Summary
Background and Objectives
Carboxypeptidase B2 (CPB2) is a basic carboxypeptidase with fibrin and complement C3a and C5a as physiological substrates. We hypothesized that in polymicrobial sepsis, CPB2-deficient mice would have sustained C5a activity, leading to disease exacerbation.
Methods
Polymicrobial sepsis was induced by cecal ligation and puncture (CLP).
Results
Contrary to our hypothesis, Cpb2−/− mice had significantly improved survival, with reduced lung edema, less liver and kidney damage, and less disseminated intravascular coagulation. Hepatic proCPB2 was induced by CLP leading to increase in proCPB2 levels. Thrombomodulin present on mesothelium supported thrombin activation of proCPB2. Both WT and Cpb2−/− animals treated with a C5a receptor antagonist had improved survival, demonstrating that C5a was detrimental in this model. Treatment with a fibrinolysis inhibitor, tranexamic acid, caused a decrease in survival in both genotypes, however, the Cpb2−/− animals still retained their survival advantage. Administration of a C3a receptor antagonist exacerbated the disease in both WT and Cpb2−/− mice, and eliminated the survival advantage of Cpb2−/− mice. C5a receptor is expressed in both peritoneal macrophages and neutrophils; in contrast, C3a receptor expression is restricted to peritoneal macrophages, and C3a induced signaling in macrophages but not neutrophils.
Conclusions
Thus while C5a exacerbates the peritonitis, resulting in a deleterious generalized inflammatory state, C3a activation of peritoneal macrophages may limit the initial infection following CLP, thereby playing a diametrically opposing protective role in this polymicrobial sepsis model.
Keywords: Anaphylatoxin, Carboxypeptidase B2, Fibrinolysis, Inflammation, Sepsis
Introduction
Procarboxypeptidase B2 (proCPB2; TAFI) is activated by the thrombin/thrombomodulin (TM) complex or plasmin to carboxypeptidase B2 (CPB2), which removes C-terminal basic amino acids [1]. CPB2 has several substrates including fibrin, complement C3a and C5a, and bradykinin (BK) [2]. Removing C-terminal lysines from partially digested fibrin prevents binding of plasminogen and tissue plasminogen activator thereby down-regulating plasmin generation and reducing fibrinolysis. Removal of C-terminal arginine residues from BK, C3a and C5a inactivates them. Generally, CPB2 substrates promote inflammation while the CPB2 cleavage products have much lower inflammatory activity, suggesting that activation of proCPB2 by the thrombin/TM complex, along with activation of protein C (PC), represents a homeostatic mechanism to regulate thrombin's inflammatory activity [3, 4].
Studies using the proCPB2-deficient (Cpb2−/−) mouse support this hypothesis [5]. Cpb2−/− mice developed much more extensive C5a-induced alveolitis than wild type (WT) mice [6]. In models of allergic bronchial asthma and acute lung injury, Cpb2−/− mice had exacerbated disease compared to WT mice that was normalized by treatment with a C5a receptor antagonist (C5aRA) [7, 8]. In an autoimmune arthritis model, Cpb2−/− mice developed severe arthritis that was abolished by anti-C5 antibody [9]. Furthermore, patients with rheumatoid arthritis (RA) who carry the Cpb2 allele variant encoding isoleucine vs. threonine at position 325 resulting in an increased plasma half-life (~16 min vs. ~8 min) have a lower risk of developing severe RA. Thus CPB2 may function as a major regulator of C5a activity in vivo.
Complement activation plays a prominent role in sepsis and C5a is a major anaphylatoxin. While C5a is a potent chemoattractant for neutrophils, excessive C5a is deleterious, contributing to immunoparalysis, thymocyte apoptosis, and dysregulation of coagulation and fibrinolysis. C5a blockade is protective in rodent models of sepsis [10, 11]. When complement is activated, two other anaphylatoxins are also generated, C4a and C3a whose potency is much lower than that of C5a [12, 13]. Increased expression of C3a protects mice against LPS challenge, but surprisingly, so does increased C3a in the absence of the C3a receptor, C3aR [14].
Reports of infectious challenge in the Cpb2−/− mouse gave conflicting observations. In Escherichia coli challenge, liver damage is reduced in Cpb2−/− mice [15] while infection with Yersinia enterocolitica exacerbated the disease [16]. The mouse cecum ligation and puncture (CLP) model, which involves a polymicrobial peritonitis, closely resembles human sepsis [17] and therefore we investigated the role of CPB2 in this model. In a rat CLP model, anti-C5a antibody is protective, consistent with the predominantly deleterious role of C5a in sepsis [18, 19]. We hypothesized that in the CLP model of polymicrobial sepsis, the Cpb2−/− mice would have excessive C5a generation, leading to increased mortality. However we found that Cpb2−/− mice had reduced mortality, contradicting our hypothesis, and C3a, rather than fibrin or C5a, was the key substrate accounting for the beneficial effect.
Materials and Methods
Mice
Cpb2−/− mice, backcrossed >11 generations onto the C57BL/6J background [20, 21], and WT C57BL/6J mice (Jackson Laboratory, Sacramento, CA) were housed at Stanford University and experiments performed under protocols approved by the Stanford University Committee of Animal Research in accordance with NIH guidelines.
CLP model
The CLP model was performed in 12 weeks old male mice [6]. All mice that were entered into the experimental protocol were accounted for. The operator was blinded with respect to the samples in all assays and mice were randomized.
In some experiments, mice received intraperitoneally 1.8 mg/kg tranexamic acid (TA) one day before CLP and then every 12 hours intraperitoneally after CLP [22]. In experiments with C5aRA (cyclic AcF(OP(D)ChaWR; Biomatik, Wilmington, DE), mice received 1 mg/kg C5aRA subcutaneously one day before CLP and each day during CLP [7]. In experiments with C3a receptor antagonist (C3aRA; EMD Millipore, Billerica, MA), mice received intraperitoneally 1 mg/kg C3aRA dissolved in PBS with SB 290157; 1.16% DMSO at 45 minutes before CLP, and then every 12 hours until sacrifice [23]. Untreated animals received the same dose of vehicle. Lung edema was determined by the wet/dry weight ratio .
Laboratory tests of blood and peritoneal lavage
Blood was collected by retro-orbital bleeding into heparinized tubes at 6 and 24 hours and by cardiac puncture at 48 hours. Peritoneal lavage at 48 hours was obtained by introducing 5 ml of PBS into the peritoneum. Blood samples were analyzed for levels of alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), creatinine and complete blood count. CPB2 level was determined by an ELISA (Sekisui Diagnostics, Stamford, CT) for human CPB2, with human CPB2 as standard [21]. Fibrinogen and fibrin(ogen) degradation products were analyzed with and without reduction by Western blot using an anti-fibrinogen antibody (Abcam, Cambridge, MA). ProCPB2 activation was detected by Western blots using an anti-CPB2 antibody (Abcam) that detects an epitope present in both proCPB2 and CPB2. The blot was scanned and the total signal from one lane normalized to 100% and then the fraction present in each band calculated. D-dimer (Kamiya Biomedical Company, Seattle, WA), IL-6 and total C5a (R&D Systems, Minneapolis, MN) were determined by ELISA [24].
Isolation of mesothelial cells (MCs) and determination of their TM cofactor activity
Primary peritoneal MCs were isolated from WT mouse peritoneum [25] and their cell surface phenotype analyzed by flow cytometry and immunofluorescence. Activation of PC by cultured MCs was monitored by chromogenic substrate and proCPB2 by chromogenic substrate and Western blot [26].
Analysis of anaphylatoxin receptor expression
Eight-week old C57BL/6J male mice were treated with thioglycollate intraperitoneally to induce peritoneal macrophages. Bone marrow cells were collected from femurs by flushing the bone cavity with PBS. Peripheral blood was collected by cardiac puncture under anesthesia and the buffy coat prepared. Expression data from thioglycollate-induced peritoneal macrophages (F4/80Hi) and blood neutrophils were from the ImmGen Consortium web page [27]. C3aR expression was detected by staining with anti-C3aR (Hycult Biotech, Plymouth Meeting, PA), C5aR1 expression by staining with anti-C5aR1 (BioLegend, San Diego, CA) and for C5aR2, cells were permeabilized with 0.2% Tween 20 before staining with anti-C5aR2 (R&D Systems). All cells were stained with FITC-conjugated anti-Gr-1 (BD Biosciences, San Jose, CA) and BV421 F4/80 (BioLegend) to define neutrophils and macrophage populations before analysis by flow cytometry.
Measurement of calcium transients and ROS induced by anaphylatoxins
Peritoneal lavage cells were stained with BV421 F4/80 to define macrophage populations and bone marrow cells were stained with Alexafluor 647 Gr-1 (BioLegend) to define neutrophils before incubation with 5 μg/mL Fluo-8 AM (AAT Bioquest, Sunnyvale CA), 25 mM probenecid, 0.04% pluronic F127 for 30 minutes. Cells were passed through a flow cytometer in which green fluorescence was recorded over 5 minutes. Human C3a (Hycult Biotech), C3a-desArg or C5a was added 60 seconds after recording started.
To detect radical oxygen species (ROS), peritoneal lavage cells were incubated with 50 mM dihydrarhodamine 123 (Life Technologies, Carlsbad, CA) for 30 minutes. Green fluorescence was read for 2 hours. Data was acquired for 1 minute to obtain baseline values before addition of C3a, C3a-desArg, or phorbol 12-myristate 13-acetate (PMA) dissolved in DMSO or vehicle.
Statistics
Data were analyzed with Prism v6 for Macintosh. Student's t test was used to compare two groups and ANOVA with post-hoc Bonferroni's correction for comparison of three or more groups. In experiments in which there was a pharmacological intervention, the data were analyzed by two-way ANOVA with post-hoc correction by Fisher's least significant difference test. Error bars show ±SEM. Kaplan-Meier survival curves were analyzed by Mantel-Cox test. p<0.05 was considered as statistically significant.
Results
Cpb2−/− mice survive longer with CLP than WT mice
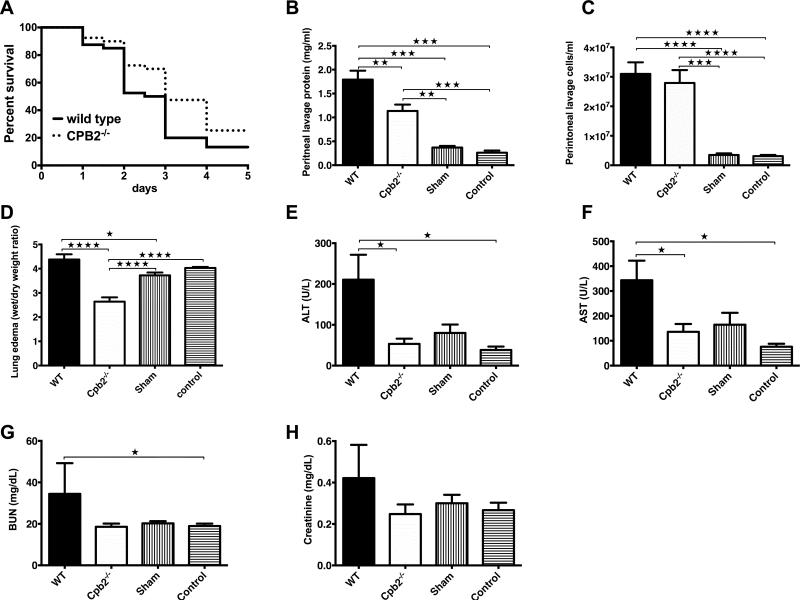
Following CLP surgery, no excessive bleeding was observed in Cpb2−/− animals. Cpb2−/− mice had a survival advantage compared to WT animals (Fig. 1A; p=0.021; n=40 in each group). On day 5 after CLP, 27% of the Cpb2−/− mice were alive while only 13% of WT mice survived. Median survival increased from 3 to 4 days (p=0.015) with a hazard ratio of 2.3 for WT animals compared to Cpb2−/− animals (95% confidence interval: 1.1–4.6). Thus CPB2 has a deleterious effect on survival in polymicrobial sepsis.
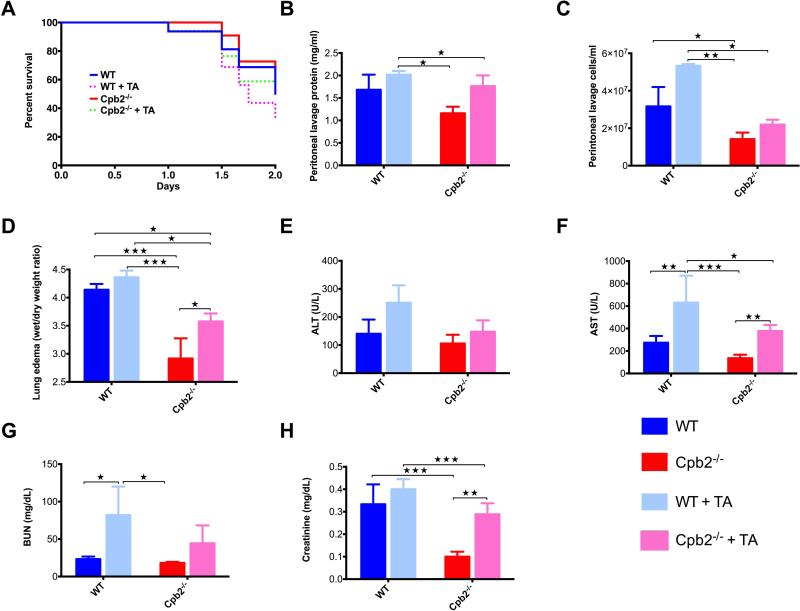
Fig. 1. Cpb2−/− mice are protected from polymicrobial sepsis compared to WT mice.
(A) Kaplan-Meier survival curves comparing Cpb2−/− to WT mice following CLP analyzed by Mantel-Cox log-rank test (p=0.021; n=40 in each group, median survival WT 3 days vs Cpb2−/− 4 days [p=0.015]; hazard ratio: 2.3 for WT vs Cpb2−/− [95% confidence interval: 1.1–4.6]). (B-H) Groups of Cpb2−/− and WT mice with CLP plus WT sham-operated and control mice were sacrificed after 48 hours. Data are shown from one of four independent experiments with similar results. (B) Protein in peritoneal lavage. (C) Cells in peritoneal lavage. (D) Lung edema (E) ALT, (F) AST, (G) BUN, (H) Creatinine in plasma at 48 h. B–H: WT n=11, Cpb2−/− n=8, sham n=8, control n=15. Data were analyzed by one-way ANOVA with post-hoc Bonferroni correction. * p<0.05; ** p<0.01; *** p<0.001; **** p < 0.0001.
Cpb2−/− protects against multiple end organ damage in CLP
In the CLP model, leakage of the microbial contents of the cecum into the peritoneum leads to a generalized abdominal peritonitis. The peritoneal lavage fluid at 48 hours was less exudative in Cpb2−/− than WT mice, with lower protein content (Fig. 1B,C). Both WT and Cpb2−/− animals had increases over WT and Cpb2−/− sham-operated or control mice, showing that the exudative lavage fluid was due to the polymicrobial sepsis.
Lung edema at 48 hours was significantly reduced in Cpb2−/− mice compared to WT (Fig. 1D). When lung edema was determined on surviving animals after 5 days, it was significantly worse in WT (n=8; 5.71±0.19) than in Cpb2−/− mice (n=15; 4.19±0.048; p<0.0001). Plasma levels of ALT and AST were increased significantly in WT mice at 48 hours compared to Cpb2−/− mice (Fig. 1E,F) showing that they had more liver damage. Similarly, renal function showed an increase in BUN levels and a trend towards increased creatinine levels in WT while there was no difference between the Cpb2−/−, sham and control animals (Fig. 1G,H). In an independent experiment, the values from control and sham WT mice were shown to be similar to those of control and sham Cpb2−/− animals (Fig. S1). To eliminate the possibility of ascertainment bias due to WT animals dying earlier than Cpb2−/−, we investigated markers at 24 and 30 hours. There was no difference at 24 hours, but at 30 hours liver markers and the exudate in the peritoneum were exacerbated in WT (Fig. S2). Taken together, these data demonstrate more extensive end organ damage at 30 and 48 hours in the WT animals, as compared to the Cpb2−/− mice.
Histological examination of lung, liver and kidney tissues at 48 or 84 hours showed no difference between Cpb2−/− and WT mice with CLP, and the tissues from mice with CLP were not different from control tissues (data not shown).
Polymicrobial sepsis leads to leukopenia
At 48 hours, animals developed significant leukopenia compared to sham and control animals, with a similar decrease in both WT and Cpb2−/− mice (Fig. S3), primarily due to a prominent reduction in lymphocytes. The increase in neutrophils over control was similar in WT, Cpb2−/− and sham animals while there was a striking reduction in monocytes in the Cpb2−/− animals. Values for CBC in WT and Cpb2−/− sham and control animals were similar.
Coagulopathy occurs in WT and Cpb2−/− mice in CLP
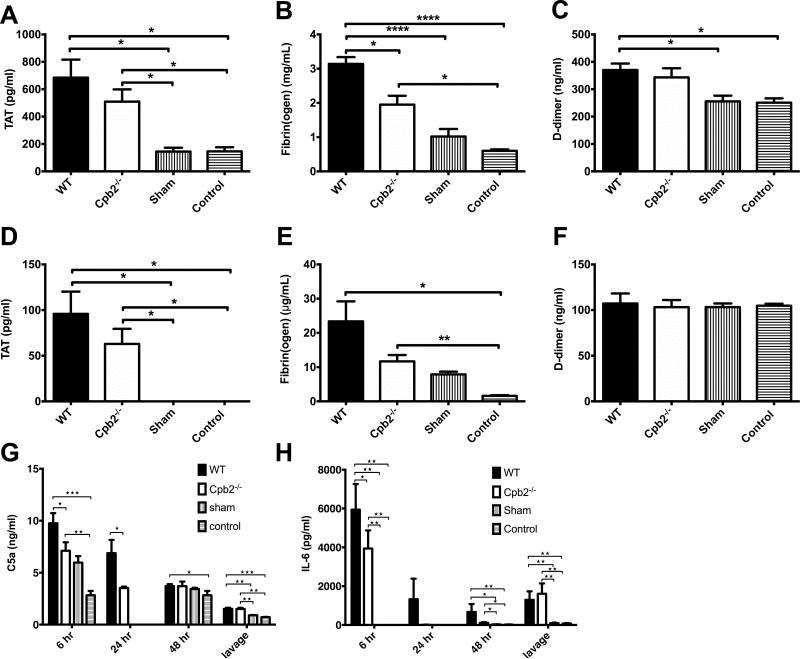
Thrombin-antithrombin (TAT) levels were increased in WT and Cpb2−/−, compared to sham and control animals (Fig 2A,D). There was a significant increase in plasma fibrin(ogen) levels in both WT and Cpb2−/− animals as expected, since fibrinogen is an acute phase protein [28]. However the total fibrin(ogen) levels were significantly higher in WT than Cpb2−/− (Fig. 2B,E), and fibrinogen was extensively degraded in all CLP samples irrespective of the genotype as demonstrated by Western blot analysis (data not shown). The level of D-dimer in plasma was elevated to a similar extent in WT and Cpb2−/− animals (Fig. 2C). Thus, coagulopathy occurred with CLP in both WT and Cpb2−/− mice.
Fig. 2. Both Cpb2−/− and WT mice develop coagulopathy during polymicrobial sepsis.
(A-F) Groups of Cpb2−/− and WT mice with CLP plus WT sham-operated and control mice were sacrificed 48 hours after CLP. Data are shown from one of two independent experiments with similar results. WT n=15, Cpb2−/− n=15, sham n=5, control n=5. Data were analyzed by one-way ANOVA with post-hoc Bonferroni correction. * p<0.05; ** p<0.01; *** p<0.001; **** p < 0.0001. (A) Plasma TAT levels. (B) Plasma fibrin(ogen) levels. (C) Plasma D-dimer levels. (D) TAT levels, in peritoneal lavage (E) Fibrin(ogen) levels, in peritoneal lavage and (F) D-dimer levels in peritoneal lavage. (G,H) Groups of Cpb2−/− and WT mice with CLP plus WT sham-operated and control mice were sacrificed 6, 24 or 48 hours after CLP. (G) C5a levels in plasma at different times after polymicrobial sepsis was initiated and in lavage at 48 hours. (H) IL-6 levels in plasma at different times after polymicrobial sepsis was initiated and in lavage at 48 hours. G,H: WT n=15 (6 hours), 5 (24 hours), 13 (48 hours) and 15 (lavage), Cpb2−/− n=14 (6 hours), 5 (24 hours), 15 (48 hours) and 14 (lavage), sham n=5 (6 hours), 5 (48 hours) and 5 (lavage), control n=5 (6 hours), 5 (48 hours) and 5 (lavage).
Inflammatory mediators are lower in Cpb2−/− than WT mice after CLP
To assess the systemic inflammatory response, two key mediators, total C5a as a marker of complement activation and IL-6 as a marker of inflammatory cytokines, were assayed in blood samples taken at 6, 24 and 48 hours. Both were significantly increased at 6 hours, with a greater increase in WT mice than Cpb2−/− mice, which returned towards the baseline subsequently (Fig. 2G,H). At 48 hours, total C3a was also increased in the plasma from WT mice compared to Cpb2−/− animals (23.1±2.85 μg/ml vs. 13.05±2.36 μg/ml, n=17 and 18 in each group respectively p=0.0101; control plasma levels 7.14 μg/ml). There was also a significant increase in C5a and IL-6 in peritoneal lavage at 48 hours in both WT and Cpb2−/− mice. Thus there is an early increase in inflammatory mediators that is greater in WT than in Cpb2−/− animals.
Production of proCPB2 by liver and its activation
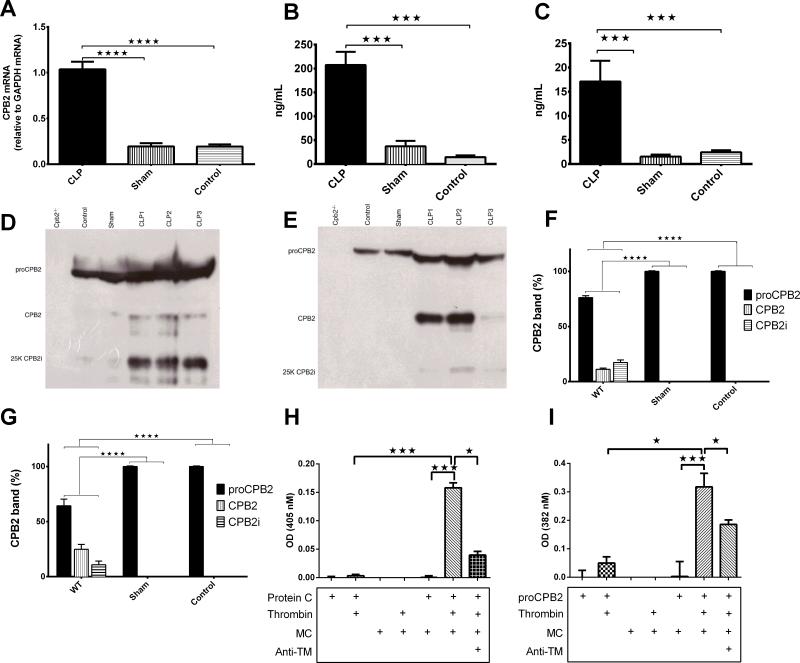
As CPB2 is an acute phase protein [29], we investigated its induction. Hepatic CPB2 mRNA was ~3 fold higher at 48 hours than that taken from sham or control mice confirming that it is an acute phase protein (Fig. 3A; p<0.0001 WT vs. either sham or control n=3-4). Residential peritoneal macrophages in control animals did not have detectable levels of CPB2 mRNA in baseline and only a trace amount was detectable at 48 hours.
Fig. 3. CPB2 mRNA and protein is induced and CPB2 activated during polymicrobial sepsis.
(A-G) Groups of Cpb2−/− and WT mice with CLP plus WT sham-operated and control mice were sacrificed 48 hours after CLP. Data are shown from one of two independent experiments with similar results. Data were analyzed by one-way ANOVA with post-hoc Bonferroni correction. * p<0.05; ** p<0.01; *** p<0.001; **** p < 0.0001. (A) CPB2 mRNA determined by qPCR from liver at 48 hours. WT n = 3, sham n=3 control n=3. (B) CPB2 levels in plasma determined by ELISA at 48 hours. (C) CPB2 levels in peritoneal lavage determined by ELISA at 48 hours. WT n=14, sham n=5, control n=5. (D,E) proCPB2 (mw = 60,000), CPB2 (mw=35,000) and CPB2i (mw=25,000) detected by Western blot in plasma (D) and lavage (E). In each case, a representative experiment is shown from one of four independent experiments. (F,G) Levels of proCPB2, CPB2 and CPB2i in (F) plasma and (G) lavage from WT (n=15), sham (n=3) and control (n=3) animals were determined by scanning the Western blots with the total signal from one lane set to 100% and then the fraction present in each band was calculated. (H,I) MCs were prepared from trypsinization of the peritoneal lavage. MCs were incubated with the reagents shown below the graph as described in Methods before stopping the reaction with hirudin and measuring the activity of aPC (H) and CPB2 (I). Baseline readings for PC alone or proCPB2 alone, due to hydrolysis of the chromogenic substrates by proCPB2 itself (51), were subtracted. n=3-11.
In animals with CLP, there was an increase in levels of proCPB2/CPB2 protein determined by ELISA in both plasma (>14 fold, p<0.001) and in the peritoneum (>6 fold, p<0.001; Fig. 3B,C). Since neither residential macrophages nor MCs produce much proCPB2 mRNA, the increase in proCPB2 levels in the peritoneum reflects an increase in production in the liver and subsequent leakage into the peritoneum.
Plasma proCPB2/CPB2 levels are positively correlated with plasma IL-6 (R2 = 0.361; p=0.0389) and fibrin(ogen) (R2 = 0.512; p=0.0089) and trend to correlate with D-dimer (R2 = 0.193; p=0.1329) and TAT (R2 = 0.242; p=0.0881; Fig. S4), suggesting that IL-6 might be inducing hepatic proCPB2 similarly to its induction of fibrinogen [30] and that levels of D-dimer and TAT levels trend to correlation with induction of proCPB2/CPB2.
We next investigated if there is active CPB2 in plasma and the peritoneum. No immunoreactive protein was detected in Cpb2−/− mice, while in plasma samples from control and sham-operated WT mice, there was a single band (60 kDa) corresponding to proCPB2 (Fig. 3D). Following CLP, proCPB2, active CPB2 (35 kDa), and an inactive fragment of CPB2 (25 kDa; CPB2i) were detected in plasma [31]. The increase in immune reactive material is consistent with the increase in total CPB2 detected by ELISA. Similar results were observed in the lavage fluid samples (Fig. 3E). Significant increases in activation of proCPB2 in WT mice with CLP was observed compared to the sham and control groups (Fig. 3F,G). Thus proCPB2 is activated during CLP in both plasma and the peritoneum, similar to the increase in activation in plasma reported in sepsis patients [32].
TM is on the mesothelium and supports thrombin activation of proCPB2
TM was located on cells lining the abdominal wall, omentum and intestinal wall (Fig. S5A). Positive TM staining was also observed in endothelial cells lining blood vessels in the abdominal wall, omentum, and in the villi of the intestinal mucosa. No TM expression was detected in the columnar epithelium lining the gut.
To investigate if the TM present in MCs is functional in supporting activation of PC and proCPB2 [1, 33], MCs were isolated from peritoneal lavage after brief trypsinization of the peritoneal cavity (Fig. S5B-G). MCs grew in culture as adherent monolayers and expressed epithelial cell markers, calretinin and pan-cytokeratin as well as TM and endothelial protein C receptor but not endothelial cell markers, P-selectin and CD31, or the leukocyte marker, CD45 (Fig. S5C–F).
MCs efficiently activated PC and proCPB2 in the presence of thrombin measured by chromogenic substrates and determination of the extent of proCPB2 activation by Western blot (Fig. 3H,I and Fig. S6). The reactions were dependent on the presence of TM, since anti-TM significantly inhibited the activation. Thus MCs that line the peritoneal cavity support the activation of proCPB2 by the thrombin/TM complex.
The key substrate for CPB2 in CLP is not C5a
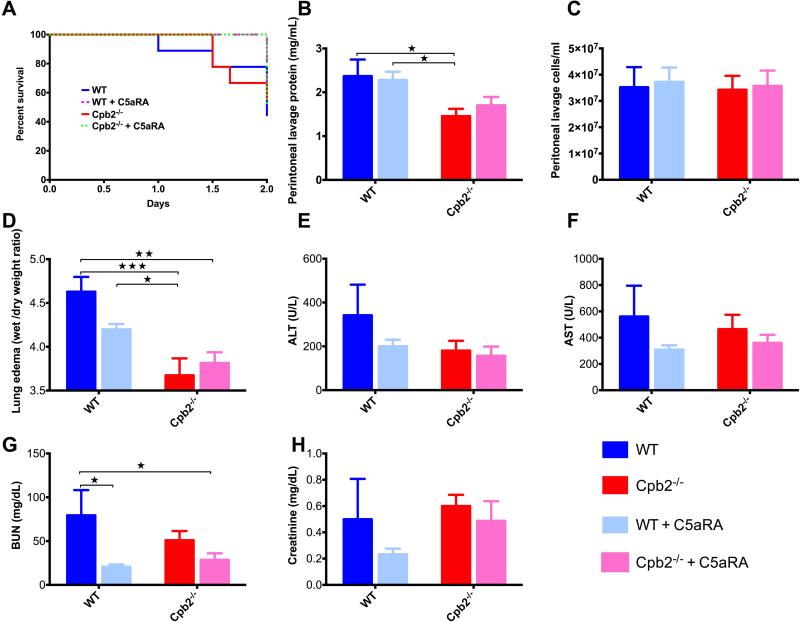
Mice treated with C5aRA survived longer than untreated animals irrespective of their genotype (Fig. 4A), consistent with the hypothesis that C5a is deleterious in this model. Protein levels were higher in peritoneal lavage of WT animals at 48 hours compared to Cpb2−/− animals. That difference in protein levels in the peritoneal lavage between WT and Cpb2−/− mice was maintained even in mice treated with C5aRA, regardless of their genotype (Fig. 4B). Treatment with C5aRA did not alter the number of infiltrating cells in the peritoneum (Fig. 4C).
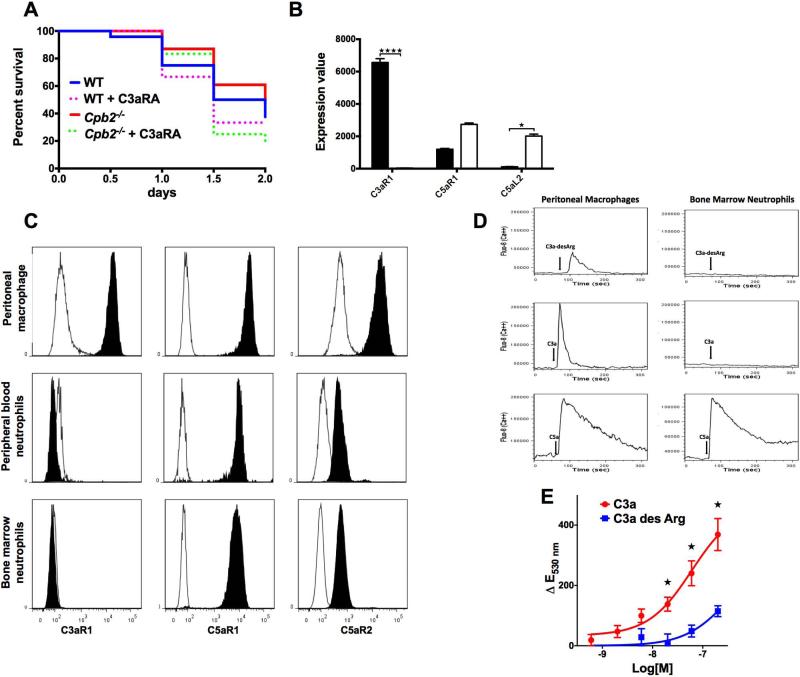
Fig. 4. Antagonism of C5a with C5aRA does not equalize outcomes for Cpb2−/− and WT mice in polymicrobial sepsis.
(A) Kaplan-Meier survival curves comparing untreated Cpb2−/−, C5aRA-treated Cpb2−/−, untreated WT to C5aRA-treated WT mice in the CLP model (n=10 each genotype; C5aRA treated vs untreated: p=0.0422). (B-H) Groups of Cpb2−/− and WT mice with CLP untreated or treated with C5aRA were sacrificed after 48 hours. Data are shown from one of two independent experiments with similar results. (B) Protein in peritoneal lavage. (C) Cells in peritoneal lavage. (D) Lung edema (E) ALT, (F) AST, (G) BUN, and (H) Creatinine in plasma at 48 hours. B–H: WT n=5, Cpb2−/− n=6, WT + C5aRA n= 6, Cpb2−/− + C5aRA n=8. Closed columns: untreated; open columns treated. Data were analyzed by two-way ANOVA with post-hoc Fisher's least significant difference correction. * p<0.05; ** p<0.01; *** p<0.001.
There was no significant change in lung edema in C5aRA treated animals in either WT or Cpb2−/− mice (Fig. 4D). There was improvement in liver and kidney functions with C5aRA administration in both groups of animals (Fig. 4E–H), while the changes were more apparent in the WT mice since the baseline organ dysfunction was more severe. Thus the data confirmed that C5a is deleterious in sepsis and both genotypes had the sepsis ameliorated by C5aRA treatment.
The key substrate for CPB2 in CLP is not fibrin
TA blocks the binding of plasminogen and tissue plasminogen activator to fibrin, resulting in suppression of fibrinolysis. We used pharmacological intervention with TA to suppress fibrinolysis [22, 34] to determine if fibrin is the key CPB2 substrate leading to protection in our CLP model.
Survival in both WT and Cpb2−/− mice was reduced by TA treatment but there was still a significant difference between WT and Cpb2−/− mice (Fig. 5A; p=0.049; n=15). In mice treated with TA, there was a worsening of peritonitis, lung edema, and liver and kidney damage that was greater in WT animals than in Cpb2−/− animals (Fig. 5B-H). Thus inhibition of fibrinolysis exacerbates the outcomes of CLP, consistent with systemic thrombosis contributing to mortality and the expedient removal of fibrin is protective. But as the survival advantage in Cpb2-/- mice and the associated end organ damage were not eliminated by TA treatment, it suggests that fibrin is not the key substrate for CPB2.
Fig. 5. Inhibition of fibrinolysis with TA does not equalize outcomes for Cpb2−/− and WT mice in polymicrobial sepsis.
(A) Kaplan-Meier survival curves comparing untreated Cpb2−/−, TA-treated Cpb2−/−, untreated WT to TA-treated WT in the CLP model (n=15 each genotype; TA treated vs untreated p=0.0241; WT + TA vs Cpb2−/−: p=0.049). (B-H) Groups of Cpb2−/− and WT mice with CLP untreated or treated with TA were sacrificed after 48 hours. Data are from one of three independent experiments with similar results. (B) Protein in peritoneal lavage. (C) Cells in peritoneal lavage (D) Lung edema (E) ALT, (F) AST, (G) BUN and (H) Creatinine in plasma at 48 hours. B – H: WT n=6, Cpb2−/− n=7, WT + TA n=5, Cpb2−/− + TA n=9. Closed columns: untreated; open columns treated. Data were analyzed by two-way ANOVA with post-hoc Fisher's least significant difference correction. * p<0.05; ** p<0.01; *** p<0.001.
The key substrate for CPB2 in CLP is C3a
C3a, another anaphylatoxin, is inactivated by CPB2 leading to C3a-desArg that is inactive in monocyte chemotaxis [35]. We used pharmacological intervention with C3aRA [23] to test if C3a is the key substrate of CPB2 leading to protection in our CLP model.
Survival in WT and Cpb2−/− mice treated with C3aRA was reduced compared to untreated animals and the difference between WT and Cpb2−/− mice was eliminated (Fig. 6A; Cpb2−/− vs. Cpb2−/− + C3aRA: p=0.026; WT vs. Cpb2−/−: p=0.047; n=24 in each group except Cpb2−/− where n=23). After 48 hours, 48% of the Cpb2−/− mice were alive while only 17% of Cpb2−/− treated with C3aRA mice survived. Treatment with C3aRA reduced the median survival of Cpb2−/− animals from 2 to 1.5 days (p=0.015) with a hazard ratio of 2.7 (95% confidence interval: 1.1–6.6) for the mice treated with C3aRA compared to untreated animals. Thus C3a has a protective effect in polymicrobial sepsis, and it is likely that C3a will have a longer half-life in Cpb2−/− mice, thus leading to an increased protective effect.
Fig. 6. C3a is the protective substrate for CPB2 in CLP.
(A) Kaplan-Meier survival curves of WT and CPB2−/− mice either untreated or treated with C3aRA (n=24 in each group except CPB2−/− where n = 23; C3aRA treated vs untreated p=0.0383; Cpb2−/− + C3aRA vs. Cpb2−/−: p=0.026; WT vs. Cpb2−/−: p=0.047) (B) Expression of C3aR, C5aR1 and C5aR2 mRNA in macrophages (■) and blood neutrophils (□). * p<0.05; **** p<0.001. Expression data from the Immgen Consortium [27]. (C) Expression of C3aR, C5aR1, and C5aR2 on peritoneal macrophages and bone marrow neutrophils. Peritoneal lavage and bone marrow cells were stained with fluorescence labeled anti-Gr-1, F4/80, C3aR, C5aR1, C5aR2, and isotype control antibodies. Peritoneal macrophages were defined as F4/80+, Gr-1- cells in the nongranulocyte gate. Bone marrow neutrophils were defined as Gr-1 high cells in the granulocyte gate. Plain histogram, isotype controls; filled black histogram, target antigens. The data are representative of cells prepared independently from 4 animals. (D) Peritoneal lavage and bone marrow cells were loaded with calcium indicator Fluo-8 prior to flow cytometry. Macrophages and neutrophils were identified by staining with GR-1 and F4/80. Human C3a, C3a-desArg, or C5a, all at 100 nM, were added at 60 secs (vertical arrow) after the start of signal acquisition. Intracellular calcium levels were detected by mean fluorescence intensity of green fluorescence of Fluo-8. Representative data showing one of four independent experiments. (E) Peritoneal macrophages were loaded with DHR 123. Various concentrations of C3a and C3a-desArg in HBSS, PMA in DMSO, and DMSO vehicle were added. Green fluorescence (excitation 488, emission 530) was read at 2 hours. Changes in fluorescent emission at 530 nm (ΔE530 nm) indicate the generation of ROS. Fluorescence values in vehicle only were subtracted from treated wells. The positive control, PMA gave a maximum response of 600 ΔE530 nm at 3 μM. n=13, data pooled from 4 independent experiments. * p<0.05 comparing C3a to C3adesArg at the same concentration.
C3aR is present and functional in peritoneal macrophages but not detectable in neutrophils
Of the three known anaphylatoxin receptors [35], the mRNA encoding the C3a receptor, C3aR, was highly expressed in macrophages but not neutrophils (Fig. 6B) [27]. Flow cytometry confirmed that C3aR was expressed on peritoneal macrophages but was undetectable in peripheral blood or bone marrow neutrophils (Fig. 6C and S7). Activated neutrophils that had migrated to the peritoneum by 24 hours, also did not express C3aR (Fig. S8) irrespective of their genotype. In contrast, the two C5a receptors, C5aR1 and C5aR2, were well expressed in neutrophils as well as macrophages.
To test if C3aR on peritoneal macrophages was functional, calcium transients and ROS induction were assessed [36]. Calcium flux was detected immediately following addition of C3a, but less with C3a-desArg (Fig. 6D). A signal was also detected upon addition of C5a. No response was detected in bone marrow neutrophils with either C3a or C3adesArg, while C5a induced a robust response. Similarly stimulation of macrophages with C3a gave a dose-dependent increase in ROS production [37] while C3a-desArg was much less potent (Fig. 6E). Thus, peritoneal macrophages, but not neutrophils, have functional C3aR responsive to C3a, while the product of C3a treatment by CPB2, C3adesArg, had much lower potency.
Discussion
As CPB2 substrates are involved in host defense mechanisms, this study sought to define its role in polymicrobial sepsis. Originally, we hypothesized that the Cpb2−/− mice would have exacerbated disease due to excessive generation of C5a [17]. To our surprise, the Cpb2−/− mice were protected from the disease in comparison to WT mice.
Our results are consistent with the data from an E. coli challenge model in which there was less liver damage in Cpb2−/− mice than WT mice [15] and with a rat model of sepsis induced by Pseudomonas aeruginosa in which treatment with a CPB2 inhibitor improved outcomes [38].
Activation of multiple protective cascades, including complement and coagulation, occurs in this polymicrobial sepsis model resulting in the generation of C3a, C5a, membrane attack complex (MAC) and thrombin respectively (Fig. 7). While the formation of the MAC is crucial in reducing the bacteremic load, C5a has been shown to be predominantly deleterious. Thrombin leads to fibrin deposition and thrombosis, contributing to coagulopathy and end organ damage.
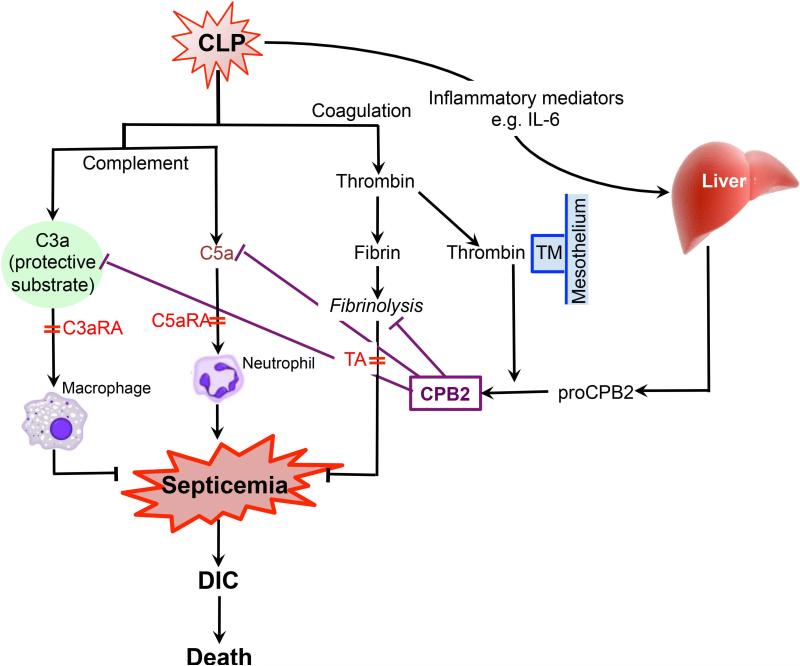
Fig. 7. Model of polymicrobial sepsis showing activation and activities of proCPB2.
Following CLP, blood from damaged vessels and bacteria from the cecum leak into the peritoneum, resulting in the activation of complement and coagulation as well as a general inflammatory reaction, producing C3a, C5a, thrombin and IL-6 amongst other inflammatory mediators. IL-6 triggers production of acute phase proteins in the liver including proCPB2 and fibrinogen. Thrombin converts fibrinogen to fibrin, which subsequently undergoes fibrinolysis. Establishment of bacterial infection systemically (septicemia) causes DIC and leads to systemic inflammatory response syndrome (SIRS), with multiple organ failure and death. Thrombin in combination with mesothelial TM activates proCPB2 to CPB2. In WT mice, CPB2 then inactivates its substrates including C3a, C5a and retards fibrinolysis. Fibrinolysis is protective as treatment with TA causes increased mortality. C5a exacerbates the disease via neutrophils as treatment with C5aRA is protective. C3a is protective via macrophages expressing C3aR1 as treatment with C3aRA exacerbates the disease. In the absence of CPB2, the levels of C3a are increased, over-riding the deleterious effect of C5a, and conferring an overall survival benefit on Cpb2−/− mice. The sites of action of the pharmacological interventions with C3aRA, C5aRA and TA are shown.
CPB2 plays a complex role in this setting because several of its known substrates such as fibrin, C3a and C5a are relevant in this model. It reduces the activity of C5a thereby minimizing its multiple deleterious side effects [2]. C5a blockade, based on either anti-C5a antibody or C5aRA, has been shown to protect animals [39, 40]. In contrast, CPB2 retards fibrinolysis, resulting in a greater thrombotic burden [41]. Here, we showed that C5aRA prolonged the survival of the animals, irrespective of the genotype, confirming the deleterious role of C5a, while TA aggravated the disease irrespective of the genotype but did not eliminate the survival benefit of Cpb2−/− mice. Monocytopenia was observed in Cpb2−/− mice, which may contribute to less coagulopathy via a reduction in monocytic tissue factor. Thus the unexpected survival benefit in the Cpb2−/− mice is not due to either increased C5a levels or enhanced fibrinolysis.
In addition to C5a and fibrin, CPB2 can cleave other substrates in vitro, including C3a and BK. BK is unlikely to be the key substrate here since an increase in active BK levels in the Cpb2−/− mice would lead to greater hypotension [2] thereby exacerbating rather than ameliorating the disease. Measurement of blood pressure in these animals did not show any difference between WT and Cpb2−/− mice (data not shown), in agreement with BK not playing a critical role in this model.
We found that C3aRA-treated Cpb2−/− mice had significantly worse survival than untreated Cpb2−/− mice, and their survival advantage compared to treated WT mice was eliminated. This is consistent with C3aR−/− mice being more sensitive to LPS challenge [42] as well as being more sensitive to Gram-negative bacteremia [43]. Thus in the Cpb2−/− mice, C3a is not inactivated by CPB2, allowing it to exert a greater protective effect, over-riding excessive C5a, and leading to the observed survival benefit (Fig. 7). This is the first experimental model in which C3a, rather than C5a, is the key substrate for CPB2 with the two anaphylatoxins having opposing effects in vivo.
It is notable that the expression pattern of the receptors for the two anaphylatoxins is quite different, with the C5a receptors expressed on peritoneal macrophages, neutrophils and other cells, while C3aR is restricted to peritoneal macrophages. Thus C3a does not function as a chemoattractant for neutrophils, and its primary role may reside in the activation of peritoneal macrophages to limit the dissemination of infection following CLP, ameliorating the subsequent peritonitis. On the other hand, when the infection can no longer be contained, excessive C5a is generated, signaling to cells expressing C5a receptors and leading to influx of neutrophils into the peritoneum, exacerbating the peritonitis and resulting in a deleterious systemic inflammatory state (Fig. 7).
In children with severe meningococcal infections, the CPB2 325 Ile/Ile genotype with the longer plasma half-life was overrepresented in patients admitted with severe DIC when compared to the 325Thr/Thr genotype, consistent with the hypothesis that CPB2 exacerbates some infections [44].
As sepsis is a common challenge in the wild and CPB2 exacerbates sepsis, the Cpb2 gene ought to be eliminated without countervailing selection pressure. One possibility is that in the face of catastrophic hemorrhage, CPB2 stabilizes the thrombus by inhibiting fibrinolysis. The Cpb2−/− mouse has more fibrinolysis in the batroxobin-induced model of pulmonary embolism [45]. Whether these mice will display a survival advantage in a severe hemorrhage model is untested.
Supplementary Material
Acknowledgments
The authors thank Dr. Gerald J. Berry (Department of Pathology, Stanford University School of Medicine) for the histology studies and Lei Zhao (Division of Hematology, Stanford University School of Medicine and VAPAHCS) for the fibrinogen Western blots. This work benefited from data assembled by the ImmGen Consortium. This work was supported by NIH grant 5RO1 HL057530 (to LL).
Footnotes
Addendum
Z. Shao performed the in vitro experiments and analysis of mouse samples, collected the data and participated in its analysis. T. Nishimura performed the in vivo mouse experiments, collected the data and participated in its analysis. L. L. K. Leung and J. Morser conceived of the project, designed the research, analyzed the results, and wrote the paper that was read by all authors.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Bajzar L, Morser J, Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombinthrombomodulin complex. J Biol Chem. 1996;271:16603–8. doi: 10.1074/jbc.271.28.16603. [DOI] [PubMed] [Google Scholar]
- 2.Myles T, Nishimura T, Yun TH, Nagashima M, Morser J, Patterson AJ, Pearl RG, Leung LL. Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. J Biol Chem. 2003;278:51059–67. doi: 10.1074/jbc.M306977200. [DOI] [PubMed] [Google Scholar]
- 3.Leung LL, Myles T, Nishimura T, Song JJ, Robinson WH. Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI). Mol Immunol. 2008;45:4080–3. doi: 10.1016/j.molimm.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung LL, Morser J. Hemostasis and Vascular Inflammation. In: Gary S, Hoffman GS, Weyand CM, Langford CA, Goronzy JJ, editors. Inflammatory Diseases of Blood Vessels. Second edn. Wiley-Blackwell Publishing Ltd; Chichester: 2012. pp. 105–14. [Google Scholar]
- 5.Morser J, Gabazza EC, Myles T, Leung LL. What has been learnt from the thrombin-activatable fibrinolysis inhibitor-deficient mouse? J Thromb Haemost. 2010;8:868–76. doi: 10.1111/j.1538-7836.2010.03787.x. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura T, Myles T, Piliponsky AM, Kao PN, Berry GJ, Leung LL. Thrombinactivatable procarboxypeptidase B regulates activated complement C5a in vivo. Blood. 2007;109:1992–7. doi: 10.1182/blood-2006-03-012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara A, Taguchi O, Takagi T, D'Alessandro-Gabazza CN, Boveda-Ruiz D, Toda M, Yasukawa A, Matsushima Y, Miyake Y, Kobayashi H, Kobayashi T, Gil-Bernabe P, Naito M, Yoshida M, Morser J, Takei Y, Gabazza EC. Role of thrombinactivatable fibrinolysis inhibitor in allergic bronchial asthma. Lung. 2012;190:189–98. doi: 10.1007/s00408-011-9337-9. [DOI] [PubMed] [Google Scholar]
- 8.Naito M, Taguchi O, Kobayashi T, Takagi T, D'Alessandro-Gabazza CN, Matsushima Y, Boveda Ruiz D, Gil-Bernabe P, Matsumoto T, Chelakkot Govindalayathil AL, Toda M, Yasukawa A, Hataji O, Morser J, Takei Y, Gabazza EC. Thrombin-Activatable Fibrinolysis Inhibitor Protects Against Acute Lung Injury by Inhibiting the Complement System. Am J Respir Cell Mol Biol. 2013;49:646–53. doi: 10.1165/rcmb.2012-0454OC. [DOI] [PubMed] [Google Scholar]
- 9.Song JJ, Hwang I, Cho KH, Garcia MA, Kim AJ, Wang TH, Lindstrom TM, Lee AT, Nishimura T, Zhao L, Morser J, Nesheim M, Goodman SB, Lee DM, Bridges SL, Jr., Gregersen PK, Leung LL, Robinson WH. Plasma carboxypeptidase B downregulates inflammatory responses in autoimmune arthritis. J Clin Invest. 2011;121:3517–27. doi: 10.1172/JCI46387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flierl MA, Stahel PF, Rittirsch D, Huber-Lang M, Niederbichler AD, Hoesel LM, Touban BM, Morgan SJ, Smith WR, Ward PA, Ipaktchi K. Inhibition of complement C5a prevents breakdown of the blood-brain barrier and pituitary dysfunction in experimental sepsis. Crit Care. 2009;13:R12. doi: 10.1186/cc7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoehlig K, Maasch C, Shushakova N, Buchner K, Huber-Lang M, Purschke WG, Vater A, Klussmann S. A Novel C5a-neutralizing Mirror-image (l−)Aptamer Prevents Organ Failure and Improves Survival in Experimental Sepsis. Mol Ther. 2013;21:2236–46. doi: 10.1038/mt.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hugli TE. Structure and function of the anaphylatoxins. Springer seminars in immunopathology. 1984;7:193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- 13.Haas PJ, van Strijp J. Anaphylatoxins: their role in bacterial infection and inflammation. Immunologic research. 2007;37:161–75. doi: 10.1007/BF02697367. [DOI] [PubMed] [Google Scholar]
- 14.Boos L, Szalai AJ, Barnum SR. C3a expressed in the central nervous system protects against LPS-induced shock. Neuroscience letters. 2005;387:68–71. doi: 10.1016/j.neulet.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Renckens R, Roelofs JJ, ter Horst SA, van 't Veer C, Havik SR, Florquin S, Wagenaar GT, Meijers JC, van der Poll T. Absence of thrombin-activatable fibrinolysis inhibitor protects against sepsis-induced liver injury in mice. J Immunol. 2005;175:6764–71. doi: 10.4049/jimmunol.175.10.6764. [DOI] [PubMed] [Google Scholar]
- 16.Luo D, Szaba FM, Kummer LW, Plow EF, Mackman N, Gailani D, Smiley ST. Protective roles for fibrin, tissue factor, plasminogen activator inhibitor-1, and thrombin activatable fibrinolysis inhibitor, but not factor XI, during defense against the gram-negative bacterium Yersinia enterocolitica. J Immunol. 2011;187:1866–76. doi: 10.4049/jimmunol.1101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flierl MA, Rittirsch D, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ward PA. Functions of the complement components C3 and C5 during sepsis. FASEB J. 2008;22:3483–90. doi: 10.1096/fj.08-110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laudes IJ, Chu JC, Huber-Lang M, Guo RF, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT, Lambris JD, Zetoune FS, Ward PA. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol. 2002;169:5962–70. doi: 10.4049/jimmunol.169.10.5962. [DOI] [PubMed] [Google Scholar]
- 19.Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–92. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 20.Bruno NE, Yano Y, Takei Y, Qin L, Suzuki T, Morser J, D'Alessandro-Gabazza CN, Mizoguchi A, Suzuki K, Taguchi O, Gabazza EC, Sumida Y. Immune complexmediated glomerulonephritis is ameliorated by thrombin-activatable fibrinolysis inhibitor deficiency. Thromb Haemost. 2008;100:90–100. doi: 10.1160/TH08-02-0092. [DOI] [PubMed] [Google Scholar]
- 21.Nagashima M, Yin ZF, Zhao L, White K, Zhu Y, Lasky N, Halks-Miller M, Broze GJ, Jr., Fay WP, Morser J. Thrombin-activatable fibrinolysis inhibitor (TAFI) deficiency is compatible with murine life. J Clin Invest. 2002;109:101–10. doi: 10.1172/JCI12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz G, Tedesco MM, Sho E, Nishimura T, Sharif S, Du X, Myles T, Morser J, Dalman RL, Leung LL. Enhanced abdominal aortic aneurysm formation in thrombinactivatable procarboxypeptidase B-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:1363–70. doi: 10.1161/ATVBAHA.109.202259. [DOI] [PubMed] [Google Scholar]
- 23.Rynkowski MA, Kim GH, Garrett MC, Zacharia BE, Otten ML, Sosunov SA, Komotar RJ, Hassid BG, Ducruet AF, Lambris JD, Connolly ES. C3a receptor antagonist attenuates brain injury after intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:98–107. doi: 10.1038/jcbfm.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kodama T, Sekine H, Takahashi M, Iwaki D, Machida T, Kanno K, Ishida Y, Endo Y, Fujita T. Role of complement in a murine model of peanut-induced anaphylaxis. Immunobiology. 2013;218:844–50. doi: 10.1016/j.imbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Bot J, Whitaker D, Vivian J, Lake R, Yao V, McCauley R. Culturing mouse peritoneal mesothelial cells. Pathology, research and practice. 2003;199:341–4. doi: 10.1078/0344-0338-00427. [DOI] [PubMed] [Google Scholar]
- 26.Bajzar L, Nesheim M, Morser J, Tracy PB. Both cellular and soluble forms of thrombomodulin inhibit fibrinolysis by potentiating the activation of thrombinactivable fibrinolysis inhibitor. J Biol Chem. 1998;273:2792–8. doi: 10.1074/jbc.273.5.2792. [DOI] [PubMed] [Google Scholar]
- 27.Heng TS, Painter MW, Immunological Genome Project C. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–4. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 28.Fish RJ, Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108:419–26. doi: 10.1160/TH12-04-0273. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Miwa T, Akatsu H, Matsukawa N, Obata K, Okada N, Campbell W, Okada H. Pro-carboxypeptidase R is an acute phase protein in the mouse, whereas carboxypeptidase N is not. J Immunol. 2000;165:1053–8. doi: 10.4049/jimmunol.165.2.1053. [DOI] [PubMed] [Google Scholar]
- 30.Huber P, Laurent M, Dalmon J. Human beta-fibrinogen gene expression. Upstream sequences involved in its tissue specific expression and its dexamethasone and interleukin. 6 stimulation. J Biol Chem. 1990;265:5695–701. [PubMed] [Google Scholar]
- 31.Bajzar L, Manuel R, Nesheim ME. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1995;270:14477–84. doi: 10.1074/jbc.270.24.14477. [DOI] [PubMed] [Google Scholar]
- 32.Park R, Song J, An SS. Elevated levels of activated and inactivated thrombinactivatable fibrinolysis inhibitor in patients with sepsis. The Korean journal of hematology. 2010;45:264–8. doi: 10.5045/kjh.2010.45.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981;78:2249–52. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longstaff C. Studies on the mechanisms of action of aprotinin and tranexamic acid as plasmin inhibitors and antifibrinolytic agents. Blood Coagul Fibrinolysis. 1994;5:537–42. [PubMed] [Google Scholar]
- 35.Klos A, Wende E, Wareham KJ, Monk PN. International Union of Pharmacology. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacological reviews. 2013;65:500–43. doi: 10.1124/pr.111.005223. [DOI] [PubMed] [Google Scholar]
- 36.Zwirner J, Gotze O, Moser A, Sieber A, Begemann G, Kapp A, Elsner J, Werfel T. Blood- and skin-derived monocytes/macrophages respond to C3a but not to C3a(desArg) with a transient release of calcium via a pertussis toxin-sensitive signal transduction pathway. Eur J Immunol. 1997;27:2317–22. doi: 10.1002/eji.1830270928. [DOI] [PubMed] [Google Scholar]
- 37.Slauch JM. How does the oxidative burst of macrophages kill bacteria? Still an open question. Molecular microbiology. 2011;80:580–3. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muto Y, Suzuki K, Iida H, Sakakibara S, Kato E, Itoh F, Kakui N, Ishii H. EF6265, a novel inhibitor of activated thrombin-activatable fibrinolysis inhibitor, protects against sepsis-induced organ dysfunction in rats. Crit Care Med. 2009;37:1744–9. doi: 10.1097/CCM.0b013e31819ffc14. [DOI] [PubMed] [Google Scholar]
- 39.Huber-Lang MS, Riedeman NC, Sarma JV, Younkin EM, McGuire SR, Laudes IJ, Lu KT, Guo RF, Neff TA, Padgaonkar VA, Lambris JD, Spruce L, Mastellos D, Zetoune FS, Ward PA. Protection of innate immunity by C5aR antagonist in septic mice. FASEB J. 2002;16:1567–74. doi: 10.1096/fj.02-0209com. [DOI] [PubMed] [Google Scholar]
- 40.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–7. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C, Dong N, da Cunha V, Martin-McNulty B, Tran K, Nagashima M, Wu Q, Morser J, Wang YX. Activated thrombin-activatable fibrinolysis inhibitor attenuates spontaneous fibrinolysis of batroxobin-induced fibrin deposition in rat lungs. Thromb Haemost. 2003;90:414–21. doi: 10.1160/TH02-09-0104. [DOI] [PubMed] [Google Scholar]
- 42.Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J Immunol. 2000;165:5406–9. doi: 10.4049/jimmunol.165.10.5406. [DOI] [PubMed] [Google Scholar]
- 43.Hollmann TJ, Mueller-Ortiz SL, Braun MC, Wetsel RA. Disruption of the C5a receptor gene increases resistance to acute Gram-negative bacteremia and endotoxic shock: opposing roles of C3a and C5a. Mol Immunol. 2008;45:1907–15. doi: 10.1016/j.molimm.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emonts M, de Bruijne EL, Guimaraes AH, Declerck PJ, Leebeek FW, de Maat MP, Rijken DC, Hazelzet JA, Gils A. Thrombin-activatable fibrinolysis inhibitor is associated with severity and outcome of severe meningococcal infection in children. J Thromb Haemost. 2008;6:268–76. doi: 10.1111/j.1538-7836.2008.02841.x. [DOI] [PubMed] [Google Scholar]
- 45.Mao SS, Holahan MA, Bailey C, Wu G, Colussi D, Carroll SS, Cook JJ. Demonstration of enhanced endogenous fibrinolysis in thrombin activatable fibrinolysis inhibitor-deficient mice. Blood Coagul Fibrinolysis. 2005;16:407–15. doi: 10.1097/01.mbc.0000181175.62437.2a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.