Abstract
Objectives
Electrographic seizures in critically ill children may be identified by continuous electroencephalographic (EEG) monitoring. We evaluated the cost-effectiveness of four electrographic seizure identification strategies (no EEG monitoring and EEG monitoring for 1 hour, 24 hours, or 48 hours).
Methods
We created a decision tree to model the relationships among variables from a societal perspective. To provide input for the model, we estimated variable costs directly related to EEG monitoring from their component parts, and we reviewed the literature to estimate the probabilities of outcomes. We calculated incremental cost-effectiveness ratios to identify the tradeoff between cost and effectiveness at different willingness-to-pay values.
Results
Our analysis found that the preferred strategy was EEG monitoring for 1 hour, 24 hours, and 48 hours if the decision maker was willing to pay <$1,666, $1,666–$22,648, and >$22,648 per critically ill child identified with electrographic seizures, respectively. The 48 hour strategy only identified 4% more children with electrographic seizures at substantially higher cost. Sensitivity analyses found that all three strategies were acceptable at lower willingness-to-pay values when children with higher electrographic seizure risk were monitored.
Conclusions
Our results support monitoring of critically ill children for 24 hours because the cost to identify a critically ill child with electrographic seizures is modest. Further study is needed to predict better which children may benefit from 48 hours of EEG monitoring since the costs are much higher.
Keywords: EEG Monitoring, Seizure, Status Epilepticus, Pediatric, Non-Convulsive Seizure, Cost-Effectiveness
Introduction
Most electrographic seizures in critically ill children with acute encephalopathy have no clinical correlate so identification requires continuous EEG monitoring.(Abend, et al. 2013, Abend, et al. 2011, Gold, et al. 2014, Greiner, et al. 2012, Jette, et al. 2006, Kirkham, et al. 2012, McCoy, et al. 2011, Piantino, et al. 2013, Schreiber, et al. 2012, Shahwan, et al. 2010, Williams, et al. 2011) The incidence of electrographic seizures varies from 10 to 40%, depending in part on the duration of EEG monitoring.(Abend, Arndt, Carpenter, et al. 2013, Abend and Dlugos 2007, Abend, Gutierrez-Colina, Topjian, et al. 2011, Abend, et al. 2009, Abend, et al. 2013, Arango, et al. 2012, Arndt, et al. 2013, Gold, Crawford, Glaser, et al. 2014, Greiner, Holland, Leach, et al. 2012, Hosain, et al. 2005, Jette, Claassen, Emerson, et al. 2006, Kirkham, Wade, McElduff, et al. 2012, McCoy, Sharma, Ochi, et al. 2011, Payne, et al. 2014, Piantino, Wainwright, Grimason, et al. 2013, Schreiber, Zelleke, Gaillard, et al. 2012, Shahwan, Bailey, Shekerdemian, et al., Tay, et al. 2006, Williams, Jarrar and Buchhalter 2011) Recent guidelines recommended monitoring at-risk encephalopathic children for 48 hours.(Brophy, et al. 2012) Unfortunately, EEG monitoring is costly because it involves expensive equipment and substantial work by technicians and physicians, and thus small changes in the duration of monitoring can have substantial resource impacts.(Gutierrez-Colina, et al. 2012) Since healthcare costs are rising and societal resources are limited, it is important to consider the consequences of implementing these guidelines.(Cassel and Guest 2012, Holloway and Ringel 2011) To provide this information, we modelled four EEG monitoring strategies for identifying electrographic seizures in critically ill children and evaluated their relative cost-effectiveness.
Methods
Model Structure and Inputs
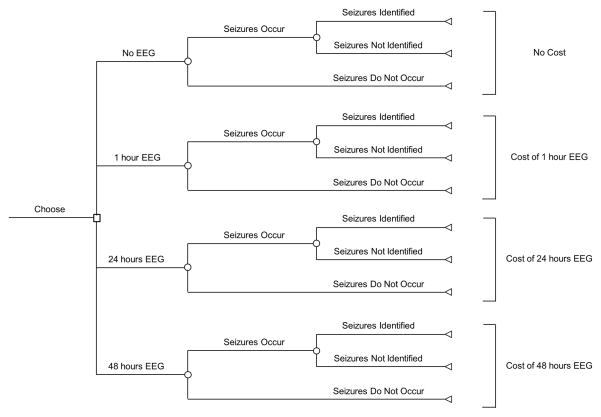
We constructed a decision tree that allowed us to estimate the costs of four strategies for monitoring a critically ill child with acute encephalopathy who might be experiencing electrographic seizures. The four strategies were: (1) no EEG monitoring, (2) 1 hour of EEG monitoring, (3) 24 hours of EEG monitoring, or (4) 48 hours of EEG monitoring (Figure 1). The model and analyses used a societal perspective.
Figure 1.
Decision tree used to represent the relationships among the variables important to monitoring strategy decisions. The decision maker may choose between the four EEG monitoring strategies (no EEG, 1 hour EEG, 24 hours EEG, or 48 hours EEG). For a given patient, electrographic seizures may or may not occur. Depending on the duration of EEG monitoring, the electrographic seizures may or may not be identified. The square signifies the choose node, circles signify the chance nodes, and triangles signify the terminal nodes.
To analyze the model, we needed information on the probability of identifying electrographic seizures with monitoring and the cost of EEG monitoring. One author (N.S.A) conducted a systematic review using PubMed keywords EEG monitoring, critical care, pediatric, and seizures to identify papers published in English related to EEG monitoring in critically ill children. The reference lists from identified publications were also reviewed. We pooled data from 17 studies which mostly reported the results of clinically indicated EEG monitoring in critically ill children and found that 740 of 2,247 (0.33) children had electrographic seizures (Supplemental Table 1). Because seizures could begin and end at different times, we used some of the same studies to determine when seizures occurred in relation to monitoring initiation (Supplemental Table 2). In children having seizures, the probability of identifying seizures was 0.55 with 1 hour of EEG monitoring; 0.85 with 24 hours of EEG monitoring; and 0.89 with 48 hours of EEG monitoring.
We used a micro-costing method to estimate the direct medical costs involved in EEG monitoring such as equipment, technologists, and physicians. We estimated how much of each component is used and assigned a cost to each unit of use. For example, the average hourly cost for technologists or physicians and the average cost per procedure for equipment. To calculate total cost, for each component we multiplied the number of units times the cost per unit, and then summed the values for all components. We used this method because it focuses attention on variable costs, which are the costs that change when more or fewer EEGs are done and when monitoring is for longer or shorter durations. We did not include fixed costs, which are the costs that do not change when the EEG monitoring strategy changes such as the overall cost of operating the intensive care unit. We did not use hospital charges in the analysis since they include fixed costs as well as costs that are not related directly to patient care, such as the costs of hospital administration and hospital debt, and they are affected by market forces, such as the hospital’s contracts with insurers. When we calculated costs for physicians and EEG technologists, we divided the yearly salaries by 1920 hours (assuming 40 hours of work per week for 48 weeks per year) to obtain an hourly cost. For personnel costs, we multiplied salaries by 1.3 to account for benefits. The interquartile ranges for costs were used to generate standard errors. Table 1 describes the costs used in the analyses.
Table 1.
Estimated costs of 1 hour, 24 hour, and 48 hour EEG monitoring strategies
| Cost Component | 1 hour EEG | 24 hours EEG | 48 hours EEG |
|---|---|---|---|
| EEG Technologist | $48.03 | $96.06 | $192.12 |
| Physician | $31.40 | $125.60 | $251.20 |
| Systems Analyst | $0.70 | $4.23 | $8.46 |
| EEG Machine | $4.01 | $24.04 | $48.08 |
| Electrodes | $0.55 | $0.55 | $1.10 |
| Total Cost | $84.69 | $250.48 | $500.96 |
Costs for physicians to interpret the EEG studies were obtained from the American Association of Medical Colleges Report on Medical School Faculty Salaries (2012–2013) (2014) for Pediatric Neurologists. This report provides the median and interquartile range for compensation at the instructor, assistant professor, associate professor, and professor levels. We averaged the four levels to obtain the average compensation (Supplemental Table 3). We used expert opinion of our colleagues to estimate that interpretation would take 0.25 hours for a 1-hour EEG, 1 hour for a 24-hour EEG, and 2 hours for a 48-hour EEG. These time estimates assume involvement of EEG technologists in screening the EEG, as described below.
Costs for EEG Technologists were derived from the Mercer Health Care System and Hospital Survey (effective date March 1, 2014) (Supplemental Table 4).(2014) Costs were converted to 2013 dollars using a consumer price index data inflation calculator. We used expert opinion of our colleagues including six EEG technologists to estimate the time spent initiating the EEG, screening the EEG tracing, periodically adjusting electrodes and equipment, and discontinuing the EEG. We summed these times to obtain a total EEG Technologist time for each strategy which were 1.5 hour for a 1-hour EEG, 3 hours for a 24-hour EEG, and 6 hours for a 48-hour EEG. This calculation assumed that an EEG technologist would remain at the patient’s bedside for a 1-hour study, but would screen the 24-hour and 48-hour studies in segments intermittently while also performing other work.
Costs for technical support personnel were based on wages for computer systems analysts (BLS code 15-1121) from the Bureau of Labor and Statistics Occupational Employment Statistics for May 2013.(2013) We estimated that on average 5 minutes of technical support time would be required each day that EEG monitoring was conducted, regardless of duration, although we recognize that technical support usually requires more time for a minority of studies and is not required for most studies.
Costs for EEG machines and electrodes were based on acquisition costs for the EEG machine and electrodes. Expert opinion of our colleagues was used to estimate their frequency of use to generate per use costs (Supplemental Tables 5 and 6).
Analyses
The primary outcome for this analysis was the incremental cost-effectiveness ratio, defined as the increase in the cost of each strategy divided by the increase in the number of children with seizures identified by each strategy. We calculated the increase in cost and the increase in the number of identified children by ranking the strategies in order of effectiveness and comparing each strategy to the closest strategy with a lower effectiveness. We did not compare each strategy to the least-effective strategy. Doing so would understate the cost required to produce additional benefits, because with each increase in the duration of monitoring the amount of benefit gained decreases relative to the cost of additional monitoring.
We performed two types of sensitivity analyses to estimate how much confidence we should have in the results. First, we performed a deterministic sensitivity analysis in which we varied the probability of experiencing electrographic seizures. Second, we performed a probabilistic sensitivity analysis using a second-order Monte Carlo simulation with 10,000 replications in which the values for all model inputs were randomly sampled from probability distributions (Table 2). The results of the probabilistic sensitivity analysis are displayed by a cost-effectiveness acceptability curve. This curve plots on the y-axis the relative frequency that the incremental cost-effectiveness ratio for each EEG strategy was cost-effective against increasing willingness-to-pay values on the x-axis. A willingness-to-pay value is the amount a decision maker is willing to pay to identify electrographic seizures in one additional child. An incremental cost-effectiveness value is cost effective when it is equal to or less than the willingness-to-pay value.
Table 2.
Variables, base case values, and distribution characteristics.
| Variable | Base Case Value | Distribution | Distribution Parameter 1 | Distribution Parameter 2 |
|---|---|---|---|---|
| Probability of Electrographic Seizures | 0.33 | Beta | 740 | 1507 |
| Probability of Electrographic Seizures Identification (1 Hour EEG) | 0.55 | Beta | 148 | 120 |
| Probability of Electrographic Seizures Identification (24 Hours EEG) | 0.85 | Beta | 229 | 39 |
| Probability of Electrographic Seizures Identification (48 Hours EEG) | 0.89 | Beta | 238 | 30 |
| Cost of EEG for 1 hour | $84.69 | Normal | $84.69 | $9.38 |
| Cost of EEG for 24 hours | $250.48 | Normal | $250.48 | $31.29 |
| Cost of EEG for 48 hours | $500.96 | Normal | $500.96 | $62.58 |
For beta distributions, parameter 1 is alpha (number with seizures) and parameter 2 is beta (number without seizures). For normal distributions, parameter 1 is mean and parameter 2 is standard error.
All analyses were performed using TreeAge Pro 2014 (TreeAge Software Inc., Williamstown, MA, USA).
Results
Table 3 describes the results from the analysis that used our single best estimate for each input value. As the duration of EEG monitoring increases, there are increases in both costs and the number of children with seizures identified. The 1 hour, 24 hour, and 48 hour EEG monitoring strategies would identify 55%, 85%, and 89% of children experiencing electrographic seizures, respectively. Thus, as the duration of EEG monitoring increases, the incremental increase in the number of children identified with seizures by each strategy compared to the prior strategy gets smaller. For example, choosing 24 hours over 1 hour identifies 30% more children with seizures while choosing 48 hours over 25 hours identifies only 4% more children with seizures. Conversely, as the duration of monitoring increases, the incremental increase in cost gets larger. As a result, as the duration of EEG monitoring increases, the incremental cost-effectiveness ratios increase rapidly. To identify one child with seizures using 1 hour of EEG monitoring costs $466, to identify one more child with 24 hours of monitoring costs $1666, and to identify one additional child with 48 hours of monitoring costs $22,648. Based on these data, if a decision maker were willing to pay more than $466 to identify a child with seizures then monitoring for 1 hour would be preferred over no monitoring. If the willingness to pay were more than $1666 to identify an additional child with seizures, then monitoring for 24 hours would be preferred over 1 hour of monitoring. Only if the decision maker were willing to pay more than $22,648 to identify an additional child with seizures would monitoring for 48 hours be preferred over monitoring for 24 hours.
Table 3.
Cost effectiveness analysis of base case values.
| Strategy | Cost | Incremental Cost | Effect | Incremental Effect | Incremental Cost Effectiveness Ratio |
|---|---|---|---|---|---|
| No EEG | 0 | - | 0 | - | - |
| 1 hour EEG | $84.69 | $84.69 | 0.18 | 0.18 | $465.67 |
| 24 hours EEG | $250.48 | $165.79 | 0.28 | 0.1 | $1,665.63 |
| 48 hours EEG | $500.96 | $250.48 | 0.29 | 0.01 | $22,648.36 |
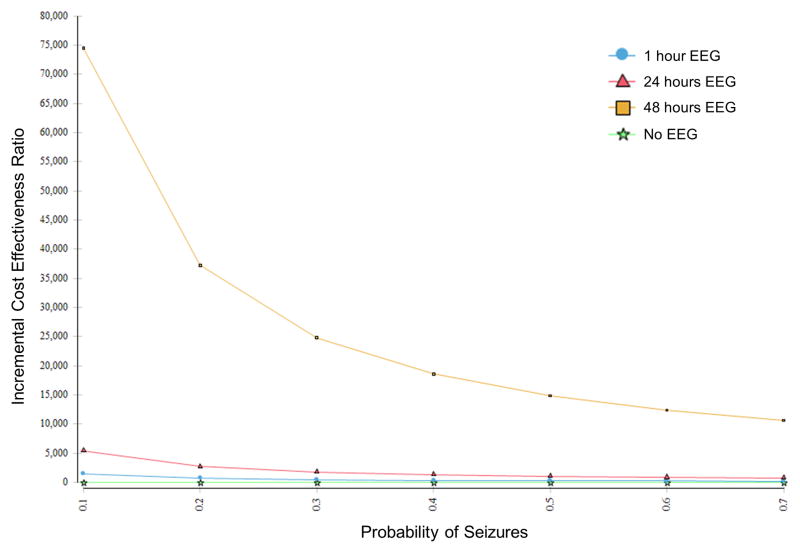
Figure 2 illustrates how the incremental cost-effectiveness ratios for the four EEG monitoring strategies change as the probability of having seizures increases. We varied the probability of electrographic seizures from 0.1 to 0.7. The lower value is the lowest value we thought possible to assign children using known risk factors, and the higher value is the highest value we thought possible to assign children using known risk factors.(Yang, et al. 2014) We used a value of 0.33 in the principal analysis. At every risk of seizures, EEG monitoring strategies with longer-duration monitoring have higher incremental cost-effectiveness ratios than strategies with shorter-duration monitoring, but the differences get smaller as the risk of seizures increases. This means that strategies with shorter-duration monitoring are more likely to meet a willingness-to-pay value when the probability of seizures is lower and that strategies with longer-duration monitoring are more likely to meet a willingness-to-pay value when the probability of seizures is higher.
Figure 2.
Change in the incremental cost-effectiveness ratios for the four EEG monitoring strategies as the probability of experiencing electrographic seizures increases. At every risk of seizures, strategies with longer-duration monitoring have higher incremental cost-effectiveness ratios than strategies with shorter-duration monitoring, but the differences get smaller as the risk of seizures increases.
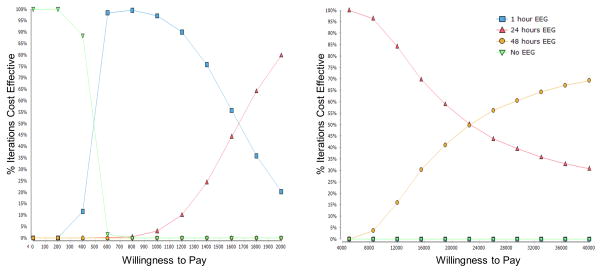
Figure 3 illustrates the relative frequency that the incremental cost-effectiveness ratios from the 10,000 replications in the probabilistic sensitivity analysis meet the willingness-to-pay value. It plots the relative frequency for which each strategy is selected as the willingness-to-pay value increases. As one strategy becomes selected more often the other strategies become selected less often. For example, as the willingness-to-pay increases above about $1,000 fewer of the individual replications show that 1-hour monitoring is cost effective and more of the replications show that 24-hour monitoring is cost effective. The number of replications which would select the 1-hour monitoring strategy decreases since the number of replications which would select the 24-hour monitoring strategy increases. When the willingness to pay reaches about $1,600, the number of cost-effective replications is roughly equal for the 1 hour and 24 hour strategies. Above a willingness-to-pay of $1,600, the number of cost-effective replications for 1-hour monitoring strategy continues to decrease while the number of cost-effective replications for 24-hour monitoring strategy continues to increase until it reaches a peak at about $4,000 when almost all replications favor the 24-hour monitoring strategy over the 1-hour monitoring strategy. The number of replications favoring the 24-hour strategy then starts to decline as the number of cost-effective replications favoring for the 48-hour strategy begins to increase, and at a willingness-to-pay of about $23,000 the number of cost-effective replications is roughly equal for the 24 hour and 48 hour monitoring strategies. These results indicate that we can be more confident that the 24-hour monitoring strategy is the preferred strategy when the willingness-to-pay value is closer to $4,000 than when the value is closer to $1,600 (when the 1-hour strategy could be preferred) or closer to $23,000 (when the 48-hour strategy could be preferred).
Figure 3.
Cost effectiveness acceptability curves. These curves describe the relative frequency that the incremental cost-effectiveness ratios from the probabilistic sensitivity analysis (y-axis) meet increasing willingness-to-pay value (x-axis). See text for explanation.
Discussion
We created a decision tree to estimate how much identifying a critically ill child having electrographic seizures costs. We determined that 1 hour of EEG monitoring was an acceptable strategy when the decision maker was willing to pay <$1,666 to identify a child experiencing electrographic seizures, 24 hours of monitoring was acceptable for a willingness-to-pay of >$1,666 to $22,648 to identify a child experiencing electrographic seizures, and 48 hours was acceptable for any willingness-to-pay value >$22,648 to identify a child experiencing electrographic seizures. The 1-hour, 24-hour, and 48-hour EEG monitoring strategies would identify 55%, 85%, and 89% of children experiencing electrographic seizures, respectively. Sensitivity analyses found that all three strategies were acceptable at lower values for willingness-to-pay when children with higher risks of seizures were monitored, but that the cost to identify a child with electrographic seizures using the 24-hour strategy was moderate even at low seizure risk. Therefore, we believe that our results support recommendations to monitor critically ill children for at least 24 hours since the cost to identify each patient experiencing electrographic seizures is modest.
This information is important because there is increasing evidence that high electrographic seizures burdens are associated with worse outcomes, even after adjustment for acute encephalopathy etiology and severity.(Abend, Arndt, Carpenter, et al. 2013, Kirkham, Wade, McElduff, et al. 2012, Payne, Zhao, Frndova, et al. 2014, Topjian, et al. 2013, Wagenman, et al. 2014) Additionally, there is some evidence that existing anti-seizure medications may terminate electrographic seizures in critically ill children.(Abend, et al. 2013) Together, these data likely explain reported increases in continuous EEG monitoring in pediatric intensive care units.(Sanchez, et al. 2013) Although studies have not evaluated whether identification and treatment of electrographic seizures and status epilepticus improve neurodevelopmental outcomes, recent guidelines recommend performing continuous EEG monitoring in many critically ill children for 48 hours to identify and manage electrographic status epilepticus.(Brophy, Bell, Claassen, et al. 2012) However, continuous EEG monitoring is resource intense since it involves expensive equipment and a substantial amount of work by technologists and physicians.
Our analysis found that 1 hour of EEG monitoring would cost about $466 to identify 1 child experiencing electrographic seizures. However, about 45% of children would not be identified because their seizures would occur after the 1-hour period. We found that, compared to the 1 hour strategy, the incremental cost to identify a child experiencing electrographic seizures with 24 hours of monitoring was $1,666. The 24-hour strategy, however, misses about 15% of children with electrographic seizures. Extending EEG monitoring to 48 hours identifies an additional 4% of children but still fails to identify about 11% of children who would subsequently experience electrographic seizures, and it requires monitoring a large number of children who will not experience electrographic seizures. Thus, there is only a small increase in effectiveness but a large increase in cost, yielding a relatively high incremental cost. Thus, while continuing EEG monitoring for 48 rather than 24 hours may seem like a small change, the incremental cost is substantial. Our analysis indicates that a decision maker would need to be willing to pay about $22,648 or more to identify one additional child with seizures to select the 48-hour strategy over the 24-hour strategy. Deciding whether such costs are appropriate may rest on future studies evaluating the extent to which electrographic seizure identification and management improves long-term neurodevelopmental outcomes.
Our results indicate that decisions regarding appropriate monitoring duration will be impacted by the probability of experiencing electrographic seizures. The costs to identify a patient with electrographic seizures using 24 hours of monitoring are relatively modest at low electrographic seizure probabilities, and thus it may be reasonable to monitor a large number of critically ill children with heterogeneous acute encephalopathy etiologies for 24 hours. However, the costs to identify a child with seizures using a 48 hour strategy are large at low seizure probabilities. Together, these data suggest prediction models for seizures are needed to help clinicians identify patients at varying risks of seizure. For example, these models might combine 24 hours of monitoring for low-risk patients and 48 hours of monitoring for high-risk patients.
While purchasing EEG equipment requires an initial expenditure, our data indicate most of the cost associated with EEG monitoring relates to personnel time and not equipment. For example, for 24 hours of EEG monitoring, only $24.59 of the cost relates to the EEG monitoring machine and electrodes while $225.89 of the cost relates to staff wages (Table 1). Thus, decisions regarding EEG monitoring infrastructure needs may be made optimally by considering EEG technologist and physician staffing and not just upfront equipment costs.
This study has several limitations. First, we estimated the probability of electrographic seizures from studies which mostly involved clinically indicated EEG monitoring. Patients deemed at high risk may have been more likely to undergo continuous EEG monitoring, and thus may be biased towards a high estimate. However, efforts to select patients requiring continuous EEG monitoring might also fail to identify some children experiencing electrographic seizures leading to a bias towards a low estimate. Further, these studies include a mixture of etiologies for acute encephalopathy. Studies are needed in which all children meeting more clearly defined inclusion criteria undergo continuous EEG monitoring. Second, we did not include in our model the possibility that identifying seizures might lead to management which could result in quicker discharge form the intensive care unit and better long-term outcomes, leading to lower costs. Alternatively, identifying electrographic seizures might lead to prolonged intensive care unit stays with no outcome benefit, thereby raising costs. Third, we considered our data in the context of a willingness-to-pay values extending up to $40,000 per child with seizures identified (Figure 3). Importantly, this analysis did not evaluate willingness-to-pay per quality adjusted life years in which $50,000-$100,000 values are often considered.(Neumann, et al. 2014) Further study is needed to extend these data to a quality-of-life analysis. If seizure identification and management led to improved outcomes this could produce a substantial increase in the number of accrued quality adjusted life years. In that context, decision makers might be willing to pay for 48 hours of EEG monitoring despite the seemingly large incremental cost-effectiveness ratio for 48 compared to 24 hours of monitoring. Further study evaluating the impact of EEG monitoring based strategies on neurodevelopmental outcome are needed to perform this more complex analysis.
Conclusions
We evaluated the cost-effectiveness of four electrographic seizure identification strategies (no monitoring and monitoring for 1 hour, 24 hours, or 48 hours) by estimating variable costs from their component parts and obtaining seizure probabilities from the literature. Our results indicated that if decision makers were willing to pay $1,666 to identify a patient with electrographic seizures, they would choose 24 hours of monitoring over 1 hour of monitoring. This is a modest cost and therefore supports 24 hours of EEG monitoring in many critically ill children to identify electrographic seizures. The incremental benefit from performing 48 hours of monitoring are small while the costs are large, and thus further study better identifying patients requiring 48 hours of monitoring are needed.
Supplementary Material
Highlights.
We performed a cost-effectiveness analysis comparing four electrographic seizure identification strategies.
The preferred strategy was monitoring for 1 hour, 24 hours, and 48 hours if the decision maker was willing to pay <$1,666, $1,666–$22,648, and >$22,648, respectively.
Cost-acceptability curves demonstrated the reliability of the choice for preferred strategy at different willingness-to-pay values.
Costs associated with monitoring critically ill children for 24 hours are modest.
References
- Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: Cohort study of risk factors and mortality. Neurology. 2013;81:383–391. doi: 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol. 2007;37:165–170. doi: 10.1016/j.pediatrneurol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend NS, Sanchez SM, Berg RA, Dlugos DJ, Topjian AA. Treatment of electrographic seizures and status epilepticus in critically ill children: A single center experience. Seizure. 2013;22:467–471. doi: 10.1016/j.seizure.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abend NS, Wusthoff CJ, Goldberg EM, Dlugos DJ. Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol. 2013;12:1170–1179. doi: 10.1016/S1474-4422(13)70246-1. [DOI] [PubMed] [Google Scholar]
- Arango JI, Deibert CP, Brown D, Bell M, Dvorchik I, Adelson PD. Posttraumatic seizures in children with severe traumatic brain injury. Childs Nerv Syst. 2012;28:1925–1929. doi: 10.1007/s00381-012-1863-0. [DOI] [PubMed] [Google Scholar]
- Arndt DH, Lerner JT, Matsumoto JH, et al. Subclinical early posttraumatic seizures detected by continuous EEG monitoring in a consecutive pediatric cohort. Epilepsia. 2013;54:1780–1788. doi: 10.1111/epi.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802. doi: 10.1001/jama.2012.476. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Crawford JR, Glaser C, Sheriff H, Wang S, Nespeca M. The role of continuous electroencephalography in childhood encephalitis. Pediatr Neurol. 2014;50:318–323. doi: 10.1016/j.pediatrneurol.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012;129:e748–755. doi: 10.1542/peds.2011-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Colina AM, Topjian AA, Dlugos DJ, Abend NS. EEG Monitoring in Critically Ill Children: Indications and Strategies. Pediatric Neurology. 2012;46:158–161. doi: 10.1016/j.pediatrneurol.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwer S, Idro R, Fegan G, et al. Continuous EEG monitoring in Kenyan children with non-traumatic coma. Arch Dis Child. 2012;97:343–349. doi: 10.1136/archdischild-2011-300935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbani DM, Topjian AA, Friess SH, et al. Nonconvulsive electrographic seizures are common in children with abusive head trauma*. Pediatr Crit Care Med. 2013;14:709–715. doi: 10.1097/PCC.0b013e3182917b83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway RG, Ringel SP. Getting to value in neurological care: a roadmap for academic neurology. Ann Neurol. 2011;69:909–918. doi: 10.1002/ana.22439. [DOI] [PubMed] [Google Scholar]
- Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol. 2005;32:162–165. doi: 10.1016/j.pediatrneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med. 2012;38:853–862. doi: 10.1007/s00134-012-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia. 2011;52:1973–1978. doi: 10.1111/j.1528-1167.2011.03291.x. [DOI] [PubMed] [Google Scholar]
- Neumann PJ, Cohen JT, Weinstein MC. Updating Cost-Effectiveness - The Curious Resilience of the $50,000-per-QALY Threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–1438. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantino JA, Wainwright MS, Grimason M, et al. Nonconvulsive Seizures Are Common in Children Treated With Extracorporeal Cardiac Life Support*. Pediatr Crit Care Med. 2013;14:601–609. doi: 10.1097/PCC.0b013e318291755a. [DOI] [PubMed] [Google Scholar]
- Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia. 2006;47:1510–1518. doi: 10.1111/j.1528-1167.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG Monitoring: Current Resources and Practice in the United States and Canada. Journal of Clinical Neurophysiology. 2013;30:156–160. doi: 10.1097/WNP.0b013e31827eda27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber JM, Zelleke T, Gaillard WD, Kaulas H, Dean N, Carpenter JL. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012;17:31–38. doi: 10.1007/s12028-012-9715-z. [DOI] [PubMed] [Google Scholar]
- Shahwan A, Bailey C, Shekerdemian L, Harvey AS. The prevalence of seizures in comatose children in the pediatric intensive care unit: A prospective video-EEG study. Epilepsia. 2010;51:1198–1204. doi: 10.1111/j.1528-1167.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia. 2006;47:1504–1509. doi: 10.1111/j.1528-1167.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Critical Care Medicine. 2013;31:215–223. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014;82:396–404. doi: 10.1212/WNL.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011;52:1130–1136. doi: 10.1111/j.1528-1167.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- Yang A, Arndt DH, Berg RA, et al. Development and validation of a seizure prediction model in critically ill children. Seizure. 2014 doi: 10.1016/j.seizure.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.