Abstract
Purpose
Current recommendations for refeeding in anorexia nervosa (AN) are conservative, beginning around 1,200 calories to avoid refeeding syndrome. We previously showed poor weight gain and long hospital stay using this approach and hypothesized that a higher calorie approach would improve outcomes.
Methods
Adolescents hospitalized for malnutrition due to AN were included in this quasi-experimental study comparing lower and higher calories during refeeding. Participants enrolled between 2002 and 2012; higher calories were prescribed starting around 2008. Daily prospective measures included weight, heart rate, temperature, hydration markers and serum phosphorus. Participants received formula only to replace refused food. Percent Median Body Mass Index (% MBMI) was calculated using 50th percentile body mass index for age and sex. Unpaired t-tests compared two groups split at 1,200 calories.
Results
Fifty-six adolescents with mean (±SEM) age 16.2 (±.3) years and admit %MBMI 79.2% (±1.5%) were hospitalized for 14.9 (±.9) days. The only significant difference between groups (N = 28 each) at baseline was starting calories (1,764 [±60] vs. 1,093 [±28], p < .001). Participants on higher calories had faster weight gain (.46 [±.04] vs. .26 [±.03] %MBMI/day, p < .001), greater daily calorie advances (122 [±8] vs. 98 [±6], p = .024), shorter hospital stay (11.9 [±1.0] vs. 17.6 [±1.2] days, p < .001), and a greater tendency to receive phosphate supplementation (12 vs. 8 participants, p = .273).
Conclusions
Higher calorie diets produced faster weight gain in hospitalized adolescents with AN as compared with the currently recommended lower calorie diets. No cases of the refeeding syndrome were seen using phosphate supplementation. These findings lend further support to the move toward more aggressive refeeding in AN.
Keywords: Anorexia nervosa, Adolescents, Refeeding syndrome, Nutrition, Weight gain, Fluid balance
Current recommendations for refeeding hospitalized patients with anorexia nervosa (AN) are conservative. The American Psychiatric Association [1,2] and the Academy of Nutrition and Dietetics [3] recommend starting with a range of 1,200 calories per day and advancing by approximately 200 calories every other day. This cautious approach is intended to avoid the refeeding syndrome, the potentially fatal shifts in electrolytes that can occur when insulin is released in response to an influx of nutrients (particularly carbohydrate) [4–7]. Despite evidence to show that refeeding risk is highest in severely malnourished patients [4–6,8,9], these lower calorie diets have been implemented widely to ensure safety.
Unfortunately, this approach is likely too conservative to maximize nutritional recovery in a large proportion of the adolescents hospitalized with AN in the United States who are only moderately malnourished, with body mass index (BMI) around 75%–85% of expected [8,10–12]. We recently reported significant initial weight loss and slow weight gain among 35 adolescents with a mean BMI of 80.1% of expected who were started on a range of calories averaging 1,200 per day and followed prospectively in our pediatric clinical research center (PCRC) [11]. This was the first study to demonstrate that the longstanding clinical observation of weight loss during early refeeding in AN was associated with the currently recommended lower calorie diets (in addition to fluid losses). These findings have contributed to recognition of the “underfeeding syndrome” [13].
In 2008, our program began increasing admission calorie prescriptions in response to our own data [11] and clinical reports of higher calorie approaches [14]. Our feeding approach is meal-based, with calorie prescriptions divided into three meals and three snacks per day with formula only as necessary to replace refused calories. In 2010, Whitelaw et al. published the first report of higher calorie meal-based refeeding showing good outcomes beginning around 1,900 calories and increasing by 500 calories in the first 5 days [9]. Yet, no studies to date have compared this higher calorie approach with the lower calorie diets that are still recommended. This gap represents a significant barrier to revising the current recommendations and moving forward with evidence-based approaches to refeeding in AN. Thus, the purpose of the present study was to compare higher and lower calorie diets during refeeding in moderately malnourished adolescents hospitalized with AN. We used the change in clinical practice in our program around 2008 to compare higher and lower calorie diets using a quasi-experimental design, with our previous study [11] participants as historical controls.
Methods
Study design and participants
This prospective observational study used a quasi-experimental design to compare a lower calorie group (800–1,200 calories) including former study participants as historical controls and higher calorie group (1,400–2,400 calories) including newly enrolled participants. Participants were age 9–20 years old, diagnosed with AN [15], met criteria for hospitalization [16] and had no previous admissions for AN. Exclusion criteria included pregnancy, diagnosis of bulimia nervosa, and/or thought disorders such as schizophrenia or other psychosis. Eligible patients and parents were consented upon hospitalization at University of California San Francisco (UCSF) Benioff Children’s Hospital between October 2002 and February 2012 and admitted to the PCRC. Race/ethnicity was self-identified upon registration for admission to hospital. Of 62 patients, three were excluded for previous admissions and three transferred care to another unit. This study was approved by the UCSF Human Subjects Protection Committee.
Refeeding protocol
Participants received three meals and three snacks served on trays at the bedside. Calorie levels were prescribed on admission by the physicians, who generally followed the lower calorie recommendations between 2002 and 2008 and began prescribing higher calorie diets starting in 2008. However, calorie levels were not strictly protocolized or randomly assigned, so it is likely that physicians used clinical judgment when assigning diets. We attempted to control for some degree of variation in clinical practice in our statistical analysis (see the following section). Macronutrient content of the diet was approximately 20% fat, 21% protein and 60% carbohydrate (Computrition, Inc., Software v.17.9.5, Chatsworth, CA). A standard high-calorie liquid supplement (“formula”) providing 1.06 calories per mL, 22% fat, 14 % protein, and 64% carbohydrate was used orally to replace refused calories; no participants received enteral feedings. All beverages were weighed and measured before serving; free water was restricted to 1 L per day. Room sitters observed all meals/snacks and 45 minutes afterwards. Daily calorie counts assessed actual calorie intake from food and formula in a subset of participants. Phosphate was supplemented as needed in packets of 250 mg phosphate, 160 mg sodium, and 280 mg potassium. Because patients are assumed to have low micronutrient stores and overall poor nutrition leading up to hospitalization, our program provides a standard supplement regimen including 500 mg calcium carbonate twice per day, zinc sulfate or zinc acetate once per day, and an adult multivitamin with minerals once per day.
Data collection
Daily measures were made from admission on day (D) 0 through D14 and day of discharge (if stay >14 days). Discharge criteria included waking heart rate (HR) >50 beats/min and temperature >36.0°C for at least 24 hours. Detailed methods for vital signs and anthropometric and laboratory measures in this study have been previously described [11]. Briefly, HR and blood pressure were assessed with continuous cardiac monitoring and temperature was measured orally. Weight was measured each morning D1–D14 and on day of discharge; height was measured on D1. Laboratory indicators of hydration status, including blood urea nitrogen (BUN), creatinine (Cr), serum sodium, and urine specific gravity, were measured within 24 hours of admission. Fluid balance was calculated daily as the difference between total intake and output recorded over 24 hours. Intake represents beverages only; output represents urine only using bedside commode. Electrolytes were measured twice per day as needed to monitor refeeding risk; only serum phosphorus is reported here.
Statistical methods
Descriptive statistics were computed for the overall study population; unpaired t-tests compared lower and higher calorie groups. Rate of calorie advancement during the first week in hospital was calculated as absolute increase in calories D1–D7 divided by seven. Discharge calories are reported as percent of energy needs, calculated retrospectively using estimated energy requirement equations from the Institute of Medicine [17]. These equations are used clinically to set goals for caloric advancement and, for the purposes of this study, they provide a standard to compare the adequacy of caloric intake across age, sex, and body size. However, they are known to underestimate energy needs in patients with anorexia [18]. Therefore we maximized these estimations by using the weight corresponding to the median body mass index (MBMI, see below) for age and sex (rather than current weight), a moderate activity factor of 1.2–1.3 (despite bed rest), and additional 500 calories (if current weight was lower than the MBMI). Low serum phosphorus was defined as ≤3 mg/dL [8]; “lowest serum phosphorus” was the lowest value recorded at any point during hospitalization.
The primary study outcome was rate of increase in %MBMI. Percent MBMI was calculated by dividing the current BMI (or BMI = 22 if >18 years old) by the MBMI for the individual participant’s age and gender [19]. Rate of increase was calculated as change in %MBMI from admission to discharge divided by stay length (D1 until discharge). D1 height was used to calculate % MBMI on all subsequent days; therefore, change in %MBMI is due to weight change. Three multivariate models examined associations between %MBMI and formula intake, serum phosphorus, and length of stay. Models were adjusted for lowest HR on admission because this primary admission criterion is often used as a clinical indicator of severity of illness and physicians may have considered it when prescribing starting calories. Analyses were performed with STATA SE 12 (Statcorp LP, College Station, TX) at significance level of p < .05. One male participant was included after analysis excluding him was not found to change the direction or significance of results.
Results
We followed 56 adolescents over a mean (±SEM) of 14.9 (±.9) days. The overall study population was 98% female, mean age 16.2 (±.3) years (12.3–20.9), 76% non-Hispanic white, 6% Asian/Pacific Islander, and 19% other race/ethnicity. Mean %MBMI was 79.2 (±1.5), mean BMI was 16.1 (±.3) kg/m2 (range 11.1–21.8). Participants were bradycardic and hypothermic consistent with hospital admission criteria [16]. Markers of hydration status were within normal limits [20]. As shown in Table 1, the only significant difference between groups on admission was the mean calorie prescription (p < .001): 1,764 (±60) calories (40.6 kcal/kg and 2.1 g protein/kg/day) in the higher calorie group versus 1,093 (±28) calories (27.0 kcal/kg and 1.4 g protein/kg/day) in the lower calorie group.
Table 1.
Comparison of study groups on admission
| Higher calorie (N = 28) | Lower calorie (N = 28) | p valuea | |
|---|---|---|---|
| Calories: | |||
| Admit calorie prescription (Kcal) | 1,764 (60) | 1,093 (28) | .000 |
| Estimated energy needs (Kcal) | 2,864 (44) | 2,793 (60) | .338 |
| Weight: | |||
| Admit BMI (kg/m2) | 16.6 (.4) | 15.8 (.5) | .188 |
| Admit %MBMIb | 81.9 (1.8) | 77.6 (2.3) | .150 |
| Age (years): | 16.1 (.4) | 16.2 (.4) | .875 |
| Vital signs: | |||
| Lowest heart rate (beats/min) | 43.0 (2.0) | 43.9 (1.7) | .880 |
| Lowest Temperature (°C) | 35.8 (.1) | 35.8 (.1) | .935 |
| Hydration status: | |||
| Serum BUN (mg/dL) | 15.3 (1.6) | 12.8 (15.3) | .241 |
| Cr (mg/dL) | .82 (.04) | .84 (.04) | .761 |
| Cr/BUN ratio | 15.7 (1.4) | 18.9 (2.3) | .239 |
| Serum sodium (mmol/L) | 139.7 (.4) | 139.9 (.5) | .769 |
| Urine-specific gravity | 1.010 (.000) | 1.011 (.002) | .806 |
Data are presented as mean (SEM).
BMI = body mass index; BUN = blood urea nitrogen; Cr = creatinine; %MBMI = percent median BMI.
p values from unpaired t-tests.
%MBMI calculated using 50th percentile BMI for age and gender [19].
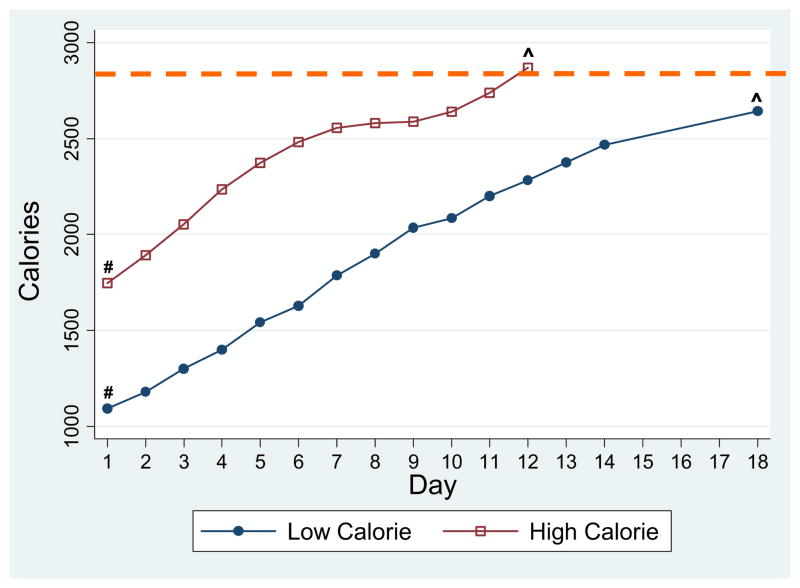
Figure 1 shows daily increases in calorie prescription for the two groups. Prescriptions were advanced faster D1–D7 in the higher calorie group: 122 (±8) versus 98 (±6) calories per day (p = .024). On discharge, calorie prescription was greater in the higher versus lower calorie group and would meet 101.1% (±1.9) versus 94.8% (±2.3) of estimated needs, respectively (p = .043). Data were available in a subset of participants showing actual calorie intake from food (N = 40) and formula (N = 34); both groups consumed 100% of their prescribed daily calories. Overall, formula intake provided 14%–15% of total calories on D1–D2, 7%–8% on D3–D9, and did not exceed 6% thereafter. There was no difference in the percent of participants in each group who took formula at any point during their stay (57.9 vs. 60.0% of participants, p = .905). There was no difference in the absolute number of calories from formula on D1–D2 (180 [±42] vs. 247 [±62] calories/day, p = .357). In a multivariate model adjusted for admission calorie prescription and lowest HR, there was a tendency for participants with the lowest %MBMI on admit to drink more formula during D1–D2 (β = −2.35; 95% CI −5.11, .410; p = .092).
Figure 1.
Comparison of increase in calorie prescription in subjects on lower calorie versus higher calorie diets. - - -Average estimated energy needs, based on calculated total energy expenditure (see Methods). #Calorie prescription was significantly greater in higher calorie group on day 1 (p < .000). ^Calorie prescription was significantly greater in the higher calorie group upon discharge (p = .043).
No clinical cases of refeeding syndrome were observed; however, 45% of participants (25/56) had a low serum phosphorus level. Lower %MBMI on admission predicted the lowest serum phosphorus in a multivariate model adjusted for admission calorie prescription and lowest HR (β = .029; 95% CI .012–.046; p = .001). Thirty-six percent of participants (20/56) received a mean of 483 (±90) mg of phosphate starting on day 1.7 (±.3). Among those, only six had a downward trend and/or low serum phosphorus suggestive of risk for hypophosphatemia on the day that supplementation commenced. There was no difference between groups in incidence of low phosphorus, lowest serum phosphorus, or the number of participants receiving phosphate supplementation (12/28 vs. 8/28, p = .273).
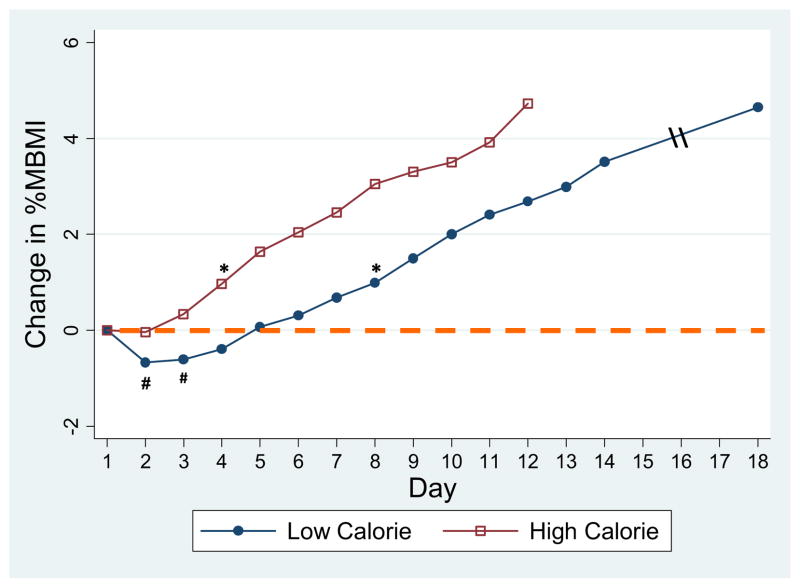
Weight gain trajectories for the two groups are shown in Figure 2. Rate of weight gain was nearly double in the higher calorie group: .46 (±.04) versus .26 (±.03) %MBMI per day, or .27 (±.03) versus .14 (±.02) kg per day (p < .001). As compared with D1, %MBMI was significantly greater as early as D4 in the higher calorie group (81.9 [±1.8] vs. 83.2% [±1.9], p = .006). As we previously showed [11], the lower calorie group initially lost weight (mean weight loss 493 [±77] g occurring on D 2.9 [±.2]) with no significant weight gain until D8. Comparing the first full 3 days in the hospital, weight change was +250 (±120) g in the higher calorie group versus −270 (±91) g in the lower calorie group (p = .001). Both groups appeared to be in negative fluid balance during hospitalization, with output significantly greater than input (p < .05) on 6 days in the higher calorie group versus 1 day in the lower calorie group. Net fluid loss D1–D3 (summed difference input-output) was nearly double in the higher calorie group (−1,102 [±243] mL) as compared with the lower calorie group (−589 [±219] mL); however, this was not significant (p = .122) due to high variation in input and output.
Figure 2.
Comparison of change in %median body mass index (%MBMI) in subjects on lower calorie versus higher calorie diets. - - -Baseline %MBMI, calculated from admit (day 1) height and weight. #%MBMI in lower calorie group on days 2 and 3 was significantly lower than day 1 (p < .05). *%MBMI on was significantly greater than day 1 on day 4 (and thereafter) in the higher calorie group and D8 in the lower calorie group (all p < .05).
As shown in Table 2, average hospital stay was 5.7 days shorter (p < .001) and discharge calorie prescription was 250 calorie greater (p = .015) in the higher versus lower calorie group. Despite faster weight gain in the higher calorie group, there was no difference between groups in overall weight gain (p = .602) or %MBMI on discharge (p = .173). Overall gain in % MBMI was significantly associated with longer hospital stay, adjusted for %MBMI, calorie prescription, and lowest HR at baseline (β = .122; 95% CI .023–.222; p = .017).
Table 2.
Comparison of study groups on discharge
| Higher calorie (N = 28) | Lower calorie (N = 28) | p value* | |
|---|---|---|---|
| Length of hospital stay (days) | 11.9 (1.0) | 17.6 (1.2) | .000 |
| Calories: | |||
| Discharge prescription (Kcal) | 2,893 (64) | 2,642 (76) | .015 |
| Weight: | |||
| Overall increase (kg) | 2.74 (.28) | 2.49 (.38) | .602 |
| Discharge %MBMIa | 85.7 (1.7) | 82.3 (1.8) | .173 |
Data are presented as mean (SEM) unless denoted otherwise.
%MBMI = percent median BMI.
p values from unpaired t-tests.
%MBMI calculated using 50th percentile BMI-for-age-and-gender [19].
Discussion
This study compared moderately malnourished adolescents with AN during refeeding on higher calorie diets starting around 1,800 calories and advancing by about 120 calories per day in the first week versus lower calorie diets starting around 1,100 calories and advancing by approximately 100 calories per day. Our previously published [11] data from participants on lower calorie diets are included here for comparison, because these diets are still recommended and widely used in the United States and internationally [1–3]. We found that participants that were started on higher calorie diets and advanced more rapidly gained weight faster and were discharged from the hospital earlier.
Since refeeding syndrome was first described in the 1950s [21,22], it has been documented in patients with AN [4–7] and conservative refeeding recommendations [1–3] have been established. Despite evidence that severely malnourished patients are at highest risk [5,8], lower calorie diets have been used widely for decades. This conservative approach may contribute to underfeeding among moderately malnourished adolescents, with BMIs between 75% and 85% of expected [10,11], who comprise a large proportion of those hospitalized with AN in the United States. A cross-section of 267 adolescents aged 14–16 years presenting at 11 outpatient eating disorders programs nationwide had an average %MBMI of 77.5–83.0; 14% were admitted to hospital at the first visit [12]. We previously showed poor weight gain and long hospital stay in such adolescents on lower calorie diets [11]. By comparison, participants in the present study starting nearly 700 calories higher and advancing 25% faster showed no initial weight loss, nearly twice the rate of weight gain, and significant weight increase within four days.
Although we cannot address safety, we did examine refeeding risk and phosphate supplementation. Almost half of all participants in the present study had a low serum phosphorus level in hospital. Whereas we did not find an association with calorie group, consistent with previous studies [5,8,9], hypophosphatemia was associated with degree of malnutrition. Thirty-six percent of participants (20/56) received phosphate supplementation. This is comparable to the only other published report of higher calorie meal-based refeeding: Whitelaw et al. reported that 43% of patients on a meal plan starting at 1,900 calories received phosphate [9]. In contrast to Whitelaw, however, supplementation in the present study appeared to be used prophylactically rather than to treat hypophosphatemia. Only 6/20 of our participants who received supplementation had a low serum phosphorus level or declining trend. These differences reflect variations in clinical approaches given the current paucity of evidence to guide best practices in this area.
The higher calorie group in the present study was discharged from hospital nearly 6 days earlier. Although patients see it as a benefit, shorter hospitalization was associated with lack of difference in discharge %MBMI between groups. Because discharge weight is an established predictor of long-term recovery [23–25], this finding could portend no long-lasting advantage to higher calorie refeeding. However, we believe that the greater calorie prescription on discharge in the higher calorie group, in combination with the faster weight gain, could lead to earlier weight recovery and perhaps other benefits. For example, medical hospitalization programs like ours have a limited mental health care component and shorter stay would enable earlier engagement in psychotherapy earlier. Clearly, studies are needed to examine long-term outcomes.
We evaluated markers of hydration and fluid status closely because they are known to impact weight. Although both groups appeared normally hydrated on admission, participants lost significant fluid during the first 10 days. Nevertheless, no net weight loss was observed in the higher calorie group and this leads us to conclude that adequate calories are the most important factor in establishing weight gain. Fluid loss during refeeding in AN [26,27] is believed to originate from an expanded extracellular water compartment [26–31]. Losses may occur at a BMI 15–16 kg/m2 threshold [26], which is consistent with our participants’ mean admit BMI of 16.1 kg/m2. Fluid loss appeared worse in the higher calorie group and, although this finding was not significant, it is interesting to note because studies in other starved populations show extracellular water loss proportional to the degree of positive nitrogen balance [32,33]. It is possible that nitrogen balance was more positive in the higher calorie group due to the 50% greater protein and calorie content, and this may have contributed to the greater fluid losses in this group.
Participants completed high-calorie meals without additional reliance on formula. There was no difference between groups in the percentage of participants who ever took formula or the number of calories coming from formula in the first 2 days. Instead, more malnourished participants tended to drink more formula regardless of calorie level. This may suggest that higher calorie meal-based approaches are more feasible for moderately malnourished patients. For more severely malnourished and chronically ill patients, enteral feedings have shown good outcomes [34–36], and may in theory attenuate the high refeeding risk in these patients by avoiding the wide glucose and insulin excursions associated with meal boluses [37].
We recognize several limitations to the current study. First, participants were moderately malnourished with no previous admissions for AN; findings are not applicable to severely and chronically malnourished patients. Second, we did not measure the known psychological [38] and physiological [39] stress related to completing meals. Third, we lack follow-up data to examine whether there is any long-term advantage to higher calorie refeeding and the retrospective data to examine factors that are likely to influence refeeding risk (such as degree of weight loss). Finally, secular trends may have influenced patient characteristics during the 10-year enrollment period. However, groups did not differ at baseline in characteristics likely to impact outcomes of interest (including weight gain and length of stay).
Despite these limitations, a major strength of this study is that it is the only prospective observational study of refeeding. Although the data from the lower calorie group were previously published [11] and reanalyzed here for the purposes of comparison, the data in both groups were collected prospectively, daily, in a protocolized fashion in a National Institutes of Health–funded PCRC by research-trained nursing staff. Comparable studies to date have been chart reviews. We also report on actual calorie intake, which addresses a major limitation of our earlier work (relying on prescribed calories) and corroborates our use of prescribed calories as marker of actual intake. Finally, the quasi-experimental study design is also a strength because it was a means to take advantage of a change in clinical practice within our own program. Randomized controlled trials remain the gold standard, and future studies should strive to compare refeeding feeding protocols within and across sites [40]. In conclusion, these findings lend support to the growing use of more aggressive refeeding in moderately malnourished hospitalized adolescents with AN.
IMPLICATIONS AND CONTRIBUTION.
Rate of weight gain was almost double on higher versus lower calorie diets for refeeding hospitalized adolescents with AN. There was no increased incidence of low serum phosphate using prophylactic supplementation. Our findings support more aggressive refeeding using a meal-based plan in moderately malnourished subjects with AN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Practice guideline for the treatment of patients with eating disorders (revision) American Psychiatric Association Work Group on Eating Disorders. Am J Psychiatry. 2000;157(1 Suppl):1–39. [PubMed] [Google Scholar]
- 2.Treatment of patients with eating disorders. American Psychiatric Association. Am J Psychiatry. (3) 2006;163(7 Suppl):4–54. [PubMed] [Google Scholar]
- 3.Position of the American Dietetic Association. Nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders. J Am Diet Assoc. 2006;106:2073–82. doi: 10.1016/j.jada.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M, Simpser E, Schneider M. Hypophosphatemia secondary to oral refeeding in anorexia nervosa. Int J Eat Disord. 2000;28:181–7. doi: 10.1002/1098-108x(200009)28:2<181::aid-eat7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Kohn MR, Golden NH, Shenker IR. Cardiac arrest and delirium: Presentations of the refeeding syndrome in severely malnourished adolescents with anorexia nervosa. J Adolesc Health. 1998;22:239–43. doi: 10.1016/S1054-139X(97)00163-8. [DOI] [PubMed] [Google Scholar]
- 6.Beumont PJ, Large M. Hypophosphataemia, delirium and cardiac arrhythmia in anorexia nervosa. Med J Aust. 1991;155:519–22. doi: 10.5694/j.1326-5377.1991.tb93887.x. [DOI] [PubMed] [Google Scholar]
- 7.Hall DE, Kahan B, Snitzer J. Delirium associated with hypophosphatemia in a patient with anorexia nervosa. J Adolesc Health. 1994;15:176–8. doi: 10.1016/1054-139x(94)90546-0. [DOI] [PubMed] [Google Scholar]
- 8.Ornstein RM, Golden NH, Jacobson MS, et al. Hypophosphatemia during nutritional rehabilitation in anorexia nervosa: Implications for refeeding and monitoring. J Adolesc Health. 2003;32:83–8. doi: 10.1016/s1054-139x(02)00456-1. [DOI] [PubMed] [Google Scholar]
- 9.Whitelaw M, Gilbertson H, Lam PY, et al. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health. 2010;46:577–82. doi: 10.1016/j.jadohealth.2009.11.207. [DOI] [PubMed] [Google Scholar]
- 10.Solanto MV, Jacobson MS, Heller L, et al. Rate of weight gain of inpatients with anorexia nervosa under two behavioral contracts. Pediatrics. 1994;93:989–91. [PubMed] [Google Scholar]
- 11.Garber AK, Michihata N, Hetnal K, et al. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J Adolesc Health. 2012;50:24–9. doi: 10.1016/j.jadohealth.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman SF, Grodin LF, Graham DA, et al. An eleven site national quality improvement evaluation of adolescent medicine-based eating disorder programs: Predictors of weight outcomes at one year and risk adjustment analyses. J Adolesc Health. 2011;49:594–600. doi: 10.1016/j.jadohealth.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 13.MARSIPAN Working Group. CR 162. Royal College Psychiatrists and Royal College of Physicians; London: Oct, 2010. Management of really sick patients with anorexia nervosa. cited 2012 June 25. Available at: http://www.rcpsych.ac.uk/files/pdfversion/CR162.pdf. [Google Scholar]
- 14.Kohn M, Madden S. Re: Critical appraisal of the management of severe malnutrition. J Paediatr Child Health. 2007;43:320. doi: 10.1111/j.1440-1754.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Washington DC: American Psychiatric Publishing Inc; 2000. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. [Google Scholar]
- 16.Golden NH, Katzman DK, Kreipe RE, et al. Eating disorders in adolescents: Position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:496–503. doi: 10.1016/s1054-139x(03)00326-4. [DOI] [PubMed] [Google Scholar]
- 17.“5 Energy.” Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: The National Academies Press; 2005. A Report of the Panel on Macronutrients, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 18.Cuerda C, Ruiz A, Velasco C, et al. How accurate are predictive formulas calculating energy expenditure in adolescent patients with anorexia nervosa? Clin Nutr. 2007;26:100–6. doi: 10.1016/j.clnu.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Data table of BMI-for-age charts. 2000 [cited 2012 Feb 13]; Available at: http://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm.
- 20.Caregaro L, Di Pascoli L, Favaro A, et al. Sodium depletion and hemo-concentration: Overlooked complications in patients with anorexia nervosa? Nutrition. 2005;21:438–45. doi: 10.1016/j.nut.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Schnitker MA, Mattman PE, Bliss TL. A clinical study of malnutrition in Japanese prisoners of war. Ann Intern Med. 1951;35:69–96. doi: 10.7326/0003-4819-35-1-69. [DOI] [PubMed] [Google Scholar]
- 22.Keys A, Brozek K, Henschel A, et al. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 23.Lock J, Litt I. What predicts maintenance of weight for adolescents medically hospitalized for anorexia nervosa? Eat Disord. 2003;11:1–7. doi: 10.1002/erv.496. [DOI] [PubMed] [Google Scholar]
- 24.Lund BC, Hernandez ER, Yates WR, et al. Rate of inpatient weight restoration predicts outcome in anorexia nervosa. Int J Eat Disord. 2009;42:301–5. doi: 10.1002/eat.20634. [DOI] [PubMed] [Google Scholar]
- 25.Baran SA, Weltzin TE, Kaye WH. Low discharge weight and outcome in anorexia nervosa. Am J Psychiatry. 1995;152:1070–2. doi: 10.1176/ajp.152.7.1070. [DOI] [PubMed] [Google Scholar]
- 26.Rigaud D, Boulier A, Tallonneau I, et al. Body fluid retention and body weight change in anorexia nervosa patients during refeeding. Clin Nutr. 2010;29:749–55. doi: 10.1016/j.clnu.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Vaisman N, Corey M, Rossi MF, et al. Changes in body composition during refeeding of patients with anorexia nervosa. J Pediatr. 1988;113:925–9. doi: 10.1016/s0022-3476(88)80033-7. [DOI] [PubMed] [Google Scholar]
- 28.Vaisman N, Rossi MF, Goldberg E, et al. Energy expenditure and body composition in patients with anorexia nervosa. J Pediatr. 1988;113:919–24. doi: 10.1016/s0022-3476(88)80032-5. [DOI] [PubMed] [Google Scholar]
- 29.Moreno MV, Djeddi DD, Jaffrin MY. Assessment of body composition in adolescent subjects with anorexia nervosa by bioimpedance. Med Eng Phys. 2008;30:783–91. doi: 10.1016/j.medengphy.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Mika C, Herpertz-Dahlmann B, Heer M, et al. Improvement of nutritional status as assessed by multifrequency BIA during 15 weeks of refeeding in adolescent girls with anorexia nervosa. J Nutr. 2004;134:3026–30. doi: 10.1093/jn/134.11.3026. [DOI] [PubMed] [Google Scholar]
- 31.Scalfi L, Marra M, Caldara A, et al. Changes in bioimpedance analysis after stable refeeding of undernourished anorexic patients. Int J Obes Relat Metab Disord. 1999;23:133–7. doi: 10.1038/sj.ijo.0800780. [DOI] [PubMed] [Google Scholar]
- 32.Starker PM, Askanazi J, Lasala PA, et al. The effect of parenteral nutritional repletion on muscle water and electrolytes. Implications for body composition. Ann Surg. 1983;198:213–7. doi: 10.1097/00000658-198308000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barac-Nieto M, Spurr GB, Lotero H, et al. Body composition during nutritional repletion of severely undernourished men. Am J Clin Nutr. 1979;32:981–91. doi: 10.1093/ajcn/32.5.981. [DOI] [PubMed] [Google Scholar]
- 34.Rigaud D, Brondel L, Poupard AT, et al. A randomized trial on the efficacy of a 2-month tube feeding regimen in anorexia nervosa: A 1-year follow-up study. Clin Nutr. 2007;26:421–9. doi: 10.1016/j.clnu.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Gentile MG, Pastorelli P, Ciceri R, et al. Specialized refeeding treatment for anorexia nervosa patients suffering from extreme undernutrition. Clin Nutr. 2010;29:627–32. doi: 10.1016/j.clnu.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Robb AS, Silber TJ, Orrell-Valente JK, et al. Supplemental nocturnal nasogastric refeeding for better short-term outcome in hospitalized adolescent girls with anorexia nervosa. Am J Psychiatry. 2002;159:1347–53. doi: 10.1176/appi.ajp.159.8.1347. [DOI] [PubMed] [Google Scholar]
- 37.Kohn MR, Madden S, Clarke SD. Refeeding in anorexia nervosa: Increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr Opin Pediatr. 2011;23:390–4. doi: 10.1097/MOP.0b013e3283487591. [DOI] [PubMed] [Google Scholar]
- 38.Steinglass JE, Sysko R, Mayer L, et al. Pre-meal anxiety and food intake in anorexia nervosa. Appetite. 2010;55:214–8. doi: 10.1016/j.appet.2010.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigaud D, Verges B, Colas-Linhart N, et al. Hormonal and psychological factors linked to the increased thermic effect of food in malnourished fasting anorexia nervosa. J Clin Endocrinol Metab. 2007;92:1623–9. doi: 10.1210/jc.2006-1319. [DOI] [PubMed] [Google Scholar]
- 40.Katzman DK. Refeeding hospitalized adolescents with anorexia nervosa: Is “start low, advance slow” urban legend or evidence based? J Adolesc Health. 2012;50:1–2. doi: 10.1016/j.jadohealth.2011.10.003. [DOI] [PubMed] [Google Scholar]