Abstract
Progress has been made over the last decade in our understanding of the brain areas and circuits involved in nicotine reward and withdrawal, leading to models of addiction that assign different addictive behaviors to distinct, yet overlapping, neural circuits (Koob and Volkow, 2010; Lobo and Nestler, 2011; Tuesta et al., 2011; Volkow et al., 2011). Recently the habenulo-interpeduncular (Hb-IPN) midbrain pathway has re-emerged as a new critical crossroad that influences the brain response to nicotine. This brain area is particularly enriched in nicotinic acetylcholine receptor (nAChR) subunits α5, α3 and β4 encoded by the CHRNA5-A3-B4 gene cluster, which has been associated with vulnerability to tobacco dependence in human genetics studies. This finding, together with studies in mice involving deletion and replacement of nAChR subunits, and investigations of the circuitry, cell types and electrophysiological properties, have begun to identify the molecular mechanisms that take place in the MHb-IPN and underlie critical aspects of nicotine dependence. In the current review we describe the anatomical and functional connections of the MHb-IPN system, as well as the contribution of specific nAChRs subtypes in nicotine-mediated behaviors. Finally, we discuss the specific electrophysiological properties of MHb-IPN neuronal populations and how nicotine exposure alters their cellular physiology, highlighting the unique role of the MHb-IPN in the context of nicotine aversion and withdrawal.
Keywords: Nicotine, Habenula, Interpeduncular Nucleus, Pacemaking, nAChR
1. Introduction
The habenula is a small bilateral structure located in the epithalamic region of the diencephalon. Together with its associated afferent and efferent tracts, it forms part of the dorsal diencephalic conduction system, which connects the limbic forebrain with nuclei in the midbrain and hindbrain (Sutherland, 1982). The habenula is phylogenetically highly conserved across vertebrates, and serves to connect more recently evolved structures involved in executive function with ancient brain areas that process pain and reward. In fish, amphibian, and reptiles, the right and left sides of the habenula exhibit a remarkable asymmetry in size, molecular properties, connectivity and associated behaviors (Aizawa et al., 2005; Concha and Wilson, 2001; Dadda et al., 2010). In mammals, the habenula is symmetric and is located on the posterior medial end of the dorsal thalamus, adjacent to the third ventricle. Highlighting its ancient origin, a recent study of human fetuses demonstrated that the habenulo-interpeduncular (Hb-IPN) tract is one of the first major fiber tracts to form in the developing brain, present as early as eight weeks gestation (Cho et al., 2014).
The habenula is subdivided into the medial habenula (MHb) and the lateral habenula (LHb) (Andres et al., 1999), each having different anatomical connections and serving different functions (Herkenham and Nauta, 1977, 1979; Klemm, 2004; Lecourtier and Kelly, 2007). In this review, we will discuss the current understanding of the MHb and its role in nicotine dependence. The MHb receives input mainly from the septum through the stria medularis and projects to the interpeduncular nucleus (IPN) through the fasciculus retroflexus (FR) (Herkenham and Nauta, 1979; Qin and Luo, 2009; Swanson and Cowan, 1979) (Fig. 1). Besides the peculiar anatomical traits of the MHb-IPN pathway, such as the remarkable high density of cell bodies in the MHb, the long axons that bundle together to form the FR and terminate in an ipsilateral manner in the IPN (Fig. 2A), the MHb-IPN tract also highly expresses a unique subset of nicotinic acetylcholine receptor (nAChR) subunits, the α5, α3 and β4 subunits encoded by the CHRNA5-A3-B4 gene cluster (Fig, 2A,D). This gene cluster has been associated with higher levels of nicotine consumption and dependence in human genetics studies (Berrettini et al., 2008; Bierut et al., 2008; Lips et al., 2010; Liu et al., 2010; Ware et al., 2011). In agreement with these association studies in smokers, cumulative evidence from animal models points to the MHb-IPN pathway as a key modulator of nicotine aversion and nicotine withdrawal (Fowler et al., 2011; Frahm et al., 2011; Salas et al., 2009).
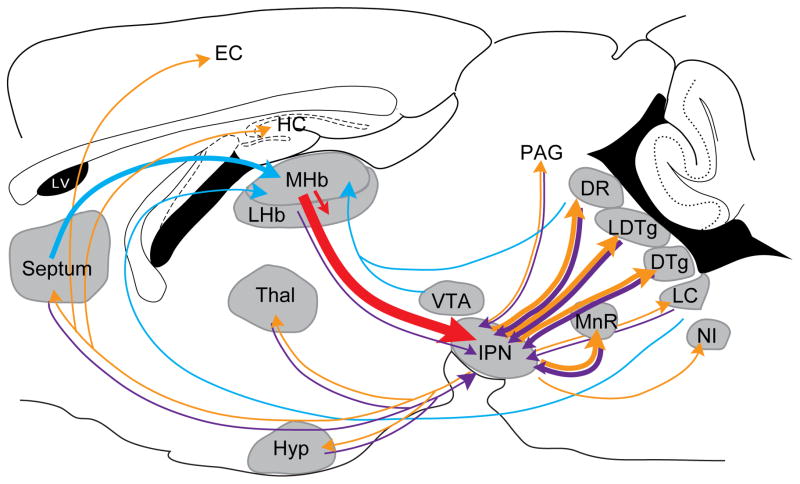
Figure 1. MHb-IPN connectivity.
Schematic sagittal view of a mouse brain showing known MHb afferents (blue), MHb efferents (red), IPN afferents (purple) and IPN efferents (orange). The thickness of the arrows reflects the strength of the connection. 3V, third ventricle; 4V, fourth ventricle; DR, dorsal raphe; DTg, dorsal tegmental nucleus; EC, entorhinal cortex; HC, hippocampus; Hyp, hypothalamus; IPN, interpeduncular nucleus; LC, locus coeruleus; LDTg, laterodorsal tegmental nucleus; LHb, lateral habenula; LV, lateral ventricle; MHb, medial habenula; MnR, median raphe; NI, nucleus incertus; PAG, periaqueductal gray; Thal, thalamus; VTA, ventral tegmental area.
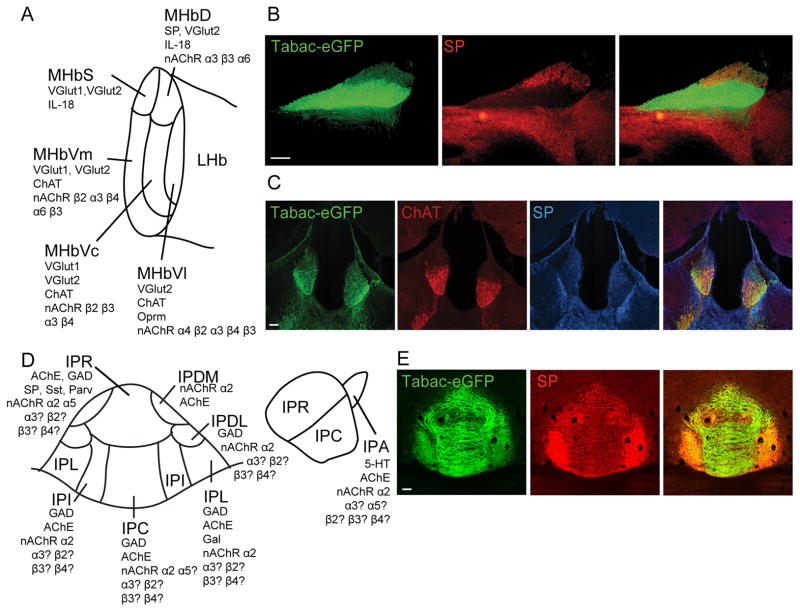
Figure 2. Subnuclei of the MHb and IPN.
(A) Schematic representation of the MHb subnuclei indicating expression of different markers based on (Aizawa et al., 2012) and (Shih et al., 2014). (B–C) Expression of α3β4-eGFP and ChAT in the ventral two-thirds of the MHb and of Substance P (SP) in the dorsal MHb in sagittal (B) and coronal (C) brain sections of Tabac mice expressing the CHRNB4-A3-eGFP-A5 gene cluster. (D) Schematic representation of the IPN subnuclei indicating expression of different makers localized to cell bodies within the IPN. The panel on the left is a coronal schematic. The right panel is a sagittal schematic. (E) Coronal sections at the level of the IPN showing α3β4-eGFP immunoreactivity (green) and SP immunoreactivity (red) in the habenular terminals of Tabac mice. (Scale bar: 100 μm.) ChAT, choline acetyltransferase; IPA, apical subnucleus of the IPN; IPC, central subnucleus of the IPN; IPDL, dorsolateral subnucleus of the IPN; IPDM, dorsomedial subnucleus of the IPN; IPI, intermediate subnucleus of the IPN; IPL, lateral subnucleus of the IPN; IPN, interpeduncular nucleus; IPR, rostral subnucleus of the IPN; LHb, lateral habenula; MHb, medial habenula; MHbD, dorsal part of the MHb, MHbS, superior part of the MHb; MHbVc, ventro-central part of the MHb; MHbVl, ventro-lateral part of the MHb; MHbVm, ventro-medial part of the MHb; SP, substance P.
2. Anatomy and connectivity: The medial habenula and its output to the interpeduncular nucleus
MHb afferents derive mostly from the posterior septum, specifically from the septofimbrial nucleus (SFi), the triangular septum (TS) and the bed nucleus of the anterior commissure (BAC) (Herkenham and Nauta, 1977). Topographic connections have been revealed from the TS and the BAC to the ventral and dorsal subnuclei of the medial habenula (MHbV and MHbD) respectively (Yamaguchi et al., 2013). The MHb also receives input from the medial septum (MS) and nucleus of diagonal band (NDB) in the basal forebrain; from the interfascicular nucleus of the ventral tegmental area (VTA)(Phillipson and Pycock, 1982), from the mesencephalic raphe in the midbrain (Herkenham and Nauta, 1977; Staines et al., 1988) and from the locus coeruleus (LC) and superior cervical ganglion (Gottesfeld, 1983) (Fig. 1).
The MHb has been subdivided into five subnuclei (Aizawa et al., 2012; Wagner et al., 2014) according to the expression of output neurotransmitters (Aizawa et al., 2012) (Fig. 2A). However, as many as 15 subnuclei have been described based on different ultrastructural, morphological and cytochemical properties (Andres et al., 1999; Geisler et al., 2003; Aizawa et al., 2012; Wagner et al., 2014). Neurons in the MHbD express the neuropeptide substance P (SP), also known as Tachykinin 1 (Fig. 2A–C). Neurons in the superior part of the MHb (MHbS) show strong glutamatergic character and lack of SP expression (Fig. 2A). The lower two-thirds of the MHb comprise the ventro-medial (MHbVm), the ventro-central (MHbVc) and the ventro-lateral (MHbVl) subnuclei. These three subnuclei display strong expression of the acetylcholine synthesizing enzyme choline acetyltransferase (ChAT) and the vesicular glutamate transporters 1 and 2 (VGlut1 and VGlut2) (Aizawa et al., 2012) (Fig. 2A,C). Intermingled with ChAT positive, there are also ChAT negative neurons expressing nAChRs (Shih et al., 2014). In addition to the differential expression of neurotransmitters, expression of other markers has been shown to be subnuclei specific. For instance, the μ-opioid receptor (Oprm) is only expressed in the MHbVl part, and interleukin 18 (IL-18) is only expressed in the MHbS and MHbD parts (Aizawa et al., 2012) (Fig. 2A).
The MHb efferents target the single midline IPN via the FR. The IPN can be subdivided into 3 unpaired and 4 paired subnuclei based primarily on cytoarchitecture and to a lesser extent on marker localization: the median, unpaired subnuclei are the apical (IPA), rostral (IPR) and central (IPC) nuclei, while the paired subnuclei comprise the dorsolateral (IPDL), dorsomedial (IPDM), lateral (IPL) and intermediate (IPI) subnuclei (Hemmendinger and Moore, 1984; Lenn and Hamill, 1984) (Fig. 2D). Projections from MHb to the IPN are topographically organized, such that a 90-degree lateral turn of the MHb corresponds to the target areas within the IPN (Herkenham and Nauta, 1979). SP neurons in the MHbD project to the IPR and IPL subnuclei of the IPN, while ChAT neurons in the MHbV part project to the IPC and IPI subnuclei of the IPN (Contestabile et al., 1987) and α4, ChAT negative neurons of the MHbVl project to the IPR (Shih et al., 2014) (Fig. 2E). The axons from MHb criss-cross through the entire extent of the IPN, forming en passant synapses, before terminating ipsilaterally in the IPL (Herkenham and Nauta, 1977). Within the IPI, cholinergic MHb axons terminate to form a special type of synapse, termed the crest synapse, where a single disc-shaped IPN dendrite, or “crest”, receives paired innervation from both the left and right MHb (Lenn et al., 1983). These synapses form and undergo extensive remodeling during the post-natal period in rodents (Lenn, 1978b), and it is possible that in birds and mammals, this is the predominant form of asymmetry within the habenulo-interpeduncular pathway. Ultrastructurally, however, the vast majority (~90%) of crest synapses receives input from both the left and right MHb (Hamill and Lenn, 1983; Lenn, 1976, 1978a; Lenn et al., 1983; Murray et al., 1979).
While the IPN receives considerable input from the MHb, this is not the only source. The IPN also receives projections from forebrain nuclei such as the septum (Contestabile and Flumerfelt, 1981; Gottesfeld and Jacobowitz, 1978; Hamill and Fass, 1984; Swanson and Cowan, 1979), the medial preoptic area(Vertes and Fass, 1988) the ventral thalamus (Moore et al., 2000), the central and ventral hypothalamus (Jennes, 1987; Villalobos and Ferssiwi, 1987), and midbrain nuclei such as the dorsal and dorsolateral tegmental nuclei (DTg and LDTg), both dorsal and median raphe nuclei, the central gray and the locus coeruleus (Cornwall et al., 1990; Groenewegen et al., 1986; Hamill and Jacobowitz, 1984; Lenn and Wong, 1980; Marchand et al., 1980; Satoh and Fibiger, 1986) (Fig. 1).
As mentioned above, the IPN can be roughly divided based on expression of markers, although it is not as discrete as the subdivision within the MHb (Fig. 2) (Groenewegen et al., 1986; Hamill et al., 1984; Kawaja et al., 1990; Kawaja et al., 1991; Zhao-Shea et al., 2013). IPN neurons are predominantly, but not exclusively GABAergic. Interestingly, many of the GABAergic cells within the IPN are projection neurons that target caudal structures such as the LDTg and DTg. Like the MHb, the IPN also expresses a unique subset of nAChR subunits (Beiranvand et al., 2014; Grady et al., 2009), most notably the α5 subunit linked to nicotine dependence. Strikingly, the only nAChRs in the IPN for which subnuclear distribution is known are α5 and α2; α2-positive cells are found in all subnuclei (Grady et al., 2009), while α5-positive cells are present in the IPR, and to a lesser extent, in the IPC (Hsu et al., 2013). Interestingly, the IPA is the only subnucleus with serotonergic cell bodies and is continuous with the raphe (Groenewegen et al., 1986).
IPN efferents are predominantly to the median and dorsal raphe nuclei, and to the dorsal and dorsolateral tegmental regions (Groenewegen et al., 1986), although they also target the nucleus incertus (Goto et al., 2001) (Fig. 1). While all subnuclei project to the DTg, the main input is from IPR and IPL, with sparse projections from IPC and IPI, and minimal from IPA. IPN fibers targeting the raphe originate predominantly in IPR and IPDM, and to a lesser extent in IPC, IPI, and IPL. The IPN also sends projections to the forebrain, namely to the septum (Montone et al., 1988; Vertes and Fass, 1988), hypothalamus (Smith et al., 1980), entorhinal cortex and thalamus (Shibata and Suzuki, 1984) and the hippocampus (Baisden et al., 1979). Additionally, there are some reports of a minor projection of ascending fibers from the IPN along either the habenulo-interpeduncular or the mammillothalamic tract (Groenewegen et al., 1986) (Fig. 1).
Although the predominant projection is from the MHb to the IPN and from the LHb to the VTA and raphe, a small subpopulation of MHb neurons has been shown to innervate the LHb (Kim and Chang, 2005), suggesting that MHb may regulate LHb activity. Likewise, a small number of LHb have been shown to project to the IPN (Contestabile and Flumerfelt, 1981; Goncalves et al., 2012; Kim, 2009), suggesting that the LHb may regulate IPN activity, providing a balance between activity in the two parallel circuits. As recent advances in viral mediated strategies facilitate tracing studies, other non-conspicuous but functionally important anatomical connections will be determined.
3. Behavioral responses to nicotine mediated by nAChRs in the MHb-IPN
Except α7, α9 and α10, the MHb-IPN densely expresses all known neuronal nAChRs subunits, sometimes at their highest expression level in the brain (Perry et al., 2002). Detailed analysis of the contribution of individual nAChRs subunits in the MHb-IPN has been possible through the use of nAChR-mouse models, including null mice (Fowler et al., 2011; Kedmi et al., 2004; Maskos et al., 2005; Picciotto et al., 1998; Salas et al., 2004a; Salas et al., 2003a; Salas et al., 2004b; Salas et al., 2003b; Salas et al., 2009), gain-of-function mice (Broide et al., 2002; Fonck et al., 2005; Tapper et al., 2004), overexpressing mice (Frahm et al., 2011; Gallego et al., 2012) and knock-in mice (Shih et al., 2014), as well as more recently with the use of viral-mediated expression of nAChR subunits or shRNAs for nAChR knockdown (Fowler et al., 2011; Frahm et al., 2011). Through the behavioral analysis of the nicotine responses of these mouse models, nAChRs highly enriched in the MHb and IPN were first identified as critical modulators of nicotine withdrawal and more recently, as key mediators of nicotine aversion, and subsequently intake. Here we will first review the studies of MHb-IPN nAChRs in nicotine withdrawal and then discuss recent findings that implicate α5 and β4 subunits encoded by the CHRNA5-CHRNA3-CHRNB4 gene cluster in nicotine aversion.
In both humans and in animal models, chronic nicotine consumption leads to withdrawal if intake is abruptly stopped or a nicotinic antagonist is administered. The nicotine withdrawal syndrome manifests as a collection of affective and physical symptoms that largely prevent success in quitting (Changeux, 2010; West et al., 1989). In humans, this includes affective symptoms such as irritability, anxiety, depressed mood, difficulty concentrating, disrupted cognition and nicotine craving; and physical symptoms such as bradycardia, gastrointestinal discomfort and increased appetite accompanied by weight gain (Dani and De Biasi, 2013). In rodent models, physical signs (often called “somatic signs”) include scratching, rearing, jumping, head nods, and body shakes (Damaj et al., 2003; Grabus et al., 2005), hyperalgesia (Grabus et al., 2005; Salas et al., 2004b), and changes in locomotor activity (Hildebrand et al., 1999; Nomikos et al., 1999); whereas affective signs include anxiety-like behaviors (Damaj et al., 2003), elevated reward thresholds (Kenny and Markou, 2001), withdrawal-induced contextual fear conditioning (Davis et al., 2005), and withdrawal-induced conditioned place aversion (CPA) (Jackson et al., 2008; Suzuki et al., 1999).
The nAChR subunits that are enriched in the Hb-IPN pathway, namely the α5 and β4 subunits, mediate many of the aversive effects of nicotine withdrawal. Mice lacking the β4 nAChR subunit (β4−/− mice) as well as null mice for α5 (α5−/− mice) have fewer somatic signs of withdrawal after chronic nicotine administration and attenuated withdrawal-induced hyperalgesia (Jackson et al., 2008; Salas et al., 2004b; Salas et al., 2009). Though not as specific for the Hb-IPN path as the CHRNA5-CHRNA3-CHRNB4 gene cluster, α2 is expressed in the IPN as well as limbic areas such as striatum (Ishii et al., 2005). Likewise the somatic manifestations of nicotine withdrawal are altered in mice null for the α2 nAChR subunit. When α2−/− mice are tested in a familiar environment, they show decreased somatic signs of withdrawal (Salas et al., 2009); whereas α2−/− mice tested in a novel environment display increased number of somatic signs and increased affective signs of nicotine withdrawal, as measured by the cued fear conditioning test (Lotfipour et al., 2013). β2* and α6* nAChRs, present in the Hb-IPN pathway, though more highly expressed in the VTA, are involved in the affective component of nicotine withdrawal. Deletion of the β2 nAChR subunit induces a loss of anxiety-related behavior during withdrawal and a loss of aversion in the CPA model but no alterations in somatic signs of nicotine withdrawal (Jackson et al., 2008). Blockade of the α6 nAChR subunit with a selective antagonist diminishes the expression of withdrawal-induced CPA and anxiety-related behavior. However somatic signs of withdrawal and withdrawal-induced hyperalgesia are not affected by administration of the α6 antagonist (Jackson et al., 2009). Altogether these animal studies indicate that α5, α2 and β4 containing nAChRs contribute to the physical symptoms of nicotine withdrawal whereas α2, β2 and α6 contribute to the affective component of withdrawal.
α5 and α2 nAChR subunits are highly expressed in a subset of neurons in the IPN (Hsu et al., 2013; Ishii et al., 2005). The β4 subunit shows high levels of expression in both the MHb and IPN (Görlich et al., 2013; Quick et al., 1999; Salas et al., 2003b; Sheffield et al., 2000; Shih et al., 2014). β2 and α6 subunits are also expressed in a subset of MHb neurons among other brain areas (Shih et al., 2014). Thus, cumulative evidence points to nAChRs in the MHb-IPN pathway as critical regulators of nicotine withdrawal. Consistently, microinjection of the nAChR antagonist mecamylamine into the habenula and IPN, but not in other brain areas such as the VTA, cortex or hippocampus is sufficient to precipitate physical nicotine withdrawal symptoms in mice chronically treated with nicotine (Salas et al., 2009). In agreement with these studies, Zhao-Shea and colleagues showed that chronic nicotine treatment upregulated gene expression of β3 and β4 nAChR subunits in somatostatin positive neurons in the IPN and, further, that infusion of the β4 nAChR antagonist SR16584 in the IPN elicited somatic signs of nicotine withdrawal (Zhao-Shea et al., 2013). In summary it is clear that the somatic manifestations of nicotine withdrawal are mediated by the MHb-IPN pathway.
Even though somatic signs of withdrawal contribute less to relapse than affective signs of nicotine withdrawal (Markou et al., 1998), few studies have analyzed which brain areas and nAChRs subunits are involved in the affective aspect of withdrawal. As mentioned above, α5, α2 and β4 containing nAChRs have been shown to contribute mainly to the physical symptoms of nicotine withdrawal whereas the β2 and α6 subunits contribute to the affective component of withdrawal. Given that the β2 nAChR subunit is almost ubiquitously expressed and the α6 subunit is strongly expressed in the VTA, substantia nigra, and locus coeruleus (Le Novere et al., 1996), it is likely that other brain regions besides the MHb and IPN are involved in the affective component of nicotine withdrawal. Another possibility is that distinct neuronal populations within the MHb and IPN, expressing different nAChRs subunit compositions are differentially involved in somatic and affective signs of withdrawal. Evidence supporting this possibility has been observed following optogenetic stimulation of a subset of neurons in the IPN. Light stimulation of GAD2-positive GABAergic neurons in the IPN induced physical withdrawal signs not only in chronic-nicotine-treated mice, but also in nicotine-naive mice; however, activation of these neurons did not induce anxiety-related behaviors (Zhao-Shea et al., 2013). Studies using an antagonist of HCN pacemaker channels in habenular neurons induced both somatic and affective signs of withdrawal in nicotine-naïve mice (Görlich et al., 2013), indicating that the MHb-IPN tract may mediate both aspects of withdrawal. Much work still needs to be done to reveal the distinct neuronal populations that may be differentially involved in somatic and affective signs of withdrawal. Conditional mouse models and viral-mediated modulation of nAChRs expression in specific brain areas may aid elucidating this question.
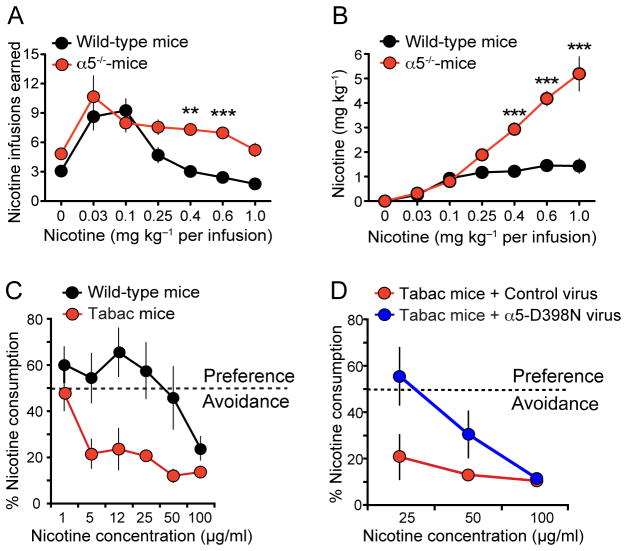
Besides their role in nicotine withdrawal, recent findings indicate that both α5 and β4 nAChRs mediate the aversive response to nicotine intake. Genetic studies have linked single nucleotide polymorphisms (SNP) within the CHRNA5-CHRNA3-CHRNB4 gene cluster to heavy smoking (Berrettini et al., 2008; Bierut et al., 2008; Lips et al., 2010; Liu et al., 2010; Ware et al., 2011). Strikingly, the most common polymorphism in CHRNA5 (rs16969968), which results in the amino acid substitution of aspartic acid to asparagine (D398N) in the α5 subunit, more than doubles the risk of tobacco dependence in those carrying two copies of the risk allele (Berrettini et al., 2008; Bierut et al., 2008; Grucza et al., 2008; Stevens et al., 2008). Consistent with these human genetic studies, α5 null mice continue to self-administer nicotine at doses that normally elicit aversion in wild type animals (Fowler et al., 2011) (Fig. 3A,B). Nicotine self-administration reverts to wild type levels when the α5 nAChR subunit is re-expressed in the MHb or the IPN (Fowler et al., 2011). Moreover, viral-mediated knockdown of the α5 nAChR subunit in the MHb–IPN tract did not alter the reward-enhancing properties of lower doses of nicotine, but significantly diminished the aversive effects of higher doses in rats (Fowler et al., 2011). Aversive responses to nicotine and reduced nicotine consumption are also observed in transgenic mice for the CHRNA5-CHRNA3-CHRNB4 gene cluster, overexpressing only the β4 nAChR subunit (called Tabac mice) (Frahm et al., 2011) (Fig. 3C). β4 was shown to be rate-limiting for the assembly of α3β4* receptors and to compete with α5 to form pentameric α3β4α5 nAChRs (Frahm et al., 2011; Slimak et al., 2014). Thus β4 overexpression led to an increased density of α3β4* receptors at the plasma membrane, as well as to a potentiation of α3β4* currents in vitro and in habenular neurons of Tabac mice (Frahm et al., 2011). Remarkably, the nicotine aversion observed in Tabac mice overexpressing β4 was reversed upon viral-mediated expression in the MHb of the α5 D398N variant associated with heavy smoking in humans (Frahm et al., 2011) (Fig. 3D). Moreover, viral-mediated overexpression of β4 in the MHb was sufficient to produce nicotine aversion (Slimak et al., 2014) (Fig. 3E,F). Functional analysis of missense β4 variants found in human genetic studies demonstrated that variants which increased nicotine-induced currents also increased aversion to nicotine in a two-bottle nicotine drinking test; whereas variants that decreased nicotine-induced currents decreased aversion (Slimak et al., 2014). In summary, these studies point to nAChRs containing α5 and β4 subunits in the MHb-IPN axis as critical regulators to signal nicotine aversion and limit nicotine intake.
Figure 3. The MHb-IPN α5 and β4 nAChRs subunits mediate nicotine aversion.
(A) α5−/− mice self-administer more nicotine than wildtype mice. Data are presented as mean (± SEM) number of nicotine infusions earned across different nicotine doses. Modified with permission from (Fowler et al., 2011) (B) Total nicotine intake at each dose analyzed in A. Modified with permission from (Fowler et al., 2011). (C) Tabac mice, which overexpress β4 in the MHb, display strong aversion to nicotine in the two-bottle choice paradigm at increasing concentrations of nicotine. Data is expressed as percent of the volume of nicotine solution consumed divided by the total fluid intake per day. Modified with permission from (Frahm et al., 2011). (D) Tabac mice stereotactically injected in the MHb with a lentivirus encoding the most frequent variant associated to heavy smoking, α5 D398N, do not display nicotine aversion at 25 μg/ml in the two-bottle choice paradigm compared to Tabac mice injected with control lentivirus. Data is expressed as percent of the volume of nicotine solution consumed divided by the total fluid intake per day. Two-way ANOVA, p<0.05 Modified with permission from (Frahm et al., 2011).
The role of the MHb-IPN pathway in nicotine aversion might be one of self-preservation, as evidenced by axonal degeneration in the central core of the fasciculus retroflexus, a significant reduction of the volume of the MHb and a decrease of ChAT in MHb neurons innervating the IPN after chronic administration of high doses of nicotine (Carlson et al., 2001; Ciani et al., 2005; Ellison, 2002). This is supported by the observation of apoptotic neurons in the MHb, after sustained high dose nicotine exposure (Ciani et al., 2005). Within the MHb, the damage was more prominent in the ventral-medial part (Carlson et al., 2001), which expresses high levels of the α3, β4, α6 and β3 receptor subtypes (Shih et al., 2014). However, it is interesting to note that nicotine acts as a neuroprotective agent in some neurological disorders, such as Alzheimer’s and Parkinson’s disease (Buckingham et al., 2009; Quik et al., 2012). Future studies are needed to understand the mechanisms by which nicotine differentially mediates neuroprotection and/or neurotoxicity and the nAChRs subtypes involved in these differential nicotine-mediated adaptative changes. These studies would help elucidate why the MHb-IPN tract is more susceptible to nicotine toxicity than other brain areas containing the same nAChRs.
4. Electrophysiological properties of the MHb-IPN circuitry
MHb neurons possess a very unique set of electrophysiological properties that confer them with the ability to sense nicotine and signal aversion and withdrawal. Here we discuss some distinctive properties of habenular neurons, including their intrinsic pacemaking activity, their receptors and neurotransmitters, synaptic inputs and outputs, and specifics of their synaptic transmission.
4.1 Pacemaking
A striking electrophysiological attribute of MHb neurons is their spontaneous pacemaking activity. Medial habenular cells generate tonic trains of action potentials (APs). In rodents, MHb neurons fire independently of their afferent inputs with a frequency of about 2–10 Hz (Görlich et al., 2013; Kim and Chang, 2005; Kim and Chung, 2007; McCormick and Prince, 1987). This rhythmic pacemaking activity is mediated by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (Görlich et al., 2013; Shih et al., 2014). Pharmacological block of HCN channels in MHb neurons with ZD7288 abolishes spontaneous AP firing in brain slices, and infusion of the same blocker into the MHb of nicotine-naïve mice precipitates somatic and affective withdrawal-like symptoms (Görlich et al., 2013). Notably, spontaneous AP activity (Görlich et al., 2013; McCormick and Prince, 1987), or induced APs by current-injection (Dao et al., 2014) are increased upon nicotine application, likely via α3β4* nAChRs (Görlich et al., 2013), and/or by α5* nAChRs and neurokinin signaling (Dao et al., 2014). While all ChAT positive neurons in the MHb where spontaneously active (Görlich et al., 2013), Shih and colleagues found nicotine-mediated increases in spontaneous activity only in the MHbVl, but not in the MHbVc (Shih et al., 2014). The major difference between these two subnuclei is the expression of α4* nAChRs in the MHbVl (Fig. 2A). From these studies it has become evident, that disruption of spontaneous AP activity or its modulation by HCN or neurokinin signaling blockers is an important factor in nicotine withdrawal symptoms (Dao et al., 2014; Görlich et al., 2013). Therefore afferents influencing MHb neuron activity are likely to affect nicotine sensitivity and nicotine withdrawal.
4.2 Synaptic Inputs to the MHb
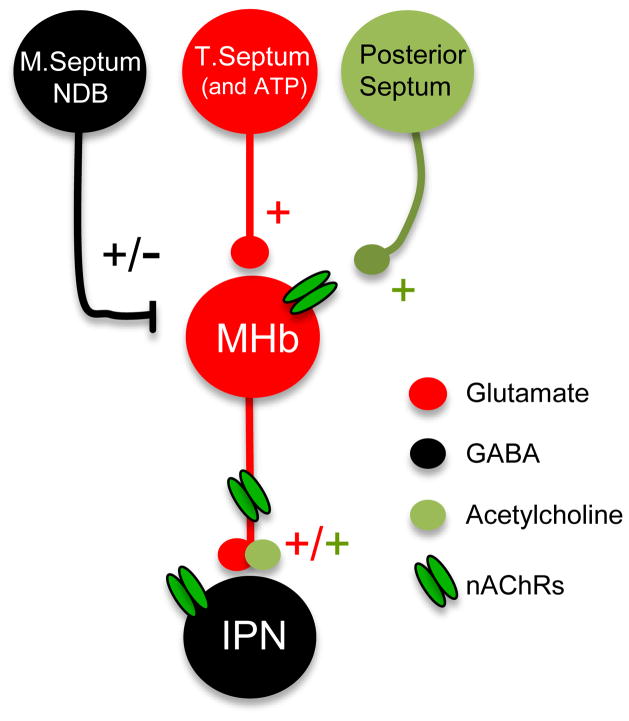
Tracing and genetic studies have shown that the MHb receives GABAergic input from the medial septum and diagonal band, and glutamatergic and ATP-ergic input from the triangular septum, (Qin and Luo, 2009; Robertson and Edwards, 1998), while the main input from the posterior septum via the stria medularis is likely to be cholinergic (Contestabile and Fonnum, 1983) (Fig. 4).
Figure 4. Neurotransmitter systems in the MHb-IPN.
Schematic illustration showing the neurotransmitter inputs to the MHb: GABAergic input from the medial septum (M. Septum) and nucleus of the diagonal band (NDB), glutamatergic and ATP-ergic input from the triangular septum (T. Septum) and cholinergic input from the posterior septum. MHb terminals in the IPN co-release glutamate and acetylcholine. Nicotinic acetylcholine receptors (nAChRs) are located in the somatodendritic compartment of MHb neurons, presynaptically in MHb axon terminals and postsynaptically in IPN neurons.
Cholinergic cells are densely packed in the ventral portion of the MHb, and innervate rostral, central, and intermediate subnuclei of the IPN (Contestabile et al., 1987; Eckenrode et al., 1987; Houser et al., 1983; Kimura et al., 1981). The MHb, together with the IPN, expresses the highest density of nicotinic binding sites in the mammalian brain (Clarke et al., 1985; Cui et al., 2003; Han et al., 2000; Han et al., 2003; Le Novere et al., 1996; Marks et al., 1992; Wada et al., 1989; Whiteaker et al., 2000; Yeh et al., 2001), but as evidenced by TRAP analysis (Görlich et al., 2013), immunoprecipitations (Grady et al., 2009; Scholze et al., 2012), physiology (Mulle et al., 1991), receptor autoradiography (Zoli et al., 1998), and knock-in studies (Shih et al., 2014), nAChR subunit compositions are quite diverse (Fig. 2A). The MHb is even more complex in terms of physiology, when taking into account the heterogenous expression of VGlut1 and VGlut2 (Aizawa et al., 2012) (Fig. 2A). Since the five molecularly distinct subdivisions of the MHb were only recently described (Aizawa et al., 2012), most electrophysiological studies on the inputs and outputs of the MHb have not taken these differences into account.
Neurons in the ventral two-thirds of the MHb are highly enriched in nAChRs, mostly containing the α3β4* subunit (Frahm et al., 2011; Grady et al., 2009). As noted earlier, in ChAT positive neurons, activation of α3β4* nAChRs by nicotine increases their pacemaking activity, but even more so in mice withdrawn from chronic nicotine (Görlich et al., 2013). On the other hand, recordings from neurons of mice in nicotine withdrawal in the MHBVl, show a reduced nicotine-mediated increase in AP firing, compared to saline treated animals (Shih et al., 2014). Within the MHb, only neurons in the MHbVl contain the α4* nAChRs, which are among the most sensitive receptors to nicotine and ACh (Chavez-Noriega et al., 1997; Gotti et al., 2009; Kuryatov et al., 2008), but likely do not express ChAT (Shih et al., 2014).
In addition to nAChRs, MHb neurons express P2X receptors, which, upon ATP release from the TS, cause a substantial Ca2+ influx into the neuron (Edwards et al., 1992; Edwards et al., 1997; Robertson et al., 1999; Rogers et al., 1997). Ca2+ is an important intracellular messenger, modulating ion channels and altering synaptic function, and α4β2* and α3β4* nAChRs show a fairly high Ca2+-permeability (Ragozzino et al., 1998; Rathouz and Berg, 1994). Together, P2X and nAChRs, likely make up for the lack of Ca2+ current mediated by NMDARs, which are not expressed in the MHb (Robertson et al., 1999; Rogers and Dani, 1995). Functionally, ATP has been shown to both depolarize and increase spontaneous AP activity in hypocretin/orexin neurons (Wollmann et al., 2005). Further, ATP facilitates HCN activation (He et al., 2014), and therefore influences pacemaking activity. Despite the plausibility, it remains unclear if ATP is directly involved in nicotine dependence and nicotine withdrawal by modulating spontaneous AP activity of MHb neurons.
Apart from the unusually high expression of α3β4* nAChRs and the uncommon use of ATP as a neurotransmitter in the MHb, the physiology of GABAergic inputs coming from the septum (Qin and Luo, 2009), is quite unique in the CNS as well. Neurons in the MHb express both GABAARs and GABABRs, which have distinct physiological roles and mediate spontaneous pacemaking activity in opposing ways. In young rats (postnatal day (P)18–25), GABA release leads to a fast excitation of MHb neurons, followed by a slow inhibition, mediated by GABAARs and GABABRs, respectively (Kim and Chung, 2007). GABAA-mediated responses are often excitatory in the early postnatal period, due to a high intracellular Cl− concentration (Cherubini et al., 1991; Friauf et al., 2011). During development, increased expression of the K+/Cl− co-transporter 2 (KCC2), which shuffles intracellular Cl− ions out of the neuron, causes GABAAR activation to hyperpolarize neurons (Clayton et al., 1998; Friauf et al., 2011; Lu et al., 1999; Rivera et al., 1999). While GABAAR-mediated excitation in MHb neurons was measured in young animals, it is likely that this persists in the adult, as expression of KCC2 has been shown to be absent in mature MHb neurons, both by immunohistochemistry (Kim and Chung, 2007; Markkanen et al., 2014), and by mRNA levels (Kanaka et al., 2001), likely leaving intracellular Cl− concentrations high in the MHb. It is noteworthy, that in some MHb neurons, GABABRs were more sensitive to GABA than GABAARs (Kim and Chung, 2007). Therefore, only high activity in the MS will cause sufficient GABA release to increase MHb pacemaking activity.
Altogether, MHb neurons express a diverse receptor composition. The nAChRs subunit compositions in the MHb subnuclei are uncommon to other brain areas (Gotti et al., 2009; Grady et al., 2009), and excitatory actions of GABA (Kim and Chung, 2007), and ATP-ergic afferents (Edwards et al., 1992; Edwards et al., 1997; Robertson et al., 1999; Rogers et al., 1997) are quite unique with respect to other CNS circuits.
4.3 Synaptic output from the MHb
As mentioned previously, the major efferents from the bilateral MHb project via the FR to neurons of the midline IPN. Along their path, MHb axons in the IPN form en passant and crest synapses. The latter are synapses on opposing sides of the dendrite (Lenn, 1976). The two opposing synapses likely each emerge from only one of the two habenula nuclei (Lenn, 1976). This makes up for a potentially very interesting physiology, but so far no studies have addressed the role of these crest synapses in synaptic transmission, much less in the context of nicotine dependence and withdrawal.
Crest synapses could function as a coincidence detector, converging the bilateral signals from each of the MHb. Neurons that serve as coincidence detectors, have been postulated for the auditory system (Carr and Konishi, 1990; Görlich et al., 2010), where they only fire APs when two excitatory postsynaptic potentials (EPSP) from two synapses arrive nearly simultaneously (Agmon-Snir et al., 1998; McAlpine and Grothe, 2003).
MHb terminals in the IPN co-release glutamate and ACh, but ACh release is only detected upon high frequency stimulation above 20 Hz (Ren et al., 2011). Spontaneous pacemaking activity in MHb slices is only up to around 10 Hz (Görlich et al., 2013; Kim and Chang, 2005; Kim and Chung, 2007; McCormick and Prince, 1987), but can easily increase up to 20 Hz in animals withdrawn from nicotine upon re-exposure to the drug (Görlich et al., 2013). In vivo AP frequencies are likely even higher. ACh is thought to be a volume-transmitter, meaning, that only high frequency stimulations cause enough ACh to be released for spillovers from remote release sites, which can be several 100 nm away from the respective receptor (Zoli et al., 1999). In the IPN, nAChRs are found both presynaptically on MHb axon terminals (Girod et al., 2000) and postsynaptically in IPN neurons. This sets up a situation in which, ACh, and potentially nicotine, not only excites postsynaptic neurons, but also facilitates presynaptic glutamate (Girod et al., 2000; Mansvelder et al., 2009), and influences GABA release (Covernton and Lester, 2002) (Fig. 4).
Volume-transmission has an essential advantage over direct neurotransmitter activation: By virtue of its dependence on the presynaptic AP frequency, synaptic and extrasynaptic ACh concentrations can change gradually with presynaptic excitation, whereas direct neurotransmission happens in an all or nothing fashion. ACh might hence gradually fine-tune MHb to IPN neurotransmission by modulating presynaptic transmitter release (Covernton and Lester, 2002; Girod et al., 2000), a mechanism that is likely disturbed in tobacco users and smokers undergoing nicotine withdrawal. Indeed, recordings from IPN neurons of mice in nicotine withdrawal, showed increased spontaneous excitatory postsynaptic currents (sEPC) (Zhao-Shea et al., 2013). Future studies of ACh volume-transmission in the IPN of mice (Ren et al., 2011) and/or zebrafish (Hong et al., 2013) might also clarify, if ACh release from one site of a crest synapse also influences function of the opposing crest synapse, as well as determine the fundamental role of the endogenous cholinergic signaling at these synapses.
5. Concluding remarks
Recent research has led to an improved understanding of the physiology and function of the MHb-IPN pathway. Several cell-types have been described in the MHb and in the IPN, and their function, and synaptic inputs have begun to be deciphered. With the application of newly developed technologies for cell specific manipulation, a more comprehensive picture of the MHb-IPN circuit is emerging. For instance, intersectional studies using Cre-dependent mice and viral tools that alter neuronal activity, such as light-activated opsins and tethered toxins, allow specific activation or silencing of particular MHb-IPN cell populations in living mice to elucidate the contribution of individual subpopulations to nicotine and non-nicotine-mediated behaviors. Also, retrograde tracing with virus in Cre-recombinase expressing mice can help decipher new connections. Importantly, the use of ribosomal tagged-EGFP subunits for Translational Ribosomal Affinity purification (TRAP) profiling provides a strategy for the identification of new molecular markers of subpopulations and epigenetic traits that can allow further dissection of the circuit and its molecular mechanisms. Given the privileged position of the MHb-IPN tract as a relay pathway where cholinergic, serotoninergic and dopaminergic signals converge, the identification of new molecules and mechanisms will accelerate progress in our understanding of this ancient brain structure, its role in the physiology of impulsive/compulsive behaviors and in the pathophysiology of schizophrenia and drug abuse. Eventually, exploratory efforts aimed at dissecting nicotine withdrawal and aversion may lead to the discovery of novel molecular events responsible for establishment of the addictive state, and result in the development of more effective therapies for smoking cessation. Because cigarette smoking is a global health problem, largely due to the difficulties associated with nicotine abstinence, developing a therapeutic strategy that specifically targets the negative symptoms of withdrawal can have a profound impact on human health.
Highlights.
Habenulo-interpeduncular system in nicotine aversion and withdrawal reviewed.
Overview of afferents and efferents of the MHb-IPN circuitry.
Role of nAChRs subtypes in nicotine withdrawal and aversion summarized.
Neurotransmitters and molecular markers of the MHb-IPN subnuclei highlighted.
Electrophysiological properties of MHb neurons discussed.
Acknowledgments
This work was supported by NIH/NIDA: grant 1P30 DA035756-01 (II-T) and by the Deutsche Forschungsgemeinschaft (DFG): grant GO 2334/1-1 (A.G)
Abbreviations
- 3V
third ventricle
- 4V
fourth ventricle
- ACh
acetylcholine
- AP
action potential
- BAC
bed nucleus of the anterior commissure
- ChAT
choline acetyltransferase
- CPA
conditioned place aversion
- DR
dorsal raphe
- DTg
dorsal tegmental nuclei
- EC
entorhinal cortex
- EPSP
excitatory postsynaptic potential
- FR
fasciculus retroflexus
- Hb-IPN
habenulo-interpeduncular
- HC
hippocampus
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- Hyp
hypothalamus
- IL-18
interleukin 18
- IPA
interpeduncular nucleus apical
- IPC
interpeduncular nucleus central
- IPDL
interpeduncular nucleus dorsolateral
- IPDM
interpeduncular nucleus dorsomedial
- IPI
interpeduncular nucleus intermediate
- IPL
interpeduncular nucleus lateral
- IPN
interpeduncular nucleus
- IPR
interpeduncular nucleus rostral
- KCC2
K+/Cl− co-transporter 2
- LC
locus coeruleus
- LDTg
laterodorsal tegmental nuclei
- LHb
lateral habenula
- LV
lateral ventricle
- MHb
medial habenula
- MHbD
medial habenula dorsal
- MHbS
medial habenula superior
- MHbV
medial habenula ventral
- MHbVc
medial habenula ventro-central
- MHbVl
medial habenula ventro-lateral
- MHbVm
medial habenula ventro-medial
- MnR
median raphe
- MS
medial septum
- nAChR
nicotinic acetylcholine receptor
- NDB
nucelus of diagonal band
- NI
nucleus incertus
- Oprm
μ-opioid receptor
- PAG
periaqueductal gray
- sEPSC
spontaenous excitatory postsynaptic current
- Sfi
septofimbrial nucleus
- SNP
single nucleotide polymorphism
- SP
substance P
- Thal
thalamus
- TRAP
translational ribosomal affinity purification
- TS
triangular septum
- VGlut
vesicular glutamate transporter
- VTA
ventral tegmental area
Footnotes
Conflict of Interest Statement:
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmon-Snir H, Carr CE, Rinzel J. The role of dendrites in auditory coincidence detection. Nature. 1998;393:268–272. doi: 10.1038/30505. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Bianco IH, Hamaoka T, Miyashita T, Uemura O, Concha ML, Russell C, Wilson SW, Okamoto H. Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr Biol. 2005;15:238–243. doi: 10.1016/j.cub.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. J Comp Neurol. 2012;520:4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- Andres KH, von During M, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407:130–150. doi: 10.1002/(sici)1096-9861(19990428)407:1<130::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Baisden RH, Hoover DB, Cowie RJ. Retrograde demonstration of hippocampal afferents from the interpeduncular and reuniens nuclei. Neurosci Lett. 1979;13:105–109. doi: 10.1016/0304-3940(79)90025-9. [DOI] [PubMed] [Google Scholar]
- Beiranvand F, Zlabinger C, Orr-Urtreger A, Ristl R, Huck S, Scholze P. Nicotinic acetylcholine receptors control acetylcholine and noradrenaline release in the rodent habenulo-interpeduncular complex. Br J Pharmacol. 2014 doi: 10.1111/bph.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J, Jr, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broide RS, Salas R, Ji D, Paylor R, Patrick JW, Dani JA, De Biasi M. Increased sensitivity to nicotine-induced seizures in mice expressing the L250T alpha 7 nicotinic acetylcholine receptor mutation. Mol Pharmacol. 2002;61:695–705. doi: 10.1124/mol.61.3.695. [DOI] [PubMed] [Google Scholar]
- Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signalling: roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J, Noguchi K, Ellison G. Nicotine produces selective degeneration in the medial habenula and fasciculus retroflexus. Brain Res. 2001;906:127–134. doi: 10.1016/s0006-8993(01)02570-7. [DOI] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cho KH, Mori S, Jang HS, Kim JH, Abe H, Rodriguez-Vazquez JF, Murakami G. The habenulo-interpeduncular and mammillothalamic tracts: early developed fiber tracts in the human fetal diencephalon. Childs Nerv Syst. 2014 doi: 10.1007/s00381-014-2432-5. [DOI] [PubMed] [Google Scholar]
- Ciani E, Severi S, Bartesaghi R, Contestabile A. Neurochemical correlates of nicotine neurotoxicity on rat habenulo-interpeduncular cholinergic neurons. Neurotoxicology. 2005;26:467–474. doi: 10.1016/j.neuro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton GH, Owens GC, Wolff JS, Smith RL. Ontogeny of cation-Cl-cotransporter expression in rat neocortex. Brain Res Dev Brain Res. 1998;109:281–292. doi: 10.1016/s0165-3806(98)00078-9. [DOI] [PubMed] [Google Scholar]
- Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J Anat. 2001;199:63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, Flumerfelt BA. Afferent connections of the interpeduncular nucleus and the topographic organization of the habenulo-interpeduncular pathway: an HRP study in the rat. J Comp Neurol. 1981;196:253–270. doi: 10.1002/cne.901960206. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Fonnum F. Cholinergic and GABAergic forebrain projections to the habenula and nucleus interpeduncularis: surgical and kainic acid lesions. Brain Res. 1983;275:287–297. doi: 10.1016/0006-8993(83)90989-7. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Oktedalen O, Fonnum F. Topography of cholinergic and substance P pathways in the habenulointerpeduncular system of the rat. An immunocytochemical and microchemical approach. Neuroscience. 1987;21:253–270. doi: 10.1016/0306-4522(87)90337-x. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Covernton PO, Lester RA. Prolonged stimulation of presynaptic nicotinic acetylcholine receptors in the rat interpeduncular nucleus has differential effects on transmitter release. Int J Dev Neurosci. 2002;20:247–258. doi: 10.1016/s0736-5748(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinemann SF. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadda M, Domenichini A, Piffer L, Argenton F, Bisazza A. Early differences in epithalamic left-right asymmetry influence lateralization and personality of adult zebrafish. Behav Brain Res. 2010;206:208–215. doi: 10.1016/j.bbr.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Mesolimbic dopamine and habenulo-interpeduncular pathways in nicotine withdrawal. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao DQ, Perez EE, Teng Y, Dani JA, De Biasi M. Nicotine Enhances Excitability of Medial Habenular Neurons via Facilitation of Neurokinin Signaling. J Neurosci. 2014;34:4273–4284. doi: 10.1523/JNEUROSCI.2736-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenrode TC, Barr GA, Battisti WP, Murray M. Acetylcholine in the interpeduncular nucleus of the rat: normal distribution and effects of deafferentation. Brain Res. 1987;418:273–286. doi: 10.1016/0006-8993(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Robertson SJ, Gibb AJ. Properties of ATP receptor-mediated synaptic transmission in the rat medial habenula. Neuropharmacology. 1997;36:1253–1268. doi: 10.1016/s0028-3908(97)00127-5. [DOI] [PubMed] [Google Scholar]
- Ellison G. Neural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitry. Eur Neuropsychopharmacol. 2002;12:287–297. doi: 10.1016/s0924-977x(02)00020-2. [DOI] [PubMed] [Google Scholar]
- Fonck C, Cohen BN, Nashmi R, Whiteaker P, Wagenaar DA, Rodrigues-Pinguet N, Deshpande P, McKinney S, Kwoh S, Munoz J, Labarca C, Collins AC, Marks MJ, Lester HA. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive alpha 4* nicotinic receptors. J Neurosci. 2005;25:11396–11411. doi: 10.1523/JNEUROSCI.3597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Friauf E, Rust MB, Schulenborg T, Hirtz JJ. Chloride cotransporters, chloride homeostasis, and synaptic inhibition in the developing auditory system. Hear Res. 2011;279:96–110. doi: 10.1016/j.heares.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Gallego X, Molas S, Amador-Arjona A, Marks MJ, Robles N, Murtra P, Armengol L, Fernandez-Montes RD, Gratacos M, Pumarola M, Cabrera R, Maldonado R, Sabria J, Estivill X, Dierssen M. Overexpression of the CHRNA5/A3/B4 genomic cluster in mice increases the sensitivity to nicotine and modifies its reinforcing effects. Amino Acids. 2012;43:897–909. doi: 10.1007/s00726-011-1149-y. [DOI] [PubMed] [Google Scholar]
- Girod R, Barazangi N, McGehee D, Role LW. Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors. Neuropharmacology. 2000;39:2715–2725. doi: 10.1016/s0028-3908(00)00145-3. [DOI] [PubMed] [Google Scholar]
- Goncalves L, Sego C, Metzger M. Differential projections from the lateral habenula to the rostromedial tegmental nucleus and ventral tegmental area in the rat. J Comp Neurol. 2012;520:1278–1300. doi: 10.1002/cne.22787. [DOI] [PubMed] [Google Scholar]
- Görlich A, Antolin-Fontes B, Ables JL, Frahm S, Slimak MA, Dougherty JD, Ibanez-Tallon I. Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons. Proc Natl Acad Sci U S A. 2013;110:17077–17082. doi: 10.1073/pnas.1313103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich A, Illy M, Friauf E, Wagner H, Luksch H, Lohrke S. Development of the delay lines in the nucleus laminaris of the chicken embryo revealed by optical imaging. Neuroscience. 2010;168:564–572. doi: 10.1016/j.neuroscience.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW, Canteras NS. Connections of the nucleus incertus. J Comp Neurol. 2001;438:86–122. doi: 10.1002/cne.1303. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z. Origin and distribution of noradrenergic innervation in the habenula: a neurochemical study. Brain Res. 1983;275:299–304. doi: 10.1016/0006-8993(83)90990-3. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z, Jacobowitz DM. Cholinergic projection of the diagonal band to the interpeduncular nucleus of the rat brain. Brain Res. 1978;156:329–332. doi: 10.1016/0006-8993(78)90513-9. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Batman AM, Tyndale RF, Sellers E, Damaj MI. Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology (Berl) 2005;178:183–192. doi: 10.1007/s00213-004-2007-3. [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J Comp Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, Saccone NL, Bucholz KK, Cloninger CR, Neuman RJ, Budde JP, Fox L, Bertelsen S, Kramer J, Hesselbrock V, Tischfield J, Nurnberger JI, Jr, Almasy L, Porjesz B, Kuperman S, Schuckit MA, Edenberg HJ, Rice JP, Goate AM, Bierut LJ. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008;64:922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill GS, Fass B. Differential distribution of diagonal band afferents to subnuclei of the interpeduncular nucleus in rats. Neurosci Lett. 1984;48:43–48. doi: 10.1016/0304-3940(84)90286-6. [DOI] [PubMed] [Google Scholar]
- Hamill GS, Jacobowitz DM. A study of afferent projections to the rat interpeduncular nucleus. Brain Res Bull. 1984;13:527–539. doi: 10.1016/0361-9230(84)90035-2. [DOI] [PubMed] [Google Scholar]
- Hamill GS, Lenn NJ. Synaptic plasticity within the interpeduncular nucleus after unilateral lesions of the habenula in neonatal rats. J Neurosci. 1983;3:2128–2145. doi: 10.1523/JNEUROSCI.03-11-02128.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill GS, Olschowka JA, Lenn NJ, Jacobowitz DM. The subnuclear distribution of substance P, cholecystokinin, vasoactive intestinal peptide, somatostatin, leu-enkephalin, dopamine-beta-hydroxylase, and serotonin in the rat interpeduncular nucleus. J Comp Neurol. 1984;226:580–596. doi: 10.1002/cne.902260410. [DOI] [PubMed] [Google Scholar]
- Han ZY, Le Novere N, Zoli M, Hill JA, Jr, Champtiaux N, Changeux JP. Localization of nAChR subunit mRNAs in the brain of Macaca mulatta. Eur J Neurosci. 2000;12:3664–3674. doi: 10.1046/j.1460-9568.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- Han ZY, Zoli M, Cardona A, Bourgeois JP, Changeux JP, Le Novere N. Localization of [3H]nicotine, [3H]cytisine, [3H]epibatidine, and [125I]alpha-bungarotoxin binding sites in the brain of Macaca mulatta. J Comp Neurol. 2003;461:49–60. doi: 10.1002/cne.10659. [DOI] [PubMed] [Google Scholar]
- He C, Chen F, Li B, Hu Z. Neurophysiology of HCN channels: from cellular functions to multiple regulations. Prog Neurobiol. 2014;112:1–23. doi: 10.1016/j.pneurobio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Hemmendinger LM, Moore RY. Interpeduncular nucleus organization in the rat: cytoarchitecture and histochemical analysis. Brain Res Bull. 1984;13:163–179. doi: 10.1016/0361-9230(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Panagis G, Svensson TH, Nomikos GG. Behavioral and biochemical manifestations of mecamylamine-precipitated nicotine withdrawal in the rat: role of nicotinic receptors in the ventral tegmental area. Neuropsychopharmacology. 1999;21:560–574. doi: 10.1016/S0893-133X(99)00055-X. [DOI] [PubMed] [Google Scholar]
- Hong E, Santhakumar K, Akitake CA, Ahn SJ, Thisse C, Thisse B, Wyart C, Mangin JM, Halpern ME. Cholinergic left-right asymmetry in the habenulo-interpeduncular pathway. Proc Natl Acad Sci U S A. 2013;110:21171–21176. doi: 10.1073/pnas.1319566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Crawford GD, Barber RP, Salvaterra PM, Vaughn JE. Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983;266:97–119. doi: 10.1016/0006-8993(83)91312-4. [DOI] [PubMed] [Google Scholar]
- Hsu YW, Tempest L, Quina LA, Wei AD, Zeng H, Turner EE. Medial Habenula Output Circuit Mediated by alpha5 Nicotinic Receptor-Expressing GABAergic Neurons in the Interpeduncular Nucleus. J Neurosci. 2013;33:18022–18035. doi: 10.1523/JNEUROSCI.2927-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K. Comparison of alpha2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J Comp Neurol. 2005;493:241–260. doi: 10.1002/cne.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther. 2009;331:547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennes L. Sites of origin of gonadotropin releasing hormone containing projections to the amygdala and the interpeduncular nucleus. Brain Res. 1987;404:339–344. doi: 10.1016/0006-8993(87)91391-6. [DOI] [PubMed] [Google Scholar]
- Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104:933–946. doi: 10.1016/s0306-4522(01)00149-x. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Flumerfelt BA, Hrycyshyn AW. Simultaneous demonstration of choline acetyltransferase and glutamic acid decarboxylase immunoreactivity in the rat interpeduncular nucleus. J Chem Neuroanat. 1990;3:165–177. [PubMed] [Google Scholar]
- Kawaja MD, Flumerfelt BA, Hunt SP, Hrycyshyn AW. Substance P immunoreactivity in the rat interpeduncular nucleus: synaptic interactions between substance P-positive profiles and choline acetyltransferase- or glutamate decarboxylase-immunoreactive structures. Neuroscience. 1991;42:739–755. doi: 10.1016/0306-4522(91)90042-m. [DOI] [PubMed] [Google Scholar]
- Kedmi M, Beaudet AL, Orr-Urtreger A. Mice lacking neuronal nicotinic acetylcholine receptor beta4-subunit and mice lacking both alpha5- and beta4-subunits are highly resistant to nicotine-induced seizures. Physiol Genomics. 2004;17:221–229. doi: 10.1152/physiolgenomics.00202.2003. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kim U. Topographic commissural and descending projections of the habenula in the rat. J Comp Neurol. 2009;513:173–187. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J Comp Neurol. 2005;483:236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- Kim U, Chung LY. Dual GABAergic synaptic response of fast excitation and slow inhibition in the medial habenula of rat epithalamus. J Neurophysiol. 2007;98:1323–1332. doi: 10.1152/jn.00575.2007. [DOI] [PubMed] [Google Scholar]
- Kimura H, McGeer PL, Peng JH, McGeer EG. The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol. 1981;200:151–201. doi: 10.1002/cne.902000202. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–273. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Zoli M, Changeux JP. Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci. 1996;8:2428–2439. doi: 10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lenn NJ. Synapses in the interpeduncular nucleus: electron microscopy of normal and habenula lesioned rats. J Comp Neurol. 1976;166:77–99. doi: 10.1002/cne.901660106. [DOI] [PubMed] [Google Scholar]
- Lenn NJ. Effect of neonatal deafferentation on synaptogenesis in the rat interpeduncular nucleus. J Comp Neurol. 1978a;181:93–115. doi: 10.1002/cne.901810106. [DOI] [PubMed] [Google Scholar]
- Lenn NJ. Postnatal synaptogenesis in the rat interpeduncular nucleus. J Comp Neurol. 1978b;181:75–91. doi: 10.1002/cne.901810105. [DOI] [PubMed] [Google Scholar]
- Lenn NJ, Hamill GS. Subdivisions of the interpeduncular nucleus: a proposed nomenclature. Brain Res Bull. 1984;13:203–204. doi: 10.1016/0361-9230(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Lenn NJ, Wong V. Electron microscopic demonstration of caudal afferents to the rat interpeduncular nucleus. Neuroscience. 1980;5:875–881. doi: 10.1016/0306-4522(80)90156-6. [DOI] [PubMed] [Google Scholar]
- Lenn NJ, Wong V, Hamill GS. Left-right pairing at the crest synapses of rat interpeduncular nucleus. Neuroscience. 1983;9:383–389. doi: 10.1016/0306-4522(83)90301-9. [DOI] [PubMed] [Google Scholar]
- Lips EH, Gaborieau V, McKay JD, Chabrier A, Hung RJ, Boffetta P, Hashibe M, Zaridze D, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Field JK, Liloglou T, Xinarianos G, McLaughlin J, Liu G, Skorpen F, Elvestad MB, Hveem K, Vatten L, Study E, Benhamou S, Lagiou P, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Castellsague X, Macfarlane TV, Barzan L, Canova C, Lowry R, Conway DI, Znaor A, Healy C, Curado MP, Koifman S, Eluf-Neto J, Matos E, Menezes A, Fernandez L, Metspalu A, Heath S, Lathrop M, Brennan P. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol. 2010;39:563–577. doi: 10.1093/ije/dyp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, Vollenweider P, Preisig M, Wareham NJ, Zhao JH, Loos RJ, Barroso I, Khaw KT, Grundy S, Barter P, Mahley R, Kesaniemi A, McPherson R, Vincent JB, Strauss J, Kennedy JL, Farmer A, McGuffin P, Day R, Matthews K, Bakke P, Gulsvik A, Lucae S, Ising M, Brueckl T, Horstmann S, Wichmann HE, Rawal R, Dahmen N, Lamina C, Polasek O, Zgaga L, Huffman J, Campbell S, Kooner J, Chambers JC, Burnett MS, Devaney JM, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Epstein S, Wilson JF, Wild SH, Campbell H, Vitart V, Reilly MP, Li M, Qu L, Wilensky R, Matthai W, Hakonarson HH, Rader DJ, Franke A, Wittig M, Schafer A, Uda M, Terracciano A, Xiao X, Busonero F, Scheet P, Schlessinger D, St Clair D, Rujescu D, Abecasis GR, Grabe HJ, Teumer A, Volzke H, Petersmann A, John U, Rudan I, Hayward C, Wright AF, Kolcic I, Wright BJ, Thompson JR, Balmforth AJ, Hall AS, Samani NJ, Anderson CA, Ahmad T, Mathew CG, Parkes M, Satsangi J, Caulfield M, Munroe PB, Farrall M, Dominiczak A, Worthington J, Thomson W, Eyre S, Barton A, Mooser V, Francks C, Marchini J. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S, Byun JS, Leach P, Fowler CD, Murphy NP, Kenny PJ, Gould TJ, Boulter J. Targeted deletion of the mouse alpha2 nicotinic acetylcholine receptor subunit gene (Chrna2) potentiates nicotine-modulated behaviors. J Neurosci. 2013;33:7728–7741. doi: 10.1523/JNEUROSCI.4731-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol. 1999;39:558–568. [PubMed] [Google Scholar]
- Mansvelder HD, Mertz M, Role LW. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin Cell Dev Biol. 2009;20:432–440. doi: 10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand ER, Riley JN, Moore RY. Interpeduncular nucleus afferents in the rat. Brain Res. 1980;193:339–352. doi: 10.1016/0006-8993(80)90169-9. [DOI] [PubMed] [Google Scholar]
- Markkanen M, Karhunen T, Llano O, Ludwig A, Rivera C, Uvarov P, Airaksinen MS. Distribution of neuronal KCC2a and KCC2b isoforms in mouse CNS. J Comp Neurol. 2014;522:1897–1914. doi: 10.1002/cne.23510. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Grothe B. Sound localization and delay lines--do mammals fit the model? Trends Neurosci. 2003;26:347–350. doi: 10.1016/S0166-2236(03)00140-1. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Acetylcholine causes rapid nicotinic excitation in the medial habenular nucleus of guinea pig, in vitro. J Neurosci. 1987;7:742–752. doi: 10.1523/JNEUROSCI.07-03-00742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montone KT, Fass B, Hamill GS. Serotonergic and nonserotonergic projections from the rat interpeduncular nucleus to the septum, hippocampal formation and raphe: a combined immunocytochemical and fluorescent retrograde labelling study of neurons in the apical subnucleus. Brain Res Bull. 1988;20:233–240. doi: 10.1016/0361-9230(88)90183-9. [DOI] [PubMed] [Google Scholar]
- Moore RY, Weis R, Moga MM. Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. J Comp Neurol. 2000;420:398–418. doi: 10.1002/(sici)1096-9861(20000508)420:3<398::aid-cne9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Mulle C, Vidal C, Benoit P, Changeux JP. Existence of different subtypes of nicotinic acetylcholine receptors in the rat habenulo-interpeduncular system. J Neurosci. 1991;11:2588–2597. doi: 10.1523/JNEUROSCI.11-08-02588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M, Zimmer J, Raisman G. Quantitative electron microscopic evidence for reinnervation in the adult rat interpeduncular nucleus after lesions of the fasciculus retroflexus. J Comp Neurol. 1979;187:447–468. doi: 10.1002/cne.901870211. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Hildebrand BE, Panagis G, Svensson TH. Nicotine withdrawal in the rat: role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroreport. 1999;10:697–702. doi: 10.1097/00001756-199903170-00007. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Phillipson OT, Pycock CJ. Dopamine neurones of the ventral tegmentum project to both medial and lateral habenula. Some implications for habenular function. Exp Brain Res. 1982;45:89–94. doi: 10.1007/BF00235766. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Qin C, Luo M. Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience. 2009;161:827–837. doi: 10.1016/j.neuroscience.2009.03.085. [DOI] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27:947–957. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Barabino B, Fucile S, Eusebi F. Ca2+ permeability of mouse and chick nicotinic acetylcholine receptors expressed in transiently transfected human cells. J Physiol. 1998;507( Pt 3):749–757. doi: 10.1111/j.1469-7793.1998.749bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathouz MM, Berg DK. Synaptic-type acetylcholine receptors raise intracellular calcium levels in neurons by two mechanisms. J Neurosci. 1994;14:6935–6945. doi: 10.1523/JNEUROSCI.14-11-06935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Burnashev N, Edwards FA. Ca2+ permeability and kinetics of glutamate receptors in rat medial habenula neurones: implications for purinergic transmission in this nucleus. J Physiol. 1999;518( Pt 2):539–549. doi: 10.1111/j.1469-7793.1999.0539p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Edwards FA. ATP and glutamate are released from separate neurones in the rat medial habenula nucleus: frequency dependence and adenosine-mediated inhibition of release. J Physiol. 1998;508( Pt 3):691–701. doi: 10.1111/j.1469-7793.1998.691bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M, Colquhoun LM, Patrick JW, Dani JA. Calcium flux through predominantly independent purinergic ATP and nicotinic acetylcholine receptors. J Neurophysiol. 1997;77:1407–1417. doi: 10.1152/jn.1997.77.3.1407. [DOI] [PubMed] [Google Scholar]
- Rogers M, Dani JA. Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophys J. 1995;68:501–506. doi: 10.1016/S0006-3495(95)80211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004a;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003a;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004b;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003b;23:6255–6263. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Fibiger HC. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol. 1986;253:277–302. doi: 10.1002/cne.902530302. [DOI] [PubMed] [Google Scholar]
- Scholze P, Koth G, Orr-Urtreger A, Huck S. Subunit composition of alpha5-containing nicotinic receptors in the rodent habenula. J Neurochem. 2012;121:551–560. doi: 10.1111/j.1471-4159.2012.07714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield EB, Quick MW, Lester RA. Nicotinic acetylcholine receptor subunit mRNA expression and channel function in medial habenula neurons. Neuropharmacology. 2000;39:2591–2603. doi: 10.1016/s0028-3908(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Shibata H, Suzuki T. Efferent projections of the interpeduncular complex in the rat, with special reference to its subnuclei: a retrograde horseradish peroxidase study. Brain Res. 1984;296:345–349. doi: 10.1016/0006-8993(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Shih PY, Engle SE, Oh G, Deshpande P, Puskar NL, Lester HA, Drenan RM. Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. J Neurosci. 2014;34:9789–9802. doi: 10.1523/JNEUROSCI.0476-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimak MA, Ables JL, Frahm S, Antolin-Fontes B, Santos-Torres J, Moretti M, Gotti C, Ibanez-Tallon I. Habenular expression of rare missense variants of the beta4 nicotinic receptor subunit alters nicotine consumption. Front Hum Neurosci. 2014;8:12. doi: 10.3389/fnhum.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith OA, Astley CA, DeVito JL, Stein JM, Walsh KE. Functional analysis of hypothalamic control of the cardiovascular responses accompanying emotional behavior. Fed Proc. 1980;39:2487–2494. [PubMed] [Google Scholar]
- Staines WA, Yamamoto T, Dewar KM, Daddona PE, Geiger JD, Nagy JI. Distribution, morphology and habenular projections of adenosine deaminase-containing neurons in the septal area of rat. Brain Res. 1988;455:72–87. doi: 10.1016/0006-8993(88)90116-3. [DOI] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in Lewis and Fischer 344 inbred rat strains. Eur J Pharmacol. 1999;369:159–162. doi: 10.1016/s0014-2999(99)00086-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. The connections of the septal region in the rat. J Comp Neurol. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol. 2011;82:984–995. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Fass B. Projections between the interpeduncular nucleus and basal forebrain in the rat as demonstrated by the anterograde and retrograde transport of WGA-HRP. Exp Brain Res. 1988;73:23–31. doi: 10.1007/BF00279657. [DOI] [PubMed] [Google Scholar]
- Villalobos J, Ferssiwi A. The differential descending projections from the anterior, central and posterior regions of the lateral hypothalamic area: an autoradiographic study. Neurosci Lett. 1987;81:95–99. doi: 10.1016/0304-3940(87)90346-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wagner F, Stroh T, Veh RW. Correlating habenular subnuclei in rat and mouse using topographical, morphological and cytochemical criteria. J Comp Neurol. 2014 doi: 10.1002/cne.23554. [DOI] [PubMed] [Google Scholar]
- Ware JJ, van den Bree MB, Munafo MR. Association of the CHRNA5-A3-B4 gene cluster with heaviness of smoking: a meta-analysis. Nicotine Tob Res. 2011;13:1167–1175. doi: 10.1093/ntr/ntr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Jimenez M, McIntosh JM, Collins AC, Marks MJ. Identification of a novel nicotinic binding site in mouse brain using [(125)I]-epibatidine. Br J Pharmacol. 2000;131:729–739. doi: 10.1038/sj.bjp.0703616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Acuna-Goycolea C, van den Pol AN. Direct excitation of hypocretin/orexin cells by extracellular ATP at P2X receptors. J Neurophysiol. 2005;94:2195–2206. doi: 10.1152/jn.00035.2005. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron. 2013;78:537–544. doi: 10.1016/j.neuron.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JJ, Yasuda RP, Davila-Garcia MI, Xiao Y, Ebert S, Gupta T, Kellar KJ, Wolfe BB. Neuronal nicotinic acetylcholine receptor alpha3 subunit protein in rat brain and sympathetic ganglion measured using a subunit-specific antibody: regional and ontogenic expression. J Neurochem. 2001;77:336–346. doi: 10.1046/j.1471-4159.2001.t01-1-00259.x. [DOI] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Pang X, Gardner PD, Tapper AR. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr Biol. 2013;23:2327–2335. doi: 10.1016/j.cub.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]