Abstract
Reprogramming of cellular energy metabolism is widely accepted to be one of the main hallmarks of cancer. Aberrant expression pattern of key regulators in the glycolysis pathway in cancer cells corroborates with the hypothesis that most cancer cells utilize aerobic glycolysis as their main ATP production method instead of mitochondrial oxidative phosphorylation. Overexpression of SLC2A1 and LDHA, both important regulators of the glycolysis pathway, was detected in the premalignant lesions and tumors of lung cancer patients, suggesting the involvements of these proteins in early carcinogenesis and tumor progression in cancer. Preclinical studies demonstrated that inhibiting SLC2A1 or LDHA led to diminished tumor growth in vitro and in vivo. SLC2A1 and LDHA inhibitors, when administered in combination with other chemotherapeutic agents, showed synergistic antitumor effects by re-sensitizing chemo-resistant cancer cells to the chemotherapies. These results indicate that disrupting SLC2A1, LDHA, or other regulators in cancer cell energetics is a very promising approach for new targeted therapies.
Background
Dependence of cancer cells on aerobic glycolysis
Cancer cells alter their primary energy metabolism pathways to support their accelerated rate of growth and proliferation (1). The reprogramming of energy production in cancer cells allows them to generate energy through aerobic glycolysis in the cytoplasm, as opposed to the preferred method of energy production in normal cells via oxidative phosphorylation in the mitochondria (2). The reasons why cancer cells primarily adopt glycolysis as their main method of energy production remains to be fully understood, but one possibility is that aerobic glycolysis provides the tumor cells with glycolytic intermediates that are required for sustained rapid growth (3). This change of cellular energetics also requires an increased uptake of glucose to compensate for the much less efficient adenosine triphosphate (ATP) production rate by glycolysis compared to mitochondrial oxidative phosphorylation (4–6). This alternative energy metabolism strategy used by tumor cells makes it a potential targetable pathway for cancer therapy.
SLC2A1 and LDHA expression is induced in the early stage of stepwise carcinogenesis
A key protein in the cellular energy metabolism pathway is solute carrier family 2 facilitated glucose transporter member 1 (SLC2A1), also known as glucose transporter 1 (GLUT1) (Fig. 1). The main function of SLC2A1 is to supply the cell with glucose by facilitated diffusion of glucose molecules across the plasma membrane when the cellular glucose concentration is low (7). Considering glucose uptake is the first rate-limiting step in aerobic glycolysis, SLC2A1 is often found to be overexpressed in various cancer types, including oral, liver, lung, breast, and endometrial cancer, due to the higher demand of glucose (8–11). In lung cancer, SLC2A1 expression is upregulated at both the RNA and protein levels in premalignant lesions of lung squamous cell carcinoma, when compared to normal lung epithelial basal cells from the same patients (12). In the same study, the expression of SLC2A1 was found to further increase when the premalignant lesions progressed to invasive carcinomas. These observations suggest that SLC2A1 plays a very important role in the initiation and progression of tumor, and could be one of the driver genes in lung cancer and possibly other cancers too.
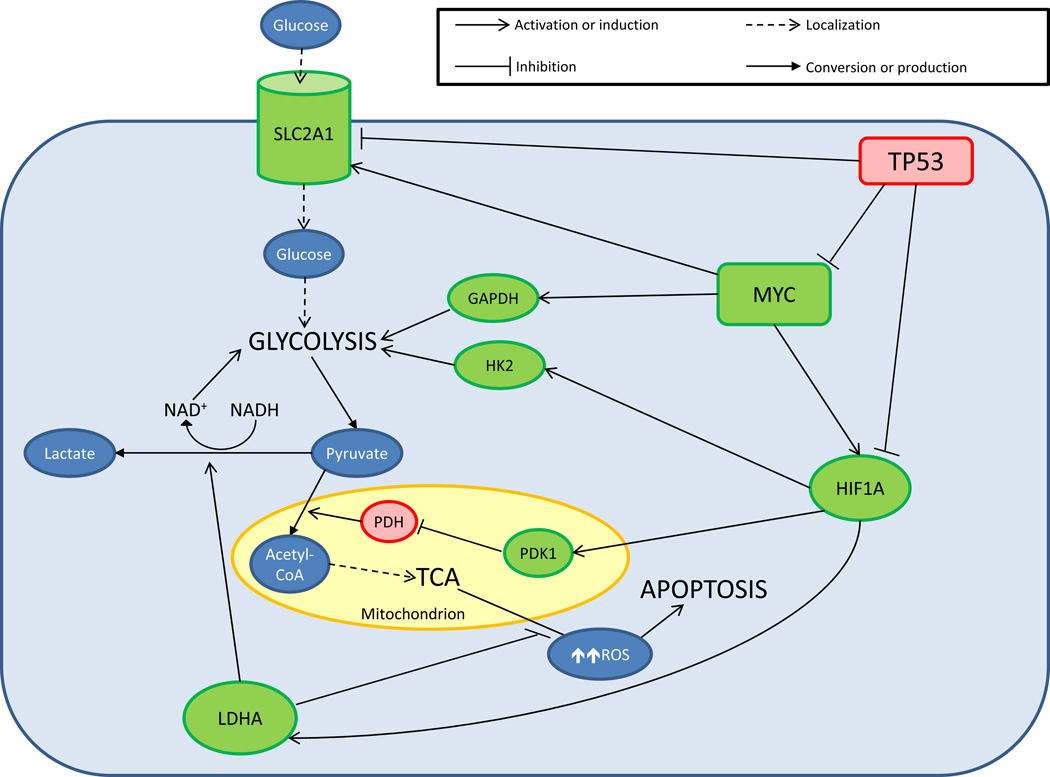
Figure 1.
Cellular energy metabolism pathways in cancer. Green molecules: upregulated or activated proteins in cancer; red molecules: downregulated or inhibited proteins in cancer. Glucose molecules enter the cell via facilitated diffusion by SLC2A1. HK2, GAPDH, and other glycolytic enzymes convert glucose to pyruvate and ATP through glycolysis. Pyruvate could either enter the mitochondria or be converted to lactate by LDHA, a process that also regenerates NAD+ required for glycolysis. Pyruvate that enters the mitochondrion is converted to acetyl-CoA by PDH prior to entering the TCA cycle. PDK1 inhibits PDH and reduces the formation of TCA cycle starting material. Increased mitochondrial energy production also generates ROS that at high levels could cause oxidative stress and subsequently apoptosis. An elevated level of LDHA is associated with reduced mitochondrial ROS production. LDHA is induced by HIF1A, which also increases the expression of PDK1 and HK2. MYC activates the expression of HIF1A, GAPDH, and SLC2A1, driving the cell to utilize aerobic glycolysis instead of mitochondrial oxidative phosphorylation. Finally, inhibition of MYC, HIF1A, and SLC2A1 by functional TP53 in a healthy cell reverses the oncogenic effects caused by overexpression of these proteins.
While increasing glucose transport could be considered as the first rate-limiting step in allowing lung carcinomas to utilize aerobic glycolysis, many other factors are involved in maintaining this reprogramming of cellular energetics. Upon being transported from the plasma membrane into the cell, glucose molecules go through glycolysis to produce ATP and pyruvate. Under normal conditions, pyruvate enters the mitochondria and goes through the tricarboxylic acid (TCA) cycle for highly efficient ATP production (Fig. 1). In cancer cells, pyruvate is rapidly converted to lactate and thus prevented from entering the mitochondria for the TCA cycle, thereby allowing the cells to continually rely on aerobic glycolysis. The conversion of pyruvate to lactate is catalyzed by the enzyme lactate dehydrogenase A (LDHA) (Fig. 1). LDHA has been shown to be overexpressed in many types of cancer, including lung cancer, breast cancer, and pancreatic cancer (13–15). Like SLC2A1, transcription of LDHA is induced in a stepwise manner from normal epithelial basal cells to premalignant lesions, and again from premalignant lesions to carcinomas in lung cancer patients (12, 16). The conversion of pyruvate to lactate by LDHA also generates NAD+ from NADH, thus replenishing the cell with the NAD+ required for glycolysis.
Other key regulators of aerobic glycolysis in cancer
LDHA is transcriptionally activated by hypoxia-inducible factor 1 alpha (HIF1A), a pro-glycolysis protein that also activates other genes of glycolytic enzymes such as hexokinase 2 (HK2) (17, 18). HK2 is an enzyme responsible for the phosphorylation of glucose to form glucose-6-phosphate in the first step of glycolysis and is found to be overexpressed in both premalignant lesions and tumors of lung cancer patients (12). HIF1A expression is induced in lung cancer, colorectal carcinoma, and other cancers, and is considered to be oncogenic, partly due to its role in activating pro-glycolytic genes and thus enabling aerobic glycolysis (12, 16, 17). HIF1A also indirectly prevents glycolytic products from entering the TCA cycle via the activation of pyruvate dehydrogenase kinase 1 (PDK1), which is an inhibitor of pyruvate dehydrogenase (PDH) (19). Pyruvate that enters the mitochondria is first converted to acetyl-CoA by PDH prior to entering the TCA cycle. Inhibition of PDH ensures that the starting molecule for the TCA cycle is never made.
MYC is generally considered to be a “master regulator” of cell growth and metabolism. Many aberrantly expressed genes in cancer are known transcriptional targets of MYC (20). Based on the changes in gene expression profiles during stepwise lung carcinogenesis, it is predicted that MYC activity is increased during the early stages as premalignant lesions are forming (12). Immunofluorescent staining also revealed that MYC remains in the cytoplasm of normal lung epithelial basal cells but localizes to the nuclei of cells in premalignant lesions and invasive carcinomas of lung cancer patients, implying the involvement of MYC in the initiation and early progression of tumor. While the oncogenic effects of MYC are often attributed to its pro-proliferative properties, MYC is involved in promoting the reprogramming of cellular energetics in cancer cells, as it is known to activate the transcription of HIF1A, as well as stabilizing the HIF1A protein (21). MYC also induces the transcription of SLC2A1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, an enzyme that catalyzes the conversion of glyceraldehyde 3-phosphate to D-glycerate 1,3-bisphosphate during the sixth step of glycolysis) (22). GAPDH expression is upregulated in numerous types of cancer including lung cancer, colorectal carcinoma, and breast cancer (12, 23, 24).
Another key regulator of energy metabolism is the tumor protein p53 (TP53), a well-recognized tumor suppressor. While TP53 is involved in a wide spectrum of cellular functions, it also plays an important role in preventing the cell from reprogramming its energetic metabolic pathway. TP53 inhibits the expression of SLC2A1, HIF1A, and MYC, and also promotes the degradation of HIF1A (25, 26). Therefore, functional wild type TP53 would reverse the effects of the pro-glycolytic events required for cancer progression. It has been known that the TP53 gene is often mutated in cancer patients (27). Alterations of TP53 target gene expression patterns in tissue samples of lung cancer patients further predict a loss of function in TP53 during carcinogenesis (12). Based on these observations, it appears that the switch to dependence on aerobic glycolysis by cancer cells as the primary energy-producing pathway is important in the biology of carcinogenesis and therefore makes this pathway an ideal target to develop cancer therapies.
Clinical-Translational Advances
Targeting SLC2A1
Most therapeutic strategies that are being developed to target the cellular energetic metabolism pathways in cancer are in the preclinical phase of drug development. Since SLC2A1 enables the cellular transport of glucose, which is the first rate-limiting step of glucose metabolism, it makes the most sense to inhibit glycolysis by perturbing the function of SLC2A1. WZB117 is a small molecule inhibitor of SLC2A1, and its feasibility and outcome in repressing the activity of SLC2A1 has been extensively tested in vitro and in vivo (28). When treated with WZB117, A549 lung cancer cells showed a rapid and complete inhibition of glucose transport starting at one-minute post treatment. WZB117 also significantly inhibited the proliferation of A549 cells by 50% (p ≤ 0.001) at 48 hours after treatment when compared to the effect observed in the NL20 non-cancerous lung cell line. Blocking glucose transport with WZB117 treatment in A549 cells reduced the rate of glycolysis and the levels of cyclins and phosphorylated retinoblastoma protein (RB1) within 6 hours. Cell cycle arrest and senescence were observed within 24 hours, which eventually led to necrosis within 48 hours (28). When tested in vivo using a human tumor xenograft model with subcutaneous injection of A549 cells into nude mice, treatment with WZB117 for 10 weeks reduced the tumor growth by 70% (p < 0.05) when compared to grafts from mock-treated mice.
Another strategy that is being tested to target SLC2A1 is by RNA interference (RNAi) using short hairpin RNA (shRNA). When tested on mouse mammary tumor cell lines, shRNA targeting SLC2A1 decreased glucose transport and consumption, reduced lactate secretion, and inhibited growth of the tumor cells on plastic as well as in soft agar gel (p < 0.05) (29). When tested in vivo by injecting the SLC2A1 shRNA-treated tumor cells into the mammary glands of athymic nude mice, growth inhibition of the treated tumor cells was observed 8 days after implantation when compared to cells treated with control shRNA (p < 0.05). Eliminating SLC2A1 expression in in transformed mammary epithelial cells by a Cre-Lox system resulted in 35% reduction of tumor size when injected into contralateral mammary glands of athymic nude mice, compared to the tumors formed by control cells after 20 days (p < 0.05)(29).
In addition to testing SLC2A1 inhibitor alone as a targeted therapy, there are also efforts to evaluate SLC2A1 inhibition in combination with other cancer therapeutics. When treated in vitro along with the anticancer drug cisplatin or paclitaxel in lung cancer cell lines A549 and H1229, and breast cancer MCF7 cells, WZB117 showed synergistic antitumor effects with each of these drugs by inhibiting the growth of the cells more than when tested alone (28). In a different study, phloretin, a natural inhibitor of SLC2A1, showed a synergistic antitumor effect when treated in combination with daunorubicin. Phloretin inhibits over 60% of glucose uptake, and improves the anticancer efficiency of daunorubicin more than 2 folds by reducing the hypoxia-enabled drug resistance in SW260 (p < 0.001) and K562 (p = 0.012) cells (30). A third study showed that inhibiting SLC2A1 in vitro with targeted antibody or shRNA sensitized Cal27 head and neck carcinoma cells to cisplatin treatment under both normoxic and hypoxic conditions (31). While these results show promising effects of SLC2A1 inhibition in combination with other anticancer drugs, more elaborate studies are needed to identify the mechanism for the synergistic antitumor activity in the combined therapies.
Targeting LDHA
A complete loss of LDHA protein in humans due to hereditary partial deletion of the LDHA gene only results in non-life threatening and relatively mild cases of exertional myoglobinuria (32). This observation proves that LDHA is a safe therapeutic target for cancer patients with increased level of LDHA expression. In A549 lung cancer cells, in vitro knockdown of LDHA by shRNA resulted in apoptosis, potentially mediated by reactive oxygen species (ROS) produced via enhanced oxidative phosphorylation in the mitochondria (33, 34). In both KRAS- and EGFR-driven lung cancer transgenic mouse models, conditional inactivation of LDHA resulted in the formation of significantly smaller tumors (p < 0.05), as well as over 50% tumor area reduction in established tumors (p < 0.01) (35). These results suggest that LDHA inhibition reduces carcinogenesis and leads to tumor regression.
In vitro treatment of FX11 (3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid), a specific LDHA inhibitor, resulted in elevated oxygen intake, ROS generation, oxidative stress, and apoptosis in P493 human lymphoma B cells (36). Further tests in the same study also suggest that FX11 modifies cellular energy metabolism via inhibition of glycolysis, thereby targeting cells that are dependent on glycolysis. FX11 also inhibits the xenograft growth of P493 lymphoma B cells and P198 human pancreatic cancer cells in mice, showing in vivo efficiency. More recently, galloflavin and N-hydroxyindole-based molecules have been discovered to inhibit LDHA (37, 38). While early results show feasibility as antitumor agents, more studies are needed to investigate their effectiveness.
As in the case with SLC2A1 inhibitors, the efficacy of combining LDHA inhibition with other chemotherapies has been evaluated. An in vitro study with paclitaxel-resistant breast cancer cells demonstrated that LDHA inactivation by small interfering RNA (siRNA) or by another LDHA inhibitor, oxamate, significantly increased the sensitivity of the cells to paclitaxel treatment resulting in 2 to 10-fold growth inhibition (p < 0.01) (39). LDHA inhibition by oxamate also significantly enhanced the therapeutic effects of trastuzumab in treating ERBB2-positive trastuzumab-resistant breast cancer cells in vitro and in vivo (p < 0.01) (40). FX11, when used in combination with FK866, which inhibits the synthesis of NAD+, resulted in significant tumor regression of lymphoma xenografts (36). Finally, inhibition of LDHA by shRNA sensitized A549 lung cancer cells to low doses of paclitaxel, leading to higher rates of apoptosis (33).
Concluding Remarks
Preclinical data suggest that SLC2A1 and LDHA are both viable drug targets for cancer therapy. Inactivating SLC2A1 or LDHA affects the glycolytic pathway in cancer cells, and eventually causes apoptosis in vitro and in vivo. With SLC2A1 inhibition, the antitumor effect is due to glucose starvation that ultimately leads to cell death. The cancer cytotoxicity effect observed in LDHA inhibition is a result of increased oxidative phosphorylation in the mitochondria, which causes oxidative stress from elevated ROS production. While the data are very promising, inhibitors for both SLC2A1 and LDHA are only in the preclinical stages of drug development, and extensive clinical trials are needed to fully evaluate their efficiencies. In addition to SLC2A1 and LDHA, there are a several other potential cancer therapies that target the cellular energetic metabolism pathway in tumor. Among them are prospective inhibitors for GAPDH, HK2, and PDK1 (41–46). Preclinical data from these inhibitors are encouraging; therefore they represent additional options for targeting the enhanced aerobic glycolysis in cancer.
Regrettably, therapies designed to target this pathway have not been fully translated to the clinic. One of the major hurdles to the success of these therapies is the potential systemic toxicity, a very common adverse effect faced by all antitumor drugs. However, advancements in imaging technology have made it possible for localized delivery of drugs to the tumor, which essentially eliminates systemic toxicity while enhancing the potency of the drugs (47). This image-assisted drug delivery technology is still a very novel approach. With further developments of this process, precise delivery of SLC2A1 or LDHA inhibitors to the tumors, either as independent therapies, adjuvant or neoadjuvant therapies, or in combination with other antitumor agents, is a highly feasible approach in treating cancer patients.
Acknowledgments
The authors dedicate this article to Lee Goodglick (1960–2014), a “close friend, collaborator, and colleague who devoted his life to cancer research and education. Lee was a constant source of inspiration for us, and his jokes and zest for life are sorely missed.”
Grant Support
B.N. Gomperts was supported by the National Heart, Lung, and Blood Institute of the NIH under award number R01HL094561, the California Institute of Regenerative Medicine (RN2-009-04), and the U.S. Department of Defense (DOD CTRA LC090615).
Footnotes
Disclosure of Potential Conflicts of Interest
A.T. Ooi is an employee of Fluidigm. No potential conflicts of interest were disclosed by the other author.
Publisher's Disclaimer: Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 4.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 5.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 6.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996;16:235–256. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, et al. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–1024. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 9.Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, et al. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544–1552. doi: 10.2353/ajpath.2009.080596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wachi S, Yoneda K, Wu R. Interactome-transcriptome analysis reveals the high centrality of genes differentially expressed in lung cancer tissues. Bioinformatics. 2005;21:4205–4208. doi: 10.1093/bioinformatics/bti688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, et al. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol Oncol Res. 2012;18:721–728. doi: 10.1007/s12253-012-9500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ooi AT, Gower AC, Zhang KX, Vick JL, Hong L, Nagao B, et al. Molecular profiling of premalignant lesions in lung squamous cell carcinomas identifies mechanisms involved in stepwise carcinogenesis. Cancer Prev Res. 2014;7:487–495. doi: 10.1158/1940-6207.CAPR-13-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koukourakis MI, Giatromanolaki A, Sivridis E. Lactate dehydrogenase isoenzymes 1 and 5: differential expression by neoplastic and stromal cells in non-small cell lung cancer and other epithelial malignant tumors. Tumour Biol. 2003;24:199–202. doi: 10.1159/000074430. [DOI] [PubMed] [Google Scholar]
- 14.Balinsky D, Platz CE, Lewis JW. Isozyme patterns of normal, benign, and malignant human breast tissues. Cancer Res. 1983;43:5895–5901. [PubMed] [Google Scholar]
- 15.Shi M, Cui J, Du J, Wei D, Jia Z, Zhang J, et al. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin Cancer Res. 2014;20:4370–4380. doi: 10.1158/1078-0432.CCR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- 18.Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem. 2001;276:43407–43412. doi: 10.1074/jbc.M108181200. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- 21.Doe MR, Ascano JM, Kaur M, Cole MD. Myc posttranscriptionally induces HIF1 protein and target gene expression in normal and cancer cells. Cancer Res. 2012;72:949–957. doi: 10.1158/0008-5472.CAN-11-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 23.Tang Z, Yuan S, Hu Y, Zhang H, Wu W, Zeng Z, et al. Over-expression of GAPDH in human colorectal carcinoma as a preferred target of 3-bromopyruvate propyl ester. J Bioenerg Biomembr. 2012;44:117–125. doi: 10.1007/s10863-012-9420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higashimura Y, Nakajima Y, Yamaji R, Harada N, Shibasaki F, Nakano Y, et al. Up-regulation of glyceraldehyde-3-phosphate dehydrogenase gene expression by HIF-1 activity depending on Sp1 in hypoxic breast cancer cells. Arch Biochem Biophys. 2011;509:1–8. doi: 10.1016/j.abb.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Zawacka-Pankau J, Grinkevich VV, Hunten S, Nikulenkov F, Gluch A, Li H, et al. Inhibition of glycolytic enzymes mediated by pharmacologically activated p53: targeting Warburg effect to fight cancer. J Biol Chem. 2011;286:41600–41615. doi: 10.1074/jbc.M111.240812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 27.Kishimoto Y, Murakami Y, Shiraishi M, Hayashi K, Sekiya T. Aberrations of the p53 tumor suppressor gene in human non-small cell carcinomas of the lung. Cancer Res. 1992;52:4799–4804. [PubMed] [Google Scholar]
- 28.Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 29.Young CD, Lewis AS, Rudolph MC, Ruehle MD, Jackman MR, Yun UJ, et al. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS One. 2011;6:e23205. doi: 10.1371/journal.pone.0023205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X, Fang L, Gibbs S, Huang Y, Dai Z, Wen P, et al. Glucose uptake inhibitor sensitizes cancer cells to daunorubicin and overcomes drug resistance in hypoxia. Cancer Chemother Pharmacol. 2007;59:495–505. doi: 10.1007/s00280-006-0291-9. [DOI] [PubMed] [Google Scholar]
- 31.Wang YD, Li SJ, Liao JX. Inhibition of glucose transporter 1 (GLUT1) chemosensitized head and neck cancer cells to cisplatin. Technol Cancer Res Treat. 2013;12:525–535. doi: 10.7785/tcrt.2012.500343. [DOI] [PubMed] [Google Scholar]
- 32.Kanno T, Sudo K, Maekawa M, Nishimura Y, Ukita M, Fukutake K. Lactate dehydrogenase M-subunit deficiency: a new type of hereditary exertional myopathy. Clin Chim Acta. 1988;173:89–98. doi: 10.1016/0009-8981(88)90359-2. [DOI] [PubMed] [Google Scholar]
- 33.Seth P, Grant A, Tang J, Vinogradov E, Wang X, Lenkinski R, et al. On-target inhibition of tumor fermentative glycolysis as visualized by hyperpolarized pyruvate. Neoplasia. 2011;13:60–71. doi: 10.1593/neo.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie H, Valera VA, Merino MJ, Amato AM, Signoretti S, Linehan WM, et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8:626–635. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P, et al. Targeting lactate dehydrogenase--a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manerba M, Vettraino M, Fiume L, Di Stefano G, Sartini A, Giacomini E, et al. Galloflavin (CAS 568-80-9): a novel inhibitor of lactate dehydrogenase. Chem Med Chem. 2012;7:311–317. doi: 10.1002/cmdc.201100471. [DOI] [PubMed] [Google Scholar]
- 38.Granchi C, Roy S, Giacomelli C, Macchia M, Tuccinardi T, Martinelli A, et al. Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. J Med Chem. 2011;54:1599–1612. doi: 10.1021/jm101007q. [DOI] [PubMed] [Google Scholar]
- 39.Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, et al. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010;9:33. doi: 10.1186/1476-4598-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Liu H, Liu Z, Ding Y, Ledoux SP, Wilson GL, et al. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011;71:4585–4597. doi: 10.1158/0008-5472.CAN-11-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birsoy K, Wang T, Possemato R, Yilmaz OH, Koch CE, Chen WW, et al. MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nat Genet. 2013;45:104–108. doi: 10.1038/ng.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumagai S, Narasaki R, Hasumi K. Glucose-dependent active ATP depletion by koningic acid kills high-glycolytic cells. Biochem Biophys Res Commun. 2008;365:362–368. doi: 10.1016/j.bbrc.2007.10.199. [DOI] [PubMed] [Google Scholar]
- 43.Jae HJ, Chung JW, Park HS, Lee MJ, Lee KC, Kim HC, et al. The antitumor effect and hepatotoxicity of a hexokinase II inhibitor 3-bromopyruvate: in vivo investigation of intraarterial administration in a rabbit VX2 hepatoma model. Korean J Radiol. 2009;10:596–603. doi: 10.3348/kjr.2009.10.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dwarakanath BS, Singh D, Banerji AK, Sarin R, Venkataramana NK, Jalali R, et al. Clinical studies for improving radiotherapy with 2-deoxy-D-glucose: present status and future prospects. J Cancer Res Ther. 2009;5(Suppl 1):S21–S26. doi: 10.4103/0973-1482.55136. [DOI] [PubMed] [Google Scholar]
- 45.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]