Abstract
Background
Korean ginseng is an ethnopharmacologically valuable herbal plant with various biological properties including anticancer, antiatherosclerosis, antidiabetic, and anti-inflammatory activities. Since there is currently no drug or therapeutic remedy derived from Korean ginseng, we developed a ginsenoside-enriched fraction (AP-SF) for prevention of various inflammatory symptoms.
Methods
The anti-inflammatory efficacy of AP-SF was tested under in vitro inflammatory conditions including nitric oxide (NO) production and inflammatory gene expression. The molecular events of inflammatory responses were explored by immunoblot analysis.
Results
AP-SF led to a significant suppression of NO production compared with a conventional Korean ginseng saponin fraction, induced by both lipopolysaccharide and zymosan A. Interestingly, AP-SF strongly downregulated the mRNA levels of genes for inducible NO synthase, tumor necrosis factor-α, and cyclooxygenase) without affecting cell viability. In agreement with these observations, AP-SF blocked the nuclear translocation of c-Jun at 2 h and also reduced phosphorylation of p38, c-Jun N-terminal kinase, and TAK-1, all of which are important for c-Jun translocation.
Conclusion
Our results suggest that AP-SF inhibits activation of c-Jun-dependent inflammatory events. Thus, AP-SF may be useful as a novel anti-inflammatory remedy.
Keywords: anti-inflammatory activity, AP-SF, c-Jun, Korean ginseng, Panax ginseng Meyer
1. Introduction
There is an increasing appreciation for the role of sustained inflammation in the development of a number of serious diseases such as cancer, diabetes, atherosclerosis, and skin disorders [1–3]. As such, many studies have focused on understanding inflammatory processes and their role in disease progression [4]. Inflammatory responses are primarily mediated by innate immune cells such as macrophages, dendritic cells, and Langerhans skin cells [5]. In particular, these cells play a critical role in protecting the body from various infectious conditions. Under such conditions, these cells produce a number of inflammatory mediators such as nitric oxide (NO) and prostaglandin E2, as well as soluble factors such as cytokines [e.g., tumor necrosis factor (TNF)-α] and chemokines [6,7]. Secretion of these molecules requires a complicated signaling cascade triggered by an interaction between surface receptors (e.g., toll-like receptors) in macrophages and ligands [e.g., lipopolysaccharide (LPS), zymosan A (ZyM A), and polynucleotides] derived from bacteria, fungi, and viruses [8]. The resulting biochemical interactions amplify cellular signaling cascades managed by mitogen activated protein kinases (p38, ERK, and c-Jun N-terminal kinase [JNK]) and protein tyrosine kinases (Src and Syk) to induce translocation of transcription factors including nuclear factor (NF)-κB (p50 and p65), activator protein, c-Fos, c-Jun, and activating transcription factor 2, and interferon regulatory transcription factor in order to increase transcriptional levels of inflammatory genes [9–11]. Due to the pathophysiological action of inflammation in humans, there is a need to develop safe and effective drugs to attenuate inflammatory responses by targeting various biochemical processes.
Korean ginseng (KG, a root of Panax ginseng Meyer, Araliaceae) is a representative herbal plant ethnopharmacologically prescribed in Korea [12,13]. The major active components of KG are well known, comprising protopanaxadiol-type and protopanaxatriol-type ginsenosides and acid polysaccharides [14,15]. These components contribute to various pharmacological activities of ginseng such as modulation of immune responses, normalization of brain functions, increased activity of the antioxidative system, and attenuation of skin troubles [15,16]. As mentioned above, the role of ginseng as an anti-inflammatory remedy has been also proposed by several reports. Red ginseng saponin fractions enriched with protopanaxadiol-type saponins were shown to suppress macrophage-mediated inflammatory responses [17]. In addition, ginsenoside (G)-Rb1, G-Rb2, and G-Rd were found to have striking anti-inflammatory activities [18]. Nonetheless, no ginseng-derived components have been developed as anti-inflammatory drugs, although Artemisia asiatica, a representative Korean herb, has been successfully developed as a specialized anti-gastritis drug. A critical reason for considering Artemisia asiatica for drug development over ginseng-derived components was because of its superior anti-inflammatory activities. Indeed, ethanol extracts of Artemisia asiatica (At-EE) strongly inhibit NO production, with an IC50 value of 44.1 μg/mL in LPS-treated RAW264.7 cells, which is significantly lower than those of ginseng-derived components [19]. By contrast, water extracts of Korean Red Ginseng are nine times less potent than At-EE [20]. Nevertheless, we firmly believe that ginseng-derived components may be just as effective as At-EE when considering their ability to modulate biochemical processes. Using phytochemical preparation techniques, we developed concentrated preparations of ginsenosides and then evaluated the anti-inflammatory strengths of these preparations on NO production, modulation of inflammatory gene expression, and inflammatory signaling cascades.
2. Materials and methods
2.1. Materials
Standard ginsenosides (G-Rg1, G-Re, G-Rb1, G-Rc, G-Rb2, and G-Rd) were purchased from Ambo Institute (Daejeon, Korea). Nω-Nitro- l-arginine methyl ester hydrochloride (l-NAME), (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and lipopolysaccharide (LPS, Escherichia coli 0111:B4) were purchased from Sigma Chemical Co. (St Louis, MO, USA). RAW264.7 cells, a BALB/c-derived murine macrophage cell line (ATCC No.: TIB-71), were obtained from American Tissue Culture Collection (ATCC, Rockville, MD, USA). Fetal bovine serum and RPMI1640 were purchased from GIBCO (Grand Island, NY, USA), and phospho-specific or total antibodies to c-Jun, c-Fos, ERK, p38, JNK, MKK3/6, TAK1, lamin A/C, and β-actin were purchased from Cell Signaling (Beverly, MA, USA). All other chemicals were purchased from Sigma Chemical Co.
2.2. Preparation of a ginsenoside-enriched fraction (AP-SF)
Dried and crushed roots of Panax ginseng (300 g) were extracted in 70% aqueous methanol at room temperature (25°C) for 2 d. The extracts were concentrated under reduced pressure at 40°C and then partitioned between petroleum ether, ethyl acetate, and n-butanol. The n-butanol fractions were further concentrated under reduced pressure at 40°C. The resulting residues were subjected to a HP-20 resin column and eluted with gradient aqueous methanol (0%, 30%, and 100%, successively). Solvent was removed from the 100% methanol fractions and analyzed by high performance liquid chromatography (HPLC). As a control, a Korean ginseng saponin fraction (KG-SF) was prepared by concentration of a 70% aqueous methanol extract.
2.3. HPLC analysis
HPLC analysis of ginsenosides in the prepared AP-SF and KG-SF was carried out on a Waters HPLC system equipped with a UV detector (Waters 2487; Waters, Milford, MA, USA), as reported previously [21,22]. Separations were performed on a Sun fire C18 column (3.5 μm, 4.6 mm × 150 mm; Waters). The elution conditions were as follows: solvent A, water; solvent B, acetonitrile; gradient, 0–22 min (18% B), 22–32 min (18–30% B), and 32–60 min (30–50% B). The flow rate was 1 mL/min, with detection at wavelength 203 nm. A total of 20 μL of AP-SF or KG-SF solutions was injected, and peaks were identified by comparing retention times with those of reference ginsenosides.
2.4. AP-SF and KG-SF treatments
Stock solutions (10 mg/mL) of AP-SF and KG-SF were prepared in dimethylsulfoxide (DMSO) and diluted to 0–100 μg/mL with media for in vitro cellular assays.
2.5. Cell culture
RAW 264.7 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, glutamine, and antibiotics (penicillin and streptomycin) at 37°C in a 5% CO2 atmosphere. For experiments, cells were detached with a cell scraper. Under the cell density conditions used in experiments (2 × 106 cells/mL), the proportion of dead cells was < 1% according to Trypan blue dye exclusion tests.
2.6. NO production
After preincubation for 18 h, RAW264.7 cells (1 × 106 cells/mL) were pretreated with AP-SF or KG-SF (0–100 μg/mL) or a standard compound (l-NAME) and incubated with LPS or ZyM A (1 μg/mL) for 24 h. The inhibitory effects of these components on NO production were determined by analyzing NO level quantified with Griess reagent, as described previously [23,24].
2.7. Cell viability test
After preincubation for 18 h, AP-SF or KG-SF (0–100 μg/mL) was added to RAW264.7 cells (1 × 106 cells/mL), followed by incubation for 24 h. The cytotoxic effects of AP-SF or KG-SF were evaluated by MTT assay, as described previously [25,26].
2.8. mRNA analysis by reverse transcriptase–polymerase chain reaction
To evaluate cytokine mRNA expression levels, RAW264.7 cells were pretreated with AP-SF (0–50 μg/mL) for 1 h and incubated with LPS (1 μg/mL) or ZyM A (400 μg/mL) for 6 h. Total RNA was isolated with TRIzol Reagent (Gibco BRL) according to the manufacturer's instructions and stored at −70°C until use. Total RNA from LPS- or ZyM A-treated RAW264.7 cells was prepared by adding TRIzol Reagent (Gibco BRL), as per the manufacturer's protocol. Semiquantitative reverse transcriptase–polymerase chain reaction reactions were conducted using MuLV reverse transcriptase as described previously [27]. The primers (Bioneer, Daejeon, Korea) described in Table 1 for inducible NO synthase (iNOS), cyclooxygenase (COX)-2, TNF-α, and glyceraldehyde 3-phosphate dehydrogenase detection were used as previously reported [28].
Table 1.
Primers used for reverse transcriptase–polymerase chain reaction
| Gene name | Sequence (5′–3′) | |
|---|---|---|
| iNOS | F | CCCTTCCGAAGTTTCTGGCAGCAG |
| R | GGCTGTCAGAGCCTCGTGGCTTTGG | |
| COX-2 | F | CACTACATCCTGACCCACTT |
| R | ATGCTCCTGCTTGAGTATGT | |
| TNF-α | F | TGCCTATGTCTCAGCCTCTTC |
| R | GAGGCCATTTGGGAACTTCT | |
| GAPDH | F | CACTCACGGCAAATTCAACGGCA |
| R | GACTCCACGACATACTCAGCAC |
COX-2, cyclooxygenase-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; iNOS, inducible NO synthase; TNF-α, tumor necrosis factor-α
2.9. Preparation of total lysates and nuclear extracts for immunoblotting analysis
RAW264.7 cells (5 × 106 cells/mL) were washed three times in cold PBS with 1 mM sodium orthovanadate and then lysed using a sonicator (Thermo Fisher Scientific, Waltham, MA, USA) or a Tissuemizer (Qiagen, Germantown, MD, USA) in lysis buffer [29] for 30 min with rotation at 4°C. Lysates were clarified by centrifugation at 16,000 × g for 10 min at 4°C and stored at −20°C until use. Nuclear fractions were prepared with RAW264.7 cell-derived lysates in a three-step procedure [30]. After treatment, cells were collected with a rubber policeman, washed once with PBS, and lysed in 500 μL of lysis buffer [31] on ice for 4 min. Lysates were centrifuged at 19,326 × g for 1 min in a microcentrifuge. The resulting pellet (nuclear fraction) was washed once in wash buffer (lysis buffer without Nonidet P-40) and then treated with extraction buffer (lysis buffer containing 500 mM KCl and 10% glycerol). The nuclei/extraction buffer mixture was frozen at −80°C, thawed on ice, and centrifuged at 19,326 × g for 5 min. The resulting supernatant was collected as the nuclear extract. Soluble cell lysates (30 μg/lane) were subjected to immunoblotting. Levels of total or phosphorylated c-Jun, c-Fos, ERK, p38, JNK, MKK3/6, lamin A/C, and β-actin were visualized as previously described [32].
2.10. Statistical analysis
In vitro experiments in this paper are presented as the mean ± standard error of three different experiments each performed using four samples. For statistical comparisons, results were analyzed using analysis of variance with Scheffe's post hoc and Kruskal–Wallis/Mann–Whitney tests. A p value < 0.05 was considered statistically significant. All statistical tests were performed using SPSS (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
Inflammation is well known to cause various diseases such as cancer, diabetes, and atherosclerosis [33]. In addition, inflammatory conditions of the skin and hair leading to skin troubles such as wrinkling and hair loss can be treated with anti-inflammatory biomaterials applied to cosmetic preparations for the purpose of antiaging, skin-protection, and hair-loss prevention [34]. Recently, our group developed a new ginsenoside-enriched preparation (AP-SF) with a higher ginsenoside content and reported that this fraction exhibits increased antioxidative and hair growth-stimulating activities. In this study, we aimed to determine whether AP-SF is able to strongly suppress various in vitro inflammatory responses.
First, in order to identify a noncytotoxic dose range of AP-SF, RAW264.7 cell viability was analyzed after exposure to AP-SF. As shown in Fig. 1A, the AP-SF fraction did not show any significant suppression of cell viability up to 100 μg/mL. Similarly, cell viability was not significantly affected by exposure to KG-SF. To confirm the phytochemical profile of AP-SF, its specific ginsenoside contents were evaluated. As shown in Fig. 1B, G-Rg1, G-Re, G-Rb1, G-Rc, G-Rb2, and G-Rd were detected as the major ginsenosides in AP-SF. Further, the levels of diol-type ginsenosides in AP-SF were significantly, up to 50-fold, higher than those of KG-SF.
Fig. 1.
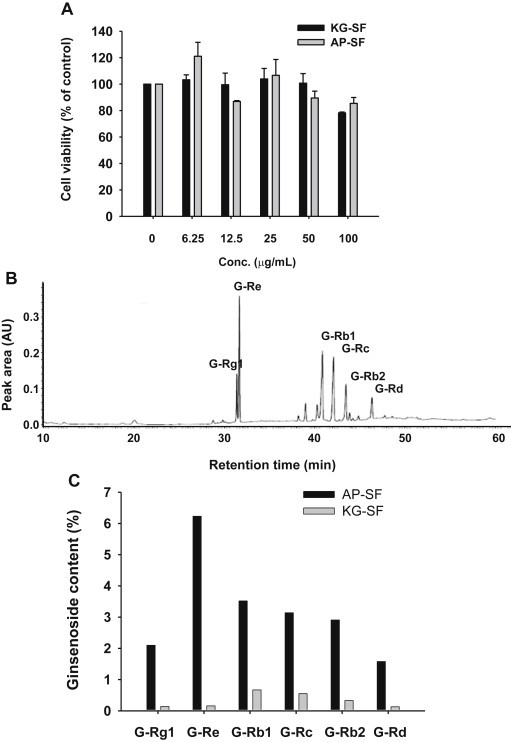
Cell viability and phytochemical profile of AP-SF. (A) The viability of RAW264.7 cells treated with either AP-SF or KG-SF in the absence of lipopolysaccharide was determined by MTT assay. (B) Phytochemical characteristics of ginsenosides in AP-SF or KG-SF were analyzed by HPLC. (C) The ginsenoside contents of AP-SF and KG-SF. AP, activator protein; HPLC, high performance liquid chromatography; KG, Korean ginseng; MTT, (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SF, saponin fraction.
We next examined the immunomodulatory effects of AP-SF by comparison with KG-SF. To this end, we employed in vitro anti-inflammatory assays, as we have shown previously that Korean Red Ginseng water extract (KRG-WE) containing saponin fractions and protopanaxadiol-type saponin fractions exhibit anti-inflammatory effects in LPS-treated RAW264.7 cells [20]. As expected, KG-SF dose-dependently inhibited NO production in LPS-treated RAW264.7 cells (Fig. 2A). Interestingly, AP-SF suppressed NO production by two-fold compared to KG-SF at the same concentration. Consistent with this observation, 50 μg/mL of AP-SF produced a similar degree of NO inhibition as KG-SF at 100 μg/mL (Fig. 2A). l-NAME inhibited LPS-induced NO production in a dose-dependent manner (Fig. 2C), as reported previously [19], indicating that our experimental conditions were well established. Furthermore, while KG-SF very strongly suppressed NO release induced by ZyM A, a RAW264.7 glucan with repeating glucose units connected by β-1,3-glycosidic linkages [35], AP-SF produced more potent suppression (Fig. 2B) according to IC50 values (Table 2). Together, these data indicate that Korean ginseng-derived saponin components strongly block fungus-derived inflammatory responses.
Fig. 2.
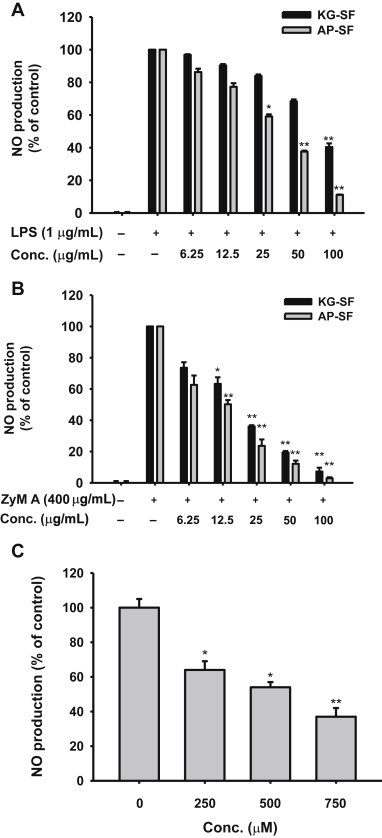
Effect of AP-SF on NO production in activated RAW264.7 cells. (A, B, and C) Levels of NO were determined from culture supernatants of RAW264.7 cells treated with (A and C) LPS (1 μg/mL) or (B) ZyM A (400 μg/mL) in the presence or absence of (A) AP-SF or KG-SF or (C) l-NAME for 24 h. *p < 0.05 and **p < 0.01 compared to the control. AP, activator protein; KG, Korean ginseng; l-NAME, Nω-Nitro-l-arginine methyl ester hydrochloride; LPS, lipopolysaccharide; NO, nitric oxide; SF, saponin fraction; ZyM A, zymosan A.
Table 2.
Inhibitory activity of AP-SF and KG-SF on nitric oxide production
| Stimulus | IC50 value (μg/mL) |
|
|---|---|---|
| AP-SF | KG-SF | |
| Lipopolysaccharide | 45.1 ± 0.7 | 82.6 ± 2.4 |
| Zymosan A | 15.3 ± 1.0 | 23.5 ± 0.2 |
It has been reported that fungus-derived immunogens can cause cellular and tissue damage, as well as various inflammatory diseases [36]. Candidiasis, codermatophytes, aspergillosis, and pneumonia are representative diseases caused by fungal infections [37,38]. In addition, various skin troubles as well as fungal-initiated hair loss can be caused by fungal infections of the skin and hair [39]. Considering the prevalence of these conditions and the strong inhibitory potential of AP-SF against inflammatory responses induced by the fungus-derived immunogen, Zym A (Fig. 2B), we hypothesized that AP-SF might be useful for treating fungal-derived dermatitis and infectious diseases as well as fungal-induced hair loss.
Because AP-SF exhibited strong NO inhibitory activity in activated RAW264.7 cells, we further characterized its anti-inflammatory effects by measuring mRNA levels of inflammatory genes. As shown in Fig. 3A, treatment with AP-SF strongly decreased mRNA levels of iNOS, COX-2, and TNF-α at 25 μg/mL and 50 μg/mL. Similar to the NO inhibitory activity observed in ZyM A-treated RAW264.7 cells, AP-SF strongly inhibited mRNA expression of inflammatory genes in cells treated with ZyM A (Fig. 3B). These results clearly indicate that AP-SF-mediated inhibition of NO production is facilitated at the level of transcription. Similar anti-inflammatory patterns were also confirmed by treatment of KRG-WE following exposure to LPS [20]. It has been also reported that compound K is able to reduce ZyM A-induced cytokine gene expression [40]. These results suggest that ginseng-derived components are capable of modulating inflammatory responses by suppressing transcriptional activation of inflammatory genes.
Fig. 3.
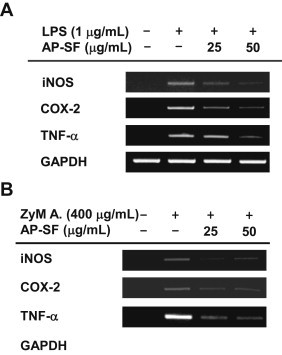
Effect of AP-SF on the transcriptional activation of inflammatory genes in activated RAW264.7 cells. (A and B) RAW264.7 cells (5 × 106 cells/mL) were incubated with LPS; 1 μg/mL) or ZyM A; 400 μg/mL) in the presence or absence of AP-SF for 6 h. The mRNA levels of iNOS, TNF-α, and COX-2 were determined by reverse transcriptase–polymerase chain reaction. AP, activator protein; COX-2, cyclooxygenase-2; iNOS, inducible NO synthase, LPS, lipopolysaccharide; SF, saponin fraction; TNF-α, tumor necrosis factor-α; ZyM A, zymosan A.
Because AP-SF was shown to block the transcriptional activation of inflammatory genes, we next aimed to determine its targeted transcription factors. Since it was previously reported that the translocation of activator protein-1 is suppressed by KRG-WE and protopanaxadiol type saponin fraction (PPD-SF) [20], we measured levels of c-Jun and c-Fos following treatment with AP-SF. As shown in Fig. 4A, the level of c-Jun in nuclear fractions at 120 min was remarkably reduced by AP-SF (50 μg/mL). In agreement with this result, the phosphorylation of p38, an upstream signaling enzyme that activates c-Jun [41], was also suppressed by AP-SF at 30 min and 45 min (Fig. 4B), indicating that the inhibition of p38 phosphorylation at these times might contribute to the suppression of c-Jun translocation. Interestingly, the phosphorylation of mitogen-activated protein kinase kinase 3/6 (MKK3/6), an upstream enzyme that phosphorylates p38 [42], at 30 min and 45 min was also inhibited by AP-SF (Fig. 4C), implying that p38 is not directly targeted by this extract. Indeed, the phosphorylation of transforming growth factor-beta–activated kinase 1 (TAK1), an upstream kinase that phosphorylates MKK3/6 [42], was also remarkably suppressed by AP-SF at 2 min, 3 min, and 5 min. These results clearly suggest that AP-SF is able to modulate c-Jun translocation by suppressing TAK1/MKK3/6-related signaling.
Fig. 4.
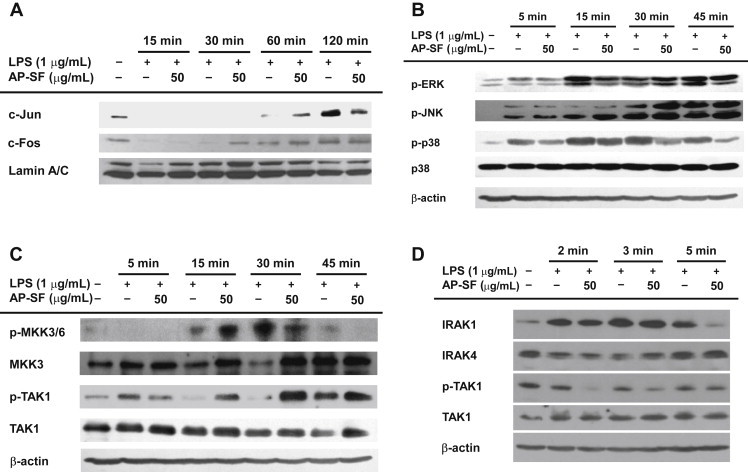
Effect of AP-SF on activator protein-1 translocation and its upstream regulatory signaling pathways. (A) The total protein levels of c-Fos and c-Jun were identified by immunoblotting analysis of lipopolysaccharide (LPS)-treated RAW264.7 cell lysates. (B, C, and D) RAW264.7 cells were incubated with LPS (1 μg/mL) in the presence or absence of AP-SF for the indicated times. Total or phospho-levels of ERK, p38, JNK, MKK3/6, MKK3, TAK1, IRAK1, and IRAK4 in whole lysates were determined by immunoblotting analysis.
The significance of the TAK1-mediated p38/c-Jun activation pathway in inflammatory responses has been widely demonstrated using pharmacological and molecular biological approaches [43]. Specifically, JLU1124 and SB203580, inhibitors of p38, are able to block LPS-induced production of NO, IL-6, and TNF-α and decrease the expression of iNOS and COX-2 in RAW264.7 macrophages [44]. In addition, toll-like receptor 2-mediated autoimmune inflammation in multiple sclerosis and experimental autoimmune encephalomyelitis is known to involve the TAK1 axis [45]. The isoform of p38 is regarded as a novel molecular target to attenuate inflammatory responses, and several pyrindinyl imidazole compounds have been developed and strongly inhibit p38α by diminishing levels of TNF-α and IL-1 [46]. Considering these findings, our observation that AP-SF was able to inhibit the TAK1/p38/c-Jun regulatory loop as summarized in Fig. 5 suggests its potential use as an anti-inflammatory remedy. Importantly, there are currently no preparations or single compounds derived from ginseng or red ginseng that exhibit therapeutic efficacy. Our data, however, have encouraged us to consider whether this preparation can be developed as a special functional food or therapeutic drug. Following additional verification of the immunopharmacological activity of AP-SF, it is our intention to evaluate this preparation using in vivo inflammation models.
Fig. 5.
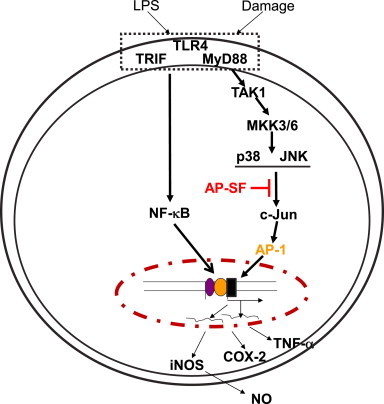
Putative inhibitory pathway of the AP-SF-mediated anti-inflammatory response. TLR4: toll like receptor 4, MyD88: myeloid differentiation primary response protein-88, AP-1: activator protein-1.
Conflicts of interest
The authors declare that there are no conflicts of interests regarding the publication of this paper.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ009544), Rural Development Administration, Republic of Korea (No. PJ009544).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Song Seok Shin, Email: ssshin@amorepacific.com.
Jae Youl Cho, Email: jaecho@skku.edu.
References
- 1.Chiurchiù V., Maccarrone M. Chronic inflammatory disorders and their redox control: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:2605–2641. doi: 10.1089/ars.2010.3547. [DOI] [PubMed] [Google Scholar]
- 2.Przemyslaw L., Boguslaw H.A., Elzbieta S., Malgorzata S.M. ADAM and ADAMTS family proteins and their role in the colorectal cancer etiopathogenesis. BMB Rep. 2013;46:139–150. doi: 10.5483/BMBRep.2013.46.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang D.H., Kang S.W. Targeting cellular antioxidant enzymes for treating atherosclerotic vascular disease. Biomol Ther (Seoul) 2013;21:89–96. doi: 10.4062/biomolther.2013.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park K., Lee S., Lee Y.M. Sphingolipids and antimicrobial peptides: function and roles in atopic dermatitis. Biomol Ther (Seoul) 2013;21:251–257. doi: 10.4062/biomolther.2013.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.W., Kim N.H., Kim J.Y., Park J.H., Shin S.Y., Kwon Y.S., Lee H.J., Kim S.S., Chun W. Aromadendrin inhibits lipopolysaccharide-induced nuclear translocation of NF-kappaB and phosphorylation of JNK in RAW 264.7 macrophage cells. Biomol Ther (Seoul) 2013;21:216–221. doi: 10.4062/biomolther.2013.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sodenkamp J., Behrends J., Förster I., Müller W., Ehlers S., Hölscher C. gp130 on macrophages/granulocytes modulates inflammation during experimental tuberculosis. Eur J Cell Biol. 2011;90:505–514. doi: 10.1016/j.ejcb.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Roberts-Thomson I.C., Fon J., Uylaki W., Cummins A.G., Barry S. Cells, cytokines and inflammatory bowel disease: a clinical perspective. Expert Rev Gastroenterol Hepatol. 2011;5:703–716. doi: 10.1586/egh.11.74. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro-Gomes F.L., Silva M.T., Dosreis G.A. Neutrophils, apoptosis and phagocytic clearance: an innate sequence of cellular responses regulating intramacrophagic parasite infections. Parasitology. 2006;132 doi: 10.1017/S0031182006000862. Suppl.:S61–8. [DOI] [PubMed] [Google Scholar]
- 9.Yu T., Yi Y.S., Yang Y., Oh J., Jeong D., Cho J.Y. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediators Inflamm. 2012;2012:979105. doi: 10.1155/2012/979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byeon S.E., Yi Y.S., Oh J., Yoo B.C., Hong S., Cho J.Y. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012;2012:512926. doi: 10.1155/2012/512926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon J.W., Park B.C., Jung J.G., Jang Y.S., Shin E.C., Park Y.W. The soluble form of the cellular prion protein enhances phagocytic activity and cytokine production by human monocytes via activation of ERK and NF-kappaB. Immune Netw. 2013;13:148–156. doi: 10.4110/in.2013.13.4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H.J., Kim P., Shin C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37:8–29. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqi M.H., Siddiqi M.Z., Ahn S., Kang S., Kim Y.J., Sathishkumar N., Yang D.U., Yang D.C. Ginseng saponins and the treatment of osteoporosis: mini literature review. J Ginseng Res. 2013;37:261–268. doi: 10.5142/jgr.2013.37.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean Red Ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim M.S., Lim H.J., Yang H.J., Lee M.S., Shin B.C., Ernst E. Ginseng for managing menopause symptoms: a systematic review of randomized clinical trials. J Ginseng Res. 2013;37:30–36. doi: 10.5142/jgr.2013.37.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yayeh T., Jung K.H., Jeong H.Y., Park J.H., Song Y.B., Kwak Y.S., Kang H.S., Cho J.Y., Oh J.W., Kim S.K. Korean Red Ginseng saponin fraction downregulates proinflammatory mediators in LPS stimulated RAW264.7 cells and protects mice against endotoxic shock. J Ginseng Res. 2012;36:263–269. doi: 10.5142/jgr.2012.36.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H.A., Kim S., Chang S.H., Hwang H.J., Choi Y.N. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int Immunopharmacol. 2007;7:1286–1291. doi: 10.1016/j.intimp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Jeong D., Yi Y.S., Sung G.H., Yang W.S., Park J.G., Yoon K., Yoon D.H., Song C., Lee Y., Rhee M.H. Anti-inflammatory activities and mechanisms of Artemisia asiatica ethanol extract. J Ethnopharmacol. 2014;152:487–496. doi: 10.1016/j.jep.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y., Yang W.S., Yu T., Sung G.H., Park K.W., Yoon K., Son Y.J., Hwang H., Kwak Y.S., Lee C.M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean Red Ginseng water extract. J Ethnopharmacol. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Kang T.H., Park H.M., Kim Y.B., Kim H., Kim N., Do J.H., Kang C., Cho Y., Kim S.Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Kim I.W., Sun W.S., Yun B.S., Kim N.R., Min D., Kim S.K. Characterizing a full spectrum of physico-chemical properties of (20S)- and (20R)-ginsenoside Rg3 to be proposed as standard reference materials. J Ginseng Res. 2013;37:124–134. doi: 10.5142/jgr.2013.37.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho J.Y., Baik K.U., Jung J.H., Park M.H. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000;398:399–407. doi: 10.1016/s0014-2999(00)00337-x. [DOI] [PubMed] [Google Scholar]
- 24.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 25.Gerlier D., Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 26.Hong Y.J., Yang K.S. Anti-inflammatory activities of crocetin derivatives from processed Gardenia jasminoides. Arch Pharm Res. 2013;36:933–940. doi: 10.1007/s12272-013-0128-0. [DOI] [PubMed] [Google Scholar]
- 27.Hong S., Kim S.H., Rhee M.H., Kim A.R., Jung J.H., Chun T., Yoo E.S., Cho J.Y. In vitro anti-inflammatory and pro-aggregative effects of a lipid compound, petrocortyne A, from marine sponges. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:448–456. doi: 10.1007/s00210-003-0848-7. [DOI] [PubMed] [Google Scholar]
- 28.Lee H.J., Hyun E.A., Yoon W.J., Kim B.H., Rhee M.H., Kang H.K., Cho J.Y., Yoo E.S. In vitro anti-inflammatory and anti-oxidative effects of Cinnamomum camphora extracts. J Ethnopharmacol. 2006;103:208–216. doi: 10.1016/j.jep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Yu T., Ahn H.M., Shen T., Yoon K., Jang H.J., Lee Y.J., Yang H.M., Kim J.H., Kim C., Han M.H. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J Ethnopharmacol. 2011;137:1197–1206. doi: 10.1016/j.jep.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 30.Kim E.H., Lee M.J., Kim I.H., Pyo S., Choi K.T., Rhee D.K. Anti-apoptotic effects of red ginseng on oxidative stress induced by hydrogen peroxide in SK-N-SH cells. J Ginseng Res. 2010;34:138–144. [Google Scholar]
- 31.Lee J.Y., Lee Y.G., Lee J., Yang K.J., Kim A.R., Kim J.Y., Won M.H., Park J., Yoo B.C., Kim S. Akt Cys-310-targeted inhibition by hydroxylated benzene derivatives is tightly linked to their immunosuppressive effects. J Biol Chem. 2010;285:9932–9948. doi: 10.1074/jbc.M109.074872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y., Kim J., Jang S., Oh S. Administration of phytoceramide enhances memory and upregulates the expression of pCREB and BDNF in hippocampus of mice. Biomol Ther (Seoul) 2013;21:229–233. doi: 10.4062/biomolther.2013.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard B.E. Inflammation as the cause of the metabolic syndrome in depression. Mod Trends Pharmacopsychiatri. 2013;28:117–126. doi: 10.1159/000343974. [DOI] [PubMed] [Google Scholar]
- 34.Caterina M.J. TRP channel cannabinoid receptors in skin sensation, homeostasis, and Inflammation. ACS Chem Neurosci. 2014 doi: 10.1021/cn5000919. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann G., Müller H.D., Nicolaus M. The chemical composition of “zymosans” from yeasts. Pharmazie. 1985;40:250–253. [In German] [PubMed] [Google Scholar]
- 36.Miceli M.H., Diaz J.A., Lee S.A. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011;11:142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Yu L. Current status and future perspectives of invasive fungal infections after hematopoietic stem cell transplantation. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19:517–522. [In Chinese] [PubMed] [Google Scholar]
- 38.Quindós G., Eraso E., López-Soria L.M., Ezpeleta G. Invasive fungal disease: conventional or molecular mycological diagnosis? Enferm Infecc Microbiol Clin. 2012;30:560–571. doi: 10.1016/j.eimc.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Mounsey A.L., Reed S.W. Diagnosing and treating hair loss. Am Fam Physician. 2009;80:356–362. [PubMed] [Google Scholar]
- 40.Cuong T.T., Yang C.S., Yuk J.M., Lee H.M., Ko S.R., Cho B.G., Jo E.K. Glucocorticoid receptor agonist compound K regulates Dectin-1-dependent inflammatory signaling through inhibition of reactive oxygen species. Life Sci. 2009;85:625–633. doi: 10.1016/j.lfs.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y., Kim S.C., Yu T., Yi Y.S., Rhee M.H., Sung G.H., Yoo B.C., Cho J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014:352371. doi: 10.1155/2014/352371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Levy R., Hooper S., Wilson R., Paterson H.F., Marshall C.J. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8:1049–1057. doi: 10.1016/s0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Li X.N., Su J., Zhao L., Xiang J.B., Wang W., Liu F., Li H.Y., Zhong J.T., Bai X., Sun L.K. The p38 MAPK inhibitor JLU1124 inhibits the inflammatory response induced by lipopolysaccharide through the MAPK-NF-κB pathway in RAW264.7 macrophages. Int Immunopharmacol. 2013;17:785–792. doi: 10.1016/j.intimp.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Xiao Y., Jin J., Chang M., Nakaya M., Hu H., Zou Q., Zhou X., Brittain G.C., Cheng X., Sun S.C. TPL2 mediates autoimmune inflammation through activation of the TAK1 axis of IL-17 signaling. J Exp Med. 2014;211:1689–1702. doi: 10.1084/jem.20132640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisk M., Gajendragadkar P.R., Maki-Petaja K.M., Wilkinson I.B., Cheriyan J. Therapeutic potential of p38 MAP kinase inhibition in the management of cardiovascular disease. Am J Cardiovasc Drugs. 2014;14:155–165. doi: 10.1007/s40256-014-0063-6. [DOI] [PubMed] [Google Scholar]


