was determined by DC protein assay kit (Bio-Rad, U.S.A) and 100 μg of protein per sample was fractionated on 4–12% Novex ®Tris-Glycine gel (Invitrogen, Carlsbad, CA) at 120 V for 2 h and transferred to nitrocellulose membrane (Invitrogen, Carlsbad, CA). The membrane was incubated for 1 h in blocking buffer (1X TBS, 0.05% Tween-20 and 5% nonfat dry milk) and then overnight in the same buffer containing the given primary antibody. The membrane was washed three times for 10 min in 1X TBST before a 2-h incubation in a buffer (1X TBST) containing horseradish peroxidase-conjugated anti-rabbit (1:3,000) (Abcam), anti-mouse (1:2000) (GE Healthcare) and anti-sheep (1:5000) IgG (EMD Millipore) secondary antibodies. The membrane was washed three times, then visualized with ECL™ prime western blotting detection reagent (GE Healthcare) and developed by autoluminography. Band densities were quantified using the free Image J software (version 10.2) from the National Institutes of Health (www.imagej.nih.gov/ij/).
Statistical Analyses
All the results are presented as mean ± standard deviation (SD). Comparisons between two was performed with Student’s t-test, Mann–Whitney’s U t -test, as appropriate as appropriate using Stat View-J 5.0 software (SAS, Cary, NC). Statistical significance was defined as p-value less than 0.05.
Acknowledgments
Funding sources
This study was in part supported by grants from: NIH-NCRR UL1 TR000153, KL2 TR000147; the Juvenile Diabetes Research Foundation International 17-2011-609.
Abbreviations
- CsA
Cyclosporine A
- DMF
Dimethyl Fumarate
- eNOS
endothelial nitric oxide synthase
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GCLC
glutamate-cysteine ligase catalytic
- HO-1
heme oxygenase-1
- iNOS
inducible nitric oxide synthase
- MDA
malondialdehyde
- MPO
myeloperoxidase
- NQO-1
NAD(P)H quinone oxidoreductase-1
- SOD
superoxide dismutase
Footnotes
All authors have no reported conflicts of interest.
References
- 1.Halloran PF. Molecular mechanisms of new immunosuppressants. Clinical transplantation. 1996;10(1 Pt 2):118–23. [PubMed] [Google Scholar]
- 2.Burdmann EA, Andoh TF, Yu L, Bennett WM. Cyclosporine nephrotoxicity. Seminars in nephrology. 2003;23(5):465–76. doi: 10.1016/s0270-9295(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 3.Tedesco D, Haragsim L. Cyclosporine: a review. Journal of transplantation. 2012;2012 doi: 10.1155/2012/230386. 230386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaziri ND, Ni Z, Zhang YP, Ruzics EP, Maleki P, Ding Y. Depressed renal and vascular nitric oxide synthase expression in cyclosporine-induced hypertension. Kidney Int. 1998;54(2):482–91. doi: 10.1046/j.1523-1755.1998.00014.x. [DOI] [PubMed] [Google Scholar]
- 5.Parra Cid T, Conejo Garcia JR, Carballo Alvarez F, de Arriba G. Antioxidant nutrients protect against cyclosporine A nephrotoxicity. Toxicology. 2003;189(1-2):99–111. doi: 10.1016/s0300-483x(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 6.Atessahin A, Ceribasi AO, Yilmaz S. Lycopene, a carotenoid, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rats. Basic & clinical pharmacology & toxicology. 2007;100(6):372–6. doi: 10.1111/j.1742-7843.2007.00060.x. [DOI] [PubMed] [Google Scholar]
- 7.Wongmekiat O, Leelarugrayub N, Thamprasert K. Beneficial effect of shallot (Allium ascalonicum L.) extract on cyclosporine nephrotoxicity in rats. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2008;46(5):1844–50. doi: 10.1016/j.fct.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Schilling S, Goelz S, Linker R, Luehder F, Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clinical and experimental immunology. 2006;145(1):101–7. doi: 10.1111/j.1365-2249.2006.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashrafian H, Czibik G, Bellahcene M, Aksentijevic D, Smith AC, Mitchell SJ, et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15(3):361–71. doi: 10.1016/j.cmet.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoreschi K, Bruck J, Kellerer C, Deng C, Peng H, Rothfuss O, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med. 2011;208(11):2291–303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(Pt 3):678–92. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 12.Meili-Butz S, Niermann T, Fasler-Kan E, Barbosa V, Butz N, John D, et al. Dimethyl fumarate, a small molecule drug for psoriasis, inhibits Nuclear Factor-kappaB and reduces myocardial infarct size in rats. Eur J Pharmacol. 2008;586(1-3):251–8. doi: 10.1016/j.ejphar.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 13.Onai Y, Suzuki J, Kakuta T, Maejima Y, Haraguchi G, Fukasawa H, et al. Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;63(1):51–9. doi: 10.1016/j.cardiores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Scannevin RH, Chollate S, Jung MY, Shackett M, Patel H, Bista P, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther. 2012;341(1):274–84. doi: 10.1124/jpet.111.190132. [DOI] [PubMed] [Google Scholar]
- 15.Chung BH, Li C, Sun BK, Lim SW, Ahn KO, Yang JH, et al. Rosiglitazone protects against cyclosporine-induced pancreatic and renal injury in rats. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):1856–67. doi: 10.1111/j.1600-6143.2005.00979.x. [DOI] [PubMed] [Google Scholar]
- 16.Wongmekiat O, Thamprasert K. Investigating the protective effects of aged garlic extract on cyclosporin-induced nephrotoxicity in rats. Fundamental & clinical pharmacology. 2005;19(5):555–62. doi: 10.1111/j.1472-8206.2005.00361.x. [DOI] [PubMed] [Google Scholar]
- 17.Amudha G, Josephine A, Varalakshmi P. Role of lipoic acid in reducing the oxidative stress induced by cyclosporine A. Clinica chimica acta; international journal of clinical chemistry. 2006;372(1-2):134–9. doi: 10.1016/j.cca.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Wongmekiat O, Gomonchareonsiri S, Thamprasert K. Caffeic acid phenethyl ester protects against oxidative stress-related renal dysfunction in rats treated with cyclosporin A. Fundamental & clinical pharmacology. 2011;25(5):619–26. doi: 10.1111/j.1472-8206.2010.00884.x. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7(3-4):385–94. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 20.Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006;28(2):169–81. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 21.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74(13):1526–39. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 22.Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology. 2008;246(1):24–33. doi: 10.1016/j.tox.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48(2):91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- 25.Kundu JK, Surh YJ. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm Res. 2010;27(6):999–1013. doi: 10.1007/s11095-010-0096-8. [DOI] [PubMed] [Google Scholar]
- 26.Shin DH, Park HM, Jung KA, Choi HG, Kim JA, Kim DD, et al. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free radical biology & medicine. 2010;48(8):1051–63. doi: 10.1016/j.freeradbiomed.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacological reviews. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 28.Hafez T, Moussa M, Nesim I, Baligh N, Davidson B, Abdul-Hadi A. The effect of intraportal prostaglandin E1 on adhesion molecule expression, inflammatory modulator function, and histology in canine hepatic ischemia/reperfusion injury. The Journal of surgical research. 2007;138(1):88–99. doi: 10.1016/j.jss.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Dogan S, Aslan M. Hepatic ischemia-reperfusion injury and therapeutic strategies to alleviate cellular damage. Hepatology research : the official journal of the Japan Society of Hepatology. 2011;41(2):103–17. doi: 10.1111/j.1872-034X.2010.00765.x. [DOI] [PubMed] [Google Scholar]
- 30.Buffoli B, Pechanova O, Kojsova S, Andriantsitohaina R, Giugno L, Bianchi R, et al. Provinol prevents CsA-induced nephrotoxicity by reducing reactive oxygen species, iNOS, and NF-kB expression. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2005;53(12):1459–68. doi: 10.1369/jhc.5A6623.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, et al. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney international. 2010;77(12):1076–85. doi: 10.1038/ki.2010.69. [DOI] [PubMed] [Google Scholar]
- 32.Bobadilla NA, Gamba G, Tapia E, Garcia-Torres R, Bolio A, Lopez-Zetina P, et al. Role of NO in cyclosporin nephrotoxicity: effects of chronic NO inhibition and NO synthases gene expression. The American journal of physiology. 1998;274(4 Pt 2):F791–8. doi: 10.1152/ajprenal.1998.274.4.F791. [DOI] [PubMed] [Google Scholar]
- 33.Kou R, Greif D, Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. The Journal of biological chemistry. 2002;277(33):29669–73. doi: 10.1074/jbc.M204519200. [DOI] [PubMed] [Google Scholar]
- 34.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 35.Erman A, Chen-Gal B, Rosenfeld J. The role of eicosanoids in cyclosporine nephrotoxicity in the rat. Biochemical pharmacology. 1989;38(13):2153–7. doi: 10.1016/0006-2952(89)90070-1. [DOI] [PubMed] [Google Scholar]
- 36.Erman A, Chen-Gal B, Zabludowski J, Rosenfeld JB. Cyclosporin A treatment enhances angiotensin converting enzyme activity in lung and serum of rats. The Journal of pharmacy and pharmacology. 1990;42(7):525–7. doi: 10.1111/j.2042-7158.1990.tb06614.x. [DOI] [PubMed] [Google Scholar]
- 37.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]


