Abstract
Crosstalk between tumor cells and their microenvironment is critical for malignant progression. Crosstalk mediators including soluble factors and direct cell contact have been identified, but roles for the interaction of physical forces between tumor cells and the bone microenvironment have not been described. Here we report preclinical evidence that tumor-generated pressure acts to modify the bone microenvironment to promote the growth of prostate cancer bone metastases. Tumors growing in mouse tibiae increased intraosseous pressure. Application of pressure to osteocytes, the main mechanotransducing cells in bone, induced PCa growth and invasion. Mechanistic investigations revealed that this process was mediated in part by upregulation of CCL5 and matrix metalloproteinases in osteocytes. Our results defined the critical contribution of physical forces to tumor cell growth in the tumor microenvironment, and they identified osteocytes as a critical mediator in the bone metastatic niche.
Keywords: Metastasis, physical force, hydrostatic pressure, osteocyte
Introduction
Prostate cancer (PCa) is the 2nd most common tumor of men in the United States with bone being the primary location of metastases. There are few therapeutic options for patients with bone metastases. Bone is a relatively inelastic tissue, with tumor growth restricted by mineralized extracellular matrix (ECM). Furthermore, PCa bone metastases are typically osteoblastic, promoting poorly organized bone formation. The lack of potential space present in bone, and continual expansion of tumor, creates a paradox in which bone must be degraded or physical pressure within the tumor will increase resultant of continuous cellular division. Therefore, we hypothesized that tumor growth in bone would increase pressure within the bone microenvironment.
Bone is comprised of osteoblasts, cells responsible for bone production, osteoclasts, cells responsible for bone resorption, and osteocytes (OCy), the cells which coordinate the responses of osteoblasts and osteoclasts. OCy are terminally differentiated osteoblast lineage cells embedded in mineralized bone (1). The primary role of OCy is maintaining bone homeostasis by translating physical forces into biochemical signals, a process called mechanotransduction (2). Alteration of physical forces in bone modulates OCy biochemical responses regulating bone remodeling. Similarly, tumor growth in bone should induce physical forces that effect OCy. The role of OCy has yet to be established in tumor biology even though OCy are the most abundant cell in bone (2). Accordingly, the goal of this study was to determine if tumor growth in bone induces physical forces and determine if these forces educate OCy to promote tumor progression.
Materials and Methods
Cell Lines
The MLO-Y4 murine OCy cell line (courtesy of Dr. Lynda Bonewald; University of Missouri, Kansas City) was maintained as previously described (3). DU145, LNCaP, and PC3 (human PCa); BPH-1 (benign prostatic hyperplasia-1); ACE1 (canine PCa; courtesy of Dr. Thomas Rosol, The Ohio State University); and H441 and A549 (human lung carcinoma) cell lines were maintained in RPMI with 10% FBS and 1× penicillin/streptomycin. MDA-MB-231 and MCF7 were cultured in DMEM based media. All cells were serially passaged by trypsinization and maintained at 37°C and 5% CO2 in a humidified atmosphere.
Animals
All animal studies were performed in an AALAC-approved facility, with approval of the University Committee on Use and Care of Animals (UCUCA). Male severe combined immunodeficiency disorder (SCID) mice 8–10 weeks of age were used for all experiments. Four mice per group were used for all in vivo experiments.
In vivo pressure measurement in tumor bearing mice
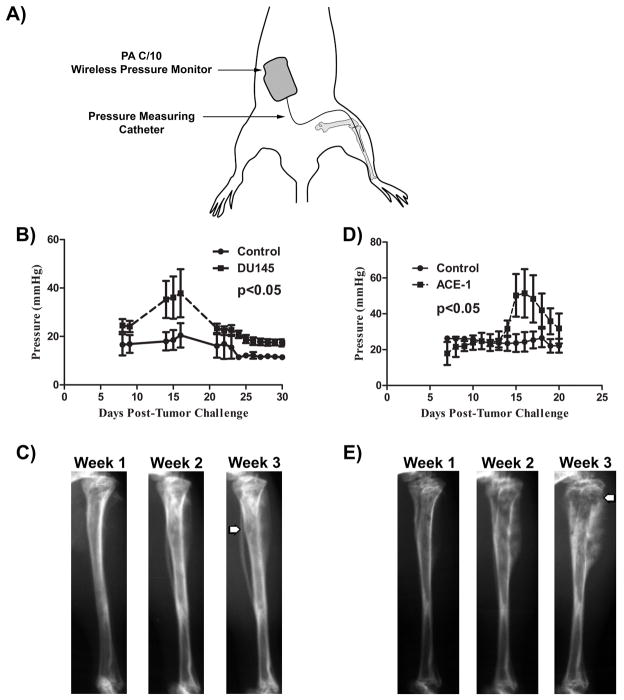
Mice were anesthetized with isoflurane and 1×106 tumor cells were injected in the intramedullary cavity of the tibia. Sham treated mice were injected with an equivalent volume of PBS. 24 hours after tumor challenge, implantation of a wireless pressure transmitter was performed similar to previous description (4). Briefly, a DSI PhysioTel PA-C10 pressure transmitter (Data Sciences International, St. Paul, MN) was implanted in the abdomen and catheter inserted into the intramedullary cavity of the tibia where tumor was previously implanted (Figure 1A).
Figure 1. Prostate cancer growth in bone leads to elevated intramedullary pressure.
A) SCID mice (n = 4 per group) were challenged with 1×106 tumor cells 24 hours prior to implantation of a wireless pressure monitoring device as depicted in the schematic. B) Mice challenged with DU145 showed a significant (p<0.05) increase in intramedullary pressure, with a peak average pressure of 37.84 mmHg 16 days after tumor challenge, compared to sham injected mice. C) Radiographs depict weakening of the cortical bone (arrow) beginning 2 weeks post tumor challenge, with loss of cortical bone 3 weeks post-challenge. Initiation of cortical thinning is associated with the drop in ImP observed. D) Mice challenged with ACE1 showed similar changes in pressure as observed in mice challenged with DU145. E) Radiographs from mouse challenged with ACE-1 show cortical bone decay similar to DU145 at 2 weeks. Together, these data provide show that PCa growth in bone leads to significant increases in intramedullary pressure. Two-way repeated measures ANOVA was utilized for statistical comparisons.
Real-time measurements of intramedullary pressure (ImP) were collected and analyzed using Dataquest A.R.T. system software (DSI international). Analysis of measurements was initiated 7-days post-catheter implant to allow for inflammation to subside. Measurements were averaged for each mouse and reported. Radiographs were obtained weekly using a Faxitron MX-20 (Wheeling, IN) at 4× magnification.
Conditioned Media (CM)
CM was prepared by incubating near confluent MLO-Y4 cells on collagen-coated plates for 24 hours with RPMI media containing 0.1% FBS and 1× penicillin/streptomycin. Supernatant was removed and centrifuged at 500×g for 5 minutes. MLO-Y4 cells were counted and CM was normalized by adjusting to a final concentration of 2×107 cells per 30 mL. BCA assay was used to measure CM protein concentration.
Application of hydrostatic pressure in vitro
Application of hydrostatic pressure to cells in vitro was accomplished as previously described (5–7) using modifications. Briefly, cells were seeded into Opticell (Thermo Scientific, Rochester, NY) cassettes and allowed to adhere overnight. Pressure was created by connecting the Opticell to an IV bag filled with cell culture media utilizing arterial line tubing. Opticell’s were clamped between perforated stainless steel sheets secured by binder clips. Hydrostatic pressure was modulated by adjusting the height of the IV bag. Pressure was determined using the following formula: P=ρ·h; where P=hydrostatic pressure, ρ=fluid density, and h=the height of the IV bag in relation to the Opticell.
Viability was assessed by pressurizing cells for 24 hours and counting live cells by trypan blue staining. CM was isolated from MLO-Y4 cells by pressurizing cells for 24 hours in RPMI containing 0.1% FBS at 0, 20, and 40 mmHg. CM was prepared as described above. BCA assay was used to quantify protein concentration of cells; no significant (p>0.05) differences were observed after normalization.
Viability
PCa cells were plated in 96-well plates at a density of 3,000 cells per well and incubated overnight. Media was aspirated and replaced with CM or control media (RPMI containing 0.1% FBS) and cells incubated for 24 hours. Viability was assessed following resazurin (Sigma Aldrich, St Louis, MO) incubation. Fluorescence was determined using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) with Softmax Pro v5.4 software. Relative viable cell number was expressed as a percentage of control treated cells.
Migration and Invasion
Screening of compounds identified in the reagents section was performed utilizing Culturex Migration and low BME Invasion assays (Trevigen, Gaithersburg, MD). Validation of these results were performed using commercially available Boyden chamber assays with (invasion) and without (migration) Matrigel according to manufacturer directions (Corning, Bedford, MA). PCa cells (ACE1=1×105; DU145=5×104; LNCaP=5×105; and PC3=1×105) were plated in the top well in 0.1% FBS RPMI. In the bottom well, control media or CM was added as a chemoattractant. Blocking or neutralizing compounds were incubated with CM or control media for 1-hour at room temperature prior to plating. To recapitulate the protein concentration of CCL5 and MMP2 found in 40 mmHg CM, recombinant respective protein was added to 0 mmHg before plating as a chemoattractant. Cell number from 5-random 400× fields were counted, averaged for each membrane, and reported as the mean±SEM.
Cytokine Array
Identification of MLO-Y4 secreted mediators was achieved using a commercially available mouse cytokine array (R&D, Minneapolis, MN) according to manufacturer instructions. Protein concentration was normalized before performing the assay. Pixel density of each spot was measured using Photoshop CS3 extended (Adobe Systems Inc, USA). Spots were averaged, background subtracted, and average values reported for each cytokine.
Reagents
The following reagents were utilized in screening effector molecules identified in the cytokine array: Rat anti-mouse CCL5 (R&D Systems, Minneapolis, MN), rat IgG2a isotype control (R&D Systems), recombinant mouse CCL5 (R&D Systems), recombinant MMP2 (R&D Systems), CCR2 antagonist (Calbiochem), rat anti-mouse CXCL10 (R&D Systems), cFMS Receptor III inhibitor (Calbiochem), Batimastat (Calbiochem).
Real-Time qPCR
RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA), purified by RNeasy Mini-Kit (Qiagen, Valencia, CA, USA), and reverse transcribed using the SuperScript III Reverse Transcriptase Kit (Invitrogen). Quantitative real time PCR was performed in triplicate using SYBR Green qPCR MasterMix (Qiagen) in a 10 μL reaction volume on a Roche LightCycler 480 (Roche, Indianapolis, IN, USA). Primers were purchased from SABiosciences (Qiagen). Measurements from triplicate Ct values were normalized to GAPDH, averaged, and reported.
ELISA
Commercially available ELISA for murine CCL5/RANTES (R&D Systems) and MMP2 (R&D Systems) were utilized according to manufacturer instructions.
Zymography
Zymography was performed using CM from OCy and PCa cell lines normalized by total protein as measured by BCA assay. 10% gelatin zymogram gels (Life Technologies, Carlsbad, CA) were used according to manufacturer directions and counterstained using SimplyBlue SafeStain (Life Technologies). Washed gels were scanned and densitometry performed using ImageJ (8).
Statistics
All experiments were repeated independently 2–3 times. Statistical analysis was performed using Prism 5 (GraphPad Software, La Jolla, CA). In vivo pressure experiments were analyzed using two-way repeated measures ANOVA with a Bonferroni post-test. Multiple groups were compared using one-way ANOVA with Bonferroni post-test. Data comprising only 2 groups was analyzed by t-test. Zymography and neutralization or pressure CM with anti-CCL5 antibody were compared using two-way ANOVA with Bonferroni post-test. For all statistical analyses, a cutoff of p<0.05 was used to assess statistical significance.
Results
Tumor growth in bone increases intramedullary pressure
The primary site of PCa metastasis is bone, in which tumor growth may induce pressure due to the lack of expansible space due to mineralized ECM. Therefore, we sought to determine if PCa growing in bone significantly altered intramedullary pressure (ImP). PCa cells were injected into the tibiae of SCID mice which were subsequently cannulated with a wireless pressure transmitter (Figure 1A) allowing for real-time monitoring of ImP. DU145 and ACE1 tumor growth significantly increased ImP, peaking approximately 15 to 16 days post-tumor challenge (Figure 1B and D). Approximately 21 days post-tumor injection, ImP declined; albeit was still increased compared to baseline. Radiographic loss of cortical bone was observed at the same time period of declining ImP (Figure 1C and E). This observation suggests that cortical bone loss may mitigate tumor-induced pressure. Challenge with the highly osteolytic PCa cell line PC3 did not induce a significant increase in ImP (Figure S1). From these data, it was determined that mice have a basal ImP of 19.12±8.18 mmHg; similar to previous reports in mice without tumors (4). An average peak ImP of 38.51±8.63 mmHg was observed in ACE1 and DU145 across all experiments. Investigation of the role of tumor-generated pressures on PCa using the values identified from the in vivo studies was then investigated in vitro.
Effects of increased ImP on OCy and PCa
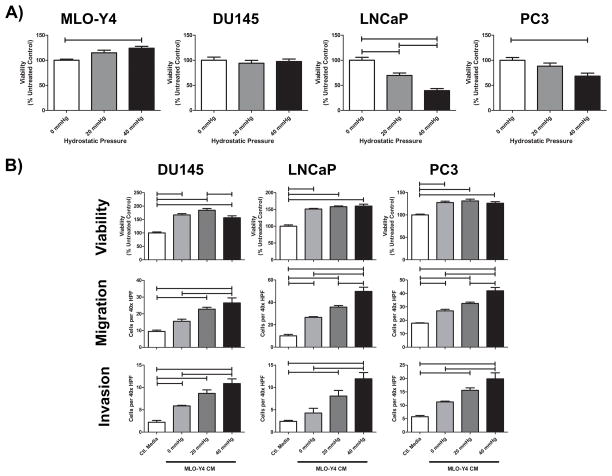
To investigate the impact of increased pressure on OCy and PCa viability in vitro, we applied hydrostatic pressure, based upon our in vivo findings, to cultures of OCy and PCa cells. Pressure increased MLO-Y4 OCy viability (Figure 2A). Even though OCy are not thought to proliferate in vivo, this observation speaks to the ability of OCy to survive stressful conditions (1,2). In contrast, pressure either had no effect (DU145) or decreased PCa (LNCaP and PC3) cell viability (Figure 2A).
Figure 2. Application of hydrostatic pressure to osteocytes promotes PCa aggressiveness.
A) Hydrostatic pressure was applied in vitro to MLO-Y4 osteocytes and DU145, LNCaP, and PC3 PCa cell lines. After 24 hours, cells were counted using trypan blue staining and live cell count depicted. B) CM was isolated from MLO-Y4 OCy under pressure at 0, 20, and 40 mmHg for 24 hours. Control media (RPMI with 0.1% FBS) was used as a negative control. OCy CM was then applied to PCa cell lines for 24 hours to determine changes in viability as measured by resazurin. CM was used as a chemoattractant in Boyden chamber assays to determine alterations in migration and invasion. One way ANOVA with Bonferroni post-test used for all analyses; bars represent significant differences of p<0.05.
OCy coordinate bone remodeling by releasing biochemical mediators. Thus, CM was collected from OCy and found to induce PCa viability (Figure S2). OCy CM also increased cell viability of breast and lung cancer cell lines, suggesting the effect is not unique to PCa (Figure S3). OCy CM induced PCa migration and invasion when utilized as a chemoattractant (Figure S2). To determine if increased pressure altered the pro-tumorigenic phenotype conferred by OCy, CM was collected from MLO-Y4 cells pressurized at 0 (normal culture conditions), 20 (physiologic baseline ImP), and 40 mmHg (peak pressure observed). CM from pressurized cells dose dependently increased PCa viability, migration, and invasion (Figure 2B). These data provide evidence that tumor-induced pressure promotes secretion of factors associated with PCa survival and motility in response to physical forces induced by tumor growth.
Identification and neutralization of CM derived CCL5
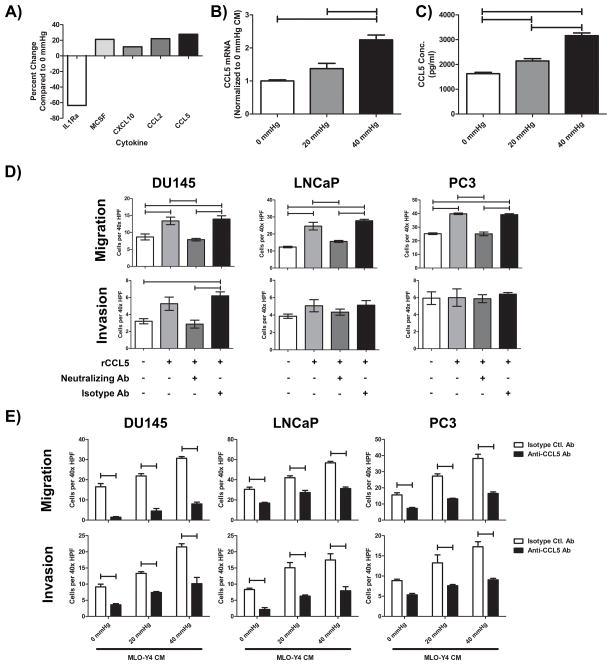
In order to identify candidate mediators of PCa pro-tumorigenic activity, the cytokine profile of 0 mmHg OCy CM was compared to CM produced at 40 mmHg pressure. Increased pressure induced numerous chemokines and cytokines (Figure 3A; detailed in Supplementary table 1). Several of the factors with altered expression were screened for function on PCa utilizing neutralizing antibodies and receptor antagonists to inhibit PCa viability, migration, and invasion. Inhibition of CCL2, MCSF, and CXCL10 were found to have no effect (data not shown). CCL5 neutralizing antibody inhibited OCy CM-induced PCa migration and invasion. Therefore, CCL5 mRNA was measured by qPCR (Figure 3B), and ELISA to measure CCL5 protein concentration in CM (Figure 3C; Figure S4A). Pressure induced a dose dependent increase in CCL5 production from MLO-Y4 cells.
Figure 3. Identification of CCL5/RANTES as an OCy secreted mediator of enhanced PCa invasion.
A) CM prepared from MLO-Y4 cells pressurized at 0 and 40 mmHg for 24 hours was compared using a cytokine screening antibody array and subjected to densitometry. Results are shown as change in optical density (OD) of the cells subjected to 40 mmHg relative to the 0 mmHg. B) Real time PCR of CCL5 from pressurized MLO-Y4 cells was performed and normalized to GAPDH. C) CCL5 secreted from OCy, normalized by protein concentration, increases as the level of hydrostatic pressure increases as measured by ELISA. D) Recombinant murine CCL5 (rCCL5; 10 μg/ml) was used as a chemoattractant for PCa migration and invasion. Neutralization of rCCL5 was accomplished by incubating with anti-CCL5 neutralizing antibody (1 μg/ml) but not with isotype control antibody (1 μg/ml). rCCL5 neutralization inhibited migration across all cell lines. However, invasion of LNCaP and PC3 was not altered by the neutralization of rCCL5. E) CM derived from MLO-Y4 cells pressurized at 0, 20, and 40 mmHg was incubated with CCL5 neutralizing antibody before being used as a chemoattractant in migration and invasion assays. Neutralization of CM derived CCL5 led to significant inhibition of PCa migration and invasion despite increasing pressure. Secretion of CCL5 by MLO-Y4 is induced by tumor generated pressure leading to subsequent increases in tumor cell invasiveness. One way ANOVA with Bonferroni post-test (C/D) or two-way ANOVA (E) was performed; bars represent significant differences where p<0.05.
CCL5 is a chemoattractant for T cells that has been implicated in promoting PCa migration and viability (9,10). To further characterize the tumor promoting role of CCL5, PCa cells were incubated with recombinant CCL5 (rCCL5). While rCCL5 promoted DU145 migration and invasion, it promoted only migration, and not invasion of LNCaP and PC3 cells (Figure 3D). Since these cells lines were tested under similar conditions it is likely there may be cell specificity to rCCL5; however, it is possible that even with the replicate experiments, there was insufficient statistical power to detect the invasion in LNCaP and PC3 cells. Neutralization of rCCL5 using an anti-CCL5 antibody negated the pro-migratory effects conferred by rCCL5 (Figure 3D). The observation that invasion was not impacted by increased CCL5 alone suggests that OCy-produced CCL5 is insufficient to promote invasion by itself.
To investigate if CCL5 in the context of OCy CM promotes PCa migration and invasion, we evaluated neutralization of OCy derived CCL5. CCL5 neutralization inhibited migration in non-pressurized CM (Figure S4B). Furthermore, neutralization of CM derived from pressurized OCy inhibited migration and invasion at all pressures examined (Figure 3E). However, inhibition of CCL5 did not completely block the pressure-induced migration and invasion indicating other factors contribute to this activity. To determine if CCL5 was sufficient to promote the pressure-induced migration and invasion, we recapitulated the concentration of CCL5 found in 40 mmHg CM (3 ng/mL) by adding rCCL5 to 0 mmHg CM. The addition of CCL5 promoted migration and invasion to levels similar to 40 mmHg CM (Figure S4C). These invasion results contrast with the studies utilizing rCCL5 alone (Figure 3D), suggesting that other factors produced by OCy contribute to promoting invasion of PCa in addition to CCL5.
Neutralization of CM derived CCL5 and MMP mitigates PCa pro-invasive phenotype
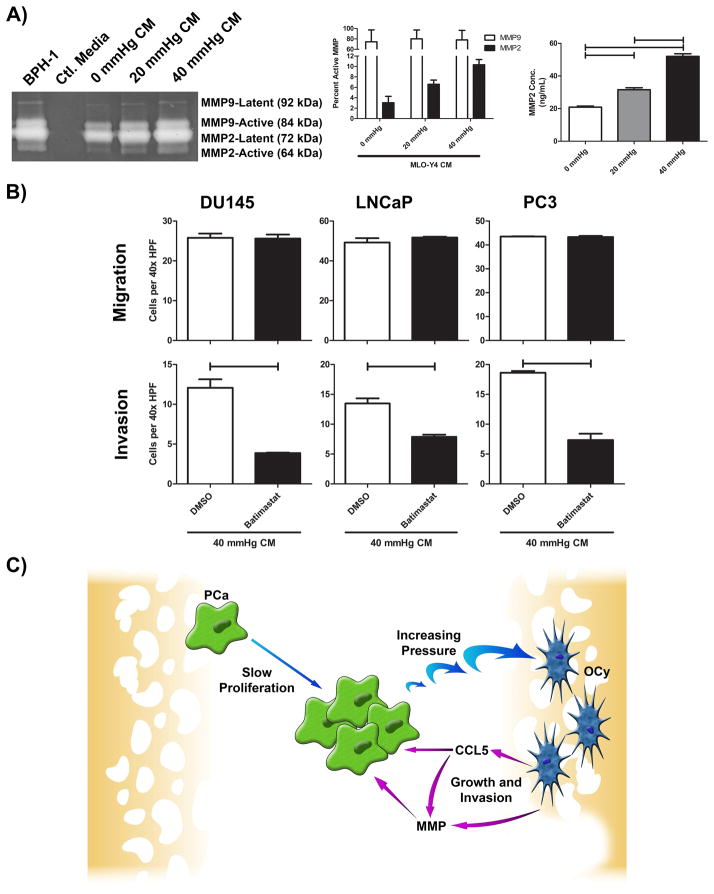
Matrix metalloproteinases (MMPs) are known to be expressed by OCy to modulate extracellular matrix during bone resorptive processes (11). To determine if OCy modulate PCa invasion through MMPs, CM from MLO-Y4 and PCa cell lines was isolated and compared using zymography. MLO-Y4 CM produced up to 67× more MMP2 than PCa cell lines (Figure S5A); MMP9 expression was elevated to a lesser degree. Pressurization of MLO-Y4 increased secretion of MMP2 and MMP9 as measured by zymography (BPH-1 was used as a positive control (12)) (Figure 4A). Quantification of latent vs active MMPs showed increased MMP2 activity associated with increased pressure (Figure 4A; Figure S5B). The effect of pressure on MMP2 and MMP9 mRNA expression in OCy was evaluated demonstrating pressure induced a dose-dependent increase of both MMP2 and MMP9 mRNA (Figure S5C). MMP2 quantitation by ELISA showed increased protein expression associated with pressure (Figure 4A). To determine if pressure promotes invasion through MMP production by OCy, we added a broad spectrum MMP inhibitor, batimastat to CM. Batimastat inhibited invasion but not migration of PCa cells in both non-pressurized CM (Figure S5D) and 40 mmHg CM (Figure 4B) cultures indicating that in addition to basal expression of MMP’s by OCy, pressure-induced MMP expression by OCy increases invasion. Furthermore, addition of recombinant MMP2 (rMMP2) to 0 mmHg CM, to recapitulate the concentration of MMP2 found in 40 mmHg CM, promoted invasion to levels similar to 40 mmHg CM alone (Figure S5E) indicating the ability of MMPs to contribute to the pressure-induced invasion. Our finding that pressure induced CCL5 (Figure 3B and 3C) along with previous reports indicating that CCL5 promotes MMP expression led us to explore if CCL5 promotes MMP expression in OCy (13,14). Accordingly, rCCL5 was added to MLO-Y4 and found to increase MMP2 and MMP9 mRNA expression (Figure S6), suggesting that pressure could induce MMP2 and MMP9, in part, through CCL5.
Figure 4. Inhibition of OCy derived MMPs mitigates pro-tumorigenic responses.
A) MLO-Y4 was subjected to 0, 20, or 40 mmHg pressure for 24 hours, CM prepared and normalized by protein concentration as measured by BCA assay prior to analysis by zymography. BPH-1 was used as a positive control for MMP expression and plain cell culture media used as a negative control. Bands were subjected to densitometry and reported as percent active MMP2 and MMP9. CM was normalized by protein concentration, as measured by BCA, and MMP2 analyzed by ELISA. B) MLO-Y4 CM was incubated with batimastat, a broad spectrum MMP inhibitor, to inhibit OCy derived MMP. 40 mmHg CM with batimastat or DMSO control where then utilized to determine alterations in PCa migration and invasion. C) A proposed model system for tumor induced pressure promoting PCa aggressiveness. PCa proliferation promotes pressure in bone leading to induction of OCy production of CCL5, which can induce MMPs, and MMPs themselves. MMPs degrade the bone matrix releasing a variety of growth factors while the combination of CCL5 and MMPs promote further invasion into bone. One way ANOVA with Bonferroni post-test used for ELISA analysis; bars represent significant differences of p<0.05. Student’s two-tailed t-test was used to analyze MMP inhibition experiments. All bars represent significant differences where p<0.05.
Discussion
The consequences of tumor-generated physical forces on the tumor microenvironment, in general, and the bone microenvironment specifically, have yet to be elucidated. Our data provide evidence that tumor growth-induced pressure promotes a pro-proliferative and pro-invasive response from OCy. It achieves this, in part, through pressure-induced OCy production of CCL5 and MMPs (model in Figure 4C). This is the first demonstration of tumor modulating the microenvironment through a physical force so that the microenvironment enhances cancer progression.
Herein, data are presented showing that tumors that produce radiographically mixed lesions (i.e. ACE1 and DU145 cells) increase ImP. However, no elevation of ImP was observed from lytic PC3 cells suggesting that osteolysis provided sufficient space for PC3 growth without inducing changes in ImP. Consistent with this possibility is the observation that breast cancer cells respond to matrix rigidity through up-regulation of MMPs (15). It is plausible that matrix rigidity, through its resistance to conformational changes, would influence the magnitude of pressure generated by a growing tumor. Furthermore, in tumors that create mixed to osteoblastic lesions, the decreased ability to resorb bone and subsequent inability to create space for tumor growth, may result in the increased ImP.
The primary role of OCy is coordination of bone production and resorption in response to physical stimuli (1,2). Our report is the first to demonstrate that OCy support cancer progression. Bone is a complex tissue, and even though OCy are the primary mechanotransducing cells, it is plausible that other cells may be impacted by altered ImP. We observed numerous cytokines produced from OCy in response to hydrostatic pressure. CCL5 was observed to have a significant response on promoting tumor invasion and migration. CCL5/RANTES is a chemokine whose primary role has been defined as a chemoattractant for immune cells. PCa cells DU145, LNCaP, and PC3 have previously been shown to express multiple receptors for CCL5 (10). CCL5 has previously been shown to promote migration of numerous tumor types, including prostate, breast, lung, and osteosarcoma (9,10,16–18). Our results are consistent with CCL5’s known activity on invasion and migration; however, as indicated in Table 1 and the observation that CCL5 only partially inhibited of migration and invasion (Fig. 3E), multiple other factors most likely also contribute to the pressure-induced migration and invasion.
In addition to CCL5, OCy MMP2 and MMP9 basal expression was higher than that of PCa cell lines and further induced by pressure. Batimastat inhibited invasion of PCa towards OCy-derived CM indicating a role for MMPs in OCy-mediated invasion. While MMPs are known to play a role in cancer invasion their expression from OCy as potentiating tumor invasion is a novel finding (19). OCy have been observed to have some bone resorption properties, and MMP expression is likely a consequence of this function (11) and MMP2 is important in OCy canalicular network formation and maintenance (20). OCy-derived MMPs provide a mechanism for nutrient release and development of a vicious cycle previously associated with tumor growth in bone (21,22). Similarly in PCa, integrins, such as αvβ6, may interact with TGFβ leading to MMP2 induction and osteolytic degradation (12). This is especially interesting in the context that MMP activation of TGFβ is important in osteoblast viability and OCy differentiation (23). Furthermore, our results show that CCL5 induces MMPs in OCy supports previous findings where CCL5 promoted MMP production in tumor cells (13,14) and provides a potential mechanism to account for pressure-induced MMP expression. In this setting, tumor promotion of bone degradation may elicit growth factor release from mineralized bone, further promoting tumor growth through initiation of a positive feedback loop. The response of OCy to tumor-generated pressure has great potential to promote the vicious cycle as OCy are the most abundant cell in bone and have a greater surface area than that of trabeculae (24).
Conclusion
The current report demonstrates that tumor growth promotes ImP and these physical forces promote OCy secretion of the tumor-promoting factors CCL5 and MMPs. Furthermore, OCy were identified as novel pro-tumorigenic cellular mediators. Exploration of OCy and their response to physical forces is necessary to develop novel therapeutic targets for the inhibition of bone metastases.
Supplementary Material
Acknowledgments
We would like to thank Dr. Lynda Bonewald at the University of Missouri-Kansas City for providing the MLO-Y4 cell line. We would like to thank Dr. John Frangos and Diana Meays of the La Jolla Bioengineering Institute for their instruction concerning the technique to measure intramedullary pressure in mice. We would also like to thank Dr. Louis D’Alecy and Steven Whitesall at the University of Michigan for their expertise in utilizing the DSI system and pressure measurement. We would also like to thank Dr. Gene DiResta at the NYU Polytechnic School of Engineering and Dr. John Healey at Memorial Sloan Kettering Cancer Center for their help with the in vitro pressure system.
Funding
This work was supported by a Department of Defense Prostate Cancer Research Program Training Award (W81XWH-12-1-0172) to JS and National Institutes of Health Grants R01CA190554, P01CA093900 and S10RR026405.
List of abbreviations
- CM
Conditioned Media
- ECM
Extracellular matrix
- ImP
Intramedullary pressure
- OCy
Osteocyte
- PCa
Prostate cancer
- rCCL5
recombinant mouse CCL5
- rMMP2
recombinant matrix metalloproteinase
- SCID
Severe combined immunodeficiency disorder
- UCUCA
University Committee on Use and Care of Animals
Footnotes
Statement of author contributions
Concept, experimental design, and development of methodology were developed by JS and EK. Acquisition of data was performed by JS, JD, HZ, and BC. Analysis of the data was performed by JS and EK. All authors contributed to the writing and review of the manuscript.
Conflict of Interest
The authors have no conflict of interest to disclose.
References
- 1.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell .. and more. Endocr Rev. 2013;34:658–90. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12:2014–23. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 4.Kwon RY, Meays DR, Tang WJ, Frangos JA. Microfluidic enhancement of intramedullary pressure increases interstitial fluid flow and inhibits bone loss in hindlimb suspended mice. J Bone Miner Res. 2010;25:1798–807. doi: 10.1002/jbmr.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diresta GR, Nathan SS, Manoso MW, Casas-Ganem J, Wyatt C, Kubo T, et al. Cell proliferation of cultured human cancer cells are affected by the elevated tumor pressures that exist in vivo. Ann Biomed Eng. 2005;33:1270–80. doi: 10.1007/s10439-005-5732-9. [DOI] [PubMed] [Google Scholar]
- 6.Nathan SS, Huvos AG, Casas-Ganem JE, Yang R, Linkov I, Sowers R, et al. Tumor interstitial fluid pressure may regulate angiogenic factors in osteosarcoma. J Orthop Res. 2008;26:1520–5. doi: 10.1002/jor.20633. [DOI] [PubMed] [Google Scholar]
- 7.Matsubara T, Diresta GR, Kakunaga S, Li D, Healey JH. Additive Influence of Extracellular pH, Oxygen Tension, and Pressure on Invasiveness and Survival of Human Osteosarcoma Cells. Front Oncol. 2013;3:199. doi: 10.3389/fonc.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldinucci D, Colombatti A. The Inflammatory Chemokine CCL5 and Cancer Progression. Mediators Inflamm. 2014;2014:292376. doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66:124–34. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- 11.Cullinane DM. The role of osteocytes in bone regulation: mineral homeostasis versus mechanoreception. J Musculoskelet Neuronal Interact. 2002;2:242–4. [PubMed] [Google Scholar]
- 12.Dutta A, Li J, Lu H, Akech J, Pratap J, Wang T, et al. Integrin alphavbeta6 promotes an osteolytic program in cancer cells by upregulating MMP2. Cancer Res. 2014;74:1598–608. doi: 10.1158/0008-5472.CAN-13-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang JY, Yang WH, Chen HT, Huang CY, Tan TW, Lin YT, et al. CCL5/CCR5 axis promotes the motility of human oral cancer cells. J Cell Physiol. 2009;220:418–26. doi: 10.1002/jcp.21783. [DOI] [PubMed] [Google Scholar]
- 14.Stormes KA, Lemken CA, Lepre JV, Marinucci MN, Kurt RA. Inhibition of metastasis by inhibition of tumor-derived CCL5. Breast Cancer Res Treat. 2005;89:209–12. doi: 10.1007/s10549-004-5328-3. [DOI] [PubMed] [Google Scholar]
- 15.Ruppender NS, Merkel AR, Martin TJ, Mundy GR, Sterling JA, Guelcher SA. Matrix rigidity induces osteolytic gene expression of metastatic breast cancer cells. PLoS One. 2010;5:e15451. doi: 10.1371/journal.pone.0015451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CY, Fong YC, Lee CY, Chen MY, Tsai HC, Hsu HC, et al. CCL5 increases lung cancer migration via PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 2009;77:794–803. doi: 10.1016/j.bcp.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Wang SW, Wu HH, Liu SC, Wang PC, Ou WC, Chou WY, et al. CCL5 and CCR5 interaction promotes cell motility in human osteosarcoma. PLoS One. 2012;7:e35101. doi: 10.1371/journal.pone.0035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaal-Hahoshen N, Shina S, Leider-Trejo L, Barnea I, Shabtai EL, Azenshtein E, et al. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin Cancer Res. 2006;12:4474–80. doi: 10.1158/1078-0432.CCR-06-0074. [DOI] [PubMed] [Google Scholar]
- 19.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue K, Mikuni-Takagaki Y, Oikawa K, Itoh T, Inada M, Noguchi T, et al. A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J Biol Chem. 2006;281:33814–24. doi: 10.1074/jbc.M607290200. [DOI] [PubMed] [Google Scholar]
- 21.Reddi AH, Roodman D, Freeman C, Mohla S. Mechanisms of tumor metastasis to the bone: challenges and opportunities. J Bone Miner Res. 2003;18:190–4. doi: 10.1359/jbmr.2003.18.2.190. [DOI] [PubMed] [Google Scholar]
- 22.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–16s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 23.Karsdal MA, Larsen L, Engsig MT, Lou H, Ferreras M, Lochter A, et al. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem. 2002;277:44061–7. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- 24.Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44:11–6. doi: 10.1016/j.bone.2008.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.