Abstract
Background
Fermentation technology is widely used to alter the effective components of ginseng. This study was carried out to analyze the characteristics and antioxidant activity of ginseng seeds fermented by Bacillus, Lactobacillus, and Pediococcus strains.
Methods
For ginseng seed fermentation, 1% of each strain was inoculated on sterilized ginseng seeds and then incubated at 30°C for 24 h in an incubator.
Results
The total sugar content, acidic polysaccharides, and phenolic compounds, including p-coumaric acid, were higher in extracts of fermented ginseng seeds compared to a nonfermented control, and highest in extracts fermented with B. subtilis KFRI 1127. Fermentation led to higher antioxidant activity. The 2,2′-azine-bis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity was higher in ginseng seeds fermented by Bacillus subtilis than by Lactobacillus and Pediococcus, but Superoxide dismutase (SOD) enzyme activity was higher in ginseng seeds fermented by Lactobacillus and Pediococcus.
Conclusion
Antioxidant activities measured by ABTS and SOD were higher in fermented ginseng seeds compared to nonfermented ginseng seeds. These results may contribute to improving the antioxidant activity and quality of ginseng subjected to fermentation treatments.
Keywords: antioxidant, fermentation strain, ginseng seed, Panax ginseng, phenolic compound
1. Introduction
Ginseng (Panax ginseng Meyer) is a half-shade plant in the Araliaceae family and has been used as a medicinal ingredient in East Asian medicine for centuries. The root is used medicinally and is widely known as a natural health food. The demand for ginseng in pharmaceuticals and functional foods has been increasing, based on the scientific and clinical evidence of its pharmacological efficacy [1,2].
Recent studies have examined the possibility of increasing active ingredients and physiological activities by fermenting ginseng with lactic acid bacteria and Bacillus subtilis [3–7]. In particular, functional fermentation products may be simply produced by skipping the soaking process during solid-state fermentation using B. subtilis [7]. Moreover, lactic acid bacteria such as lactobacilli and bifidobacteria have been reported to produce lactic acid by fermenting sugars as probiotics, promoting the growth of beneficial bacteria in the human intestine [8].
Thus, fermented ginseng provides live beneficial microbes as probiotics as well as more useful saponins than those found in fresh ginseng by transforming the structure of ginsenosides through the degradation of sugars with glycoside structure [9]. In addition, fermented ginseng improves specific compounds through fermentation with microbes and enzymes and shows antidiabetic [10] and antioxidative effects [11]. Fermented ginseng is popular as a functional food after being processed for easier digestion and absorption [12].
Ginseng fruit has recently been reported to be effective for improving blood circulation and skin tone [13] and delaying skin aging [14]. Cosmetics taking advantage of these properties are available commercially. However, studies of ginseng seeds have been conducted mainly in the context of analyzing saponin [15–18], phytosterol [19], and fatty acids [20,21], and few address the development of ginseng seed products using fermentation or processing.
In this study, total sugar content, acidic polysaccharides, total phenol content, and phenolic compounds, and physicochemical properties such as antioxidative activities were investigated in ginseng seeds fermented using Bacillus, Lactobacillus, and Pediococcus strains.
2. Materials and methods
2.1. Materials
The ginseng seeds used were from 4-year-old ginseng produced in 2012 at the Geumsan Ginseng Market in Chungcheongnam-do (Geumsan, South Korea). A total of 10 standard substances were used in the phenolic compound analysis: maltol, coumaric acid, cinnamic acid, salicylic acid, vanillic acid, syringic acid, ferulic acid, gentisic acid, Phenol standards, and caffeic acid (Sigma Aldrich, St. Louis, MO, USA). Hydroxyl benzoic acid was purchased from Junsei (Tokyo, Japan).
2.2. Strains used for ginseng seed fermentation
The strains used for ginseng seed fermentation were gram-positive Lactobacillus gasseri KCTC 3162, Pediococcus pentosaceus LY011, B. subtilis KFRI 1124, and B. subtilis KFRI 1127 obtained from the Korean Collection for Type Culture of KRIBB and KFRI. Bacillus strains were inoculated on TS broth, and Lactobacillus and Pediococcus strains were inoculated on MRS broth. All samples were then incubated at 30°C for 24 h in an incubator to be used as fermentation strains.
2.3. Fermentation and inoculation of ginseng seed
For ginseng seed fermentation, 1% of each strain was inoculated on sterilized ginseng seeds and mixed well with a sterilized spatula, and then incubated at 30°C for 24 h in an incubator. Fermented ginseng seeds were then freeze-dried until analysis.
2.4. Total sugar and acidic polysaccharide contents
Approximately 2 g of powder sample was mixed with 50 mL of distilled water, and then heat-extracted at 80°C using a reflux condenser and transferred to a 100-mL volumetric flask for constant volume. Part of the sample was collected and centrifuged at 4°C, 10,280 ×g for 20 min, and 5 mL of the supernatant was subsequently filtered through a 0.45-μm membrane filter for use as samples for the analysis of total sugar content. For acidic polysaccharide analysis, 5 mL of the supernatant obtained after extraction and centrifugation was mixed with 20 mL of cold ethanol to form acidic polysaccharides. The acidic polysaccharide precipitate obtained by discarding the supernatant from centrifugation at 4°C, 10,280 ×g for 20 min was dissolved in 5 mL distilled water and filtered through a 0.45-μm membrane filter for use as samples for the analysis of acidic polysaccharide content. The total sugar content was quantified by a phenol–sulfuric acid method using d-glucose [22]. The acidic polysaccharide content was quantified by a carbazole–sulfuric acid method using β-d-galacturonic acid [23].
2.5. Total phenolic compound contents
Approximately 1 g of powder sample was mixed with 25 mL of 80% MeOH. The soluble components were then extracted in 80°C hot water using a reflux condenser. The extract was filtered through filter paper No. 2 (Whatman, Maidstone, England) and vacuum-concentrated and subsequently dissolved in 10 mL of distilled water and filtered through a 0.45-μm membrane filter (Whatman), for use as samples for the analysis of total phenolic compound content. The total phenolic compound content was colorimetrically determined according to the Folin–Ciocalteu method using gallic acid [24].
2.6. Phenolic compound analysis
The phenolic compounds in ginseng seed oil were analyzed with high-performance liquid chromatography (PU-980; Jasco, Tokyo, Japan) under the following analytical conditions: a Waters C-18 column (5.0 μm, 4.6 mm × 250 mm; Waters, Milford, MA, USA) was used, with a mobile phase of water with 2% acetic acid (solvent A) and 50% acetonitrile with 0.5% acetic acid (solvent B) or (solvent A) with 2% acetic acid and 50% acetonitrile (solvent B) with 0.5% acetic acid; samples were developed from an initial 100% of A solvent to a 45% gradient after 70 min with a speed of 0.8 mL/min for 80 min. The sample was detected at 280 nm with a UV detector (MD-2010; Jasco) using a 20-μL sample for injection. Each 2 g sample was dissolved in 10 mL n-hexane, and 20 mL of 80% methanol was added to extract the phenolic compounds. Finally, 10 mL of n-hexane was added to the extract to eliminate the remaining lipid constituents, and the solvent in the 80% methanol layer was evaporated completely using a vacuum evaporator. The concentrated extracts were diluted with methanol to 10 mg/mL, and filtered through a 0.45-μm syringe filter (Whatman) for further analysis.
2.7. 2,2′-Azine-bis-(3-ethylbenzothiazoline-6-sulfonic acid) radical-scavenging activity
The 2,2′-azine-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical-scavenging activity of fermented ginseng seed extract was measured using a slightly modified method of van den Berg et al [25]. To 0.1M phosphate buffered saline (pH 7.4), 2.5mM ABTS and 1.0mM AAPH [2,2′-azobis(2-mehtylpropionamidine)dihydrochloride] were added and maintained for 12 min in a dark room at 68°C, then quickly cooled to generate an ABTS radical solution. Each 20 μL of fermented ginseng seed extract dissolved in phosphate buffered saline was added to 980 μL of ABTS radical solution and incubated for 10 min at 37°C, whereupon absorbance was measured at 734 nm.
2.8. Antioxidant enzyme activity
The superoxide anion scavenging activity of plant extracts was determined with the WST (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) reduction method, using the Superoxide dismutase (SOD) assay Kit-WST (Dojindo Lab, Kumamoto, Japan). In this method, O2•− reduces WST-1 to produce the yellow formazan, which is measured spectrophotometrically at 450 nm. Antioxidants are able to inhibit yellow WST formation. All measurements were done in triplicate. The percentage of inhibition of superoxide radicals was calculated using the above formula.
2.9. Statistical analysis
All expressed values are the means of triplicate determinations. All statistical analyses were performed using the SAS version 9.3 (SAS Institute, Cary, NC, USA). The statistical significance of differences was determined using Duncan's multiple tests and one-way analysis of variance, evaluating significant differences at p < 0.05. All data are at the 5% significance level and are reported as means ± standard deviation.
3. Results and discussion
3.1. Total sugar content
Table 1 shows that the total sugar content in ginseng seeds fermented by B. subtilis KFRI 1124 (35.41 mg/g) and KFRI 1127 (45.32 mg/g), P. pentosaceus LY 011 (37.63 mg/g), and L. gasseri KCTC 3162 (40.14 mg/g) was higher than that of the nonfermentation control group (31.94 mg/g). According to Gui and Ryu [26], the total sugar content of white ginseng treated with extrusion was 297.0 mg/g and was different from that of ginseng seed, which is attributed to the varying total sugar content dependent on the ginseng part. In that same report, the total sugar content increased when pressure or heat was applied to white ginseng; this was similar to our results on ginseng seeds fermented after sterilization. In particular, fermentation with B. subtilis KFRI 1127 led to the highest total sugar content. These results imply that the fermentation capability of B. subtilis strains to produce sugars by degrading carbohydrates is better than that of the other bacteria tested.
Table 1.
Total sugar, acidic polysaccharides and total phenolic compound contents of fermented ginseng seed on the different strains
| Strain | Total sugar (mg/g) | Acidic polysaccharides (mg/g) | Total phenolic compound (mg/g) |
|---|---|---|---|
| Control | 31.94 ± 2.15d | 2.15 ± 0.17d | 0.81 ± 0.02c |
| Bacillus subtilis KFRI 1124 | 35.41 ± 0.54cd | 5.72 ± 0.18b | 1.28 ± 0.01a |
| Bacillus subtilis KFRI 1127 | 45.32 ± 2.67a | 8.74 ± 0.12a | 1.31 ± 0.04a |
| Pediococcus pentosaceus LY 011 | 37.63 ± 3.78bc | 5.05 ± 0.06c | 1.08 ± 0.02b |
| Lactobacillus gasseri KCTC 3162 | 40.14 ± 1.85b | 5.08 ± 0.05c | 1.05 ± 0.03b |
Data are presented as mean ± SD in triplicate determinations. Means with the same letters in each column are not significantly different at p < 0.05 using Duncan's multiple range test
3.2. Acidic polysaccharides contents
Table 1 shows that the acidic polysaccharide content of extracts from fermented ginseng seeds was 2.5–4 times higher than that of the nonfermentation control group (2.15 mg/g). The acidic polysaccharide content in extracts fermented by B. subtilis KFRI 1127 (8.74 mg/g) was higher compared with that in extracts fermented by B. subtilis KFRI 1124 (5.72 mg/g), P. pentosaceus LY 011 (5.05 mg/g), and L. gasseri KCTC 3162 (5.08 mg/g). These results suggest that acidic polysaccharides become soluble during heat treatments such as steaming, and they have been reported to occur in generally higher content in steam-treated red ginseng compared to fresh ginseng and white ginseng [23].
3.3. Total phenolic compound contents
Table 1 shows that the total phenolic compound contents of extracts from seeds fermented with B. subtilis KFRI 1127 (1.31 mg/g), B. subtilis KFRI 1124 (1.28 mg/g), P. pentosaceus LY 011 (1.08 mg/g), and L. gasseri KCTC 3162 (1.05 mg/g), were higher than those of the nonfermented control group (0.81 mg/g). The results suggest that total phenolic compound content differed according to the fermentation strains used. Polyphenols in antioxidants have been reported to increase with high-temperature treatments along with antioxidative effects [27,28]. In other words, the sterilization process during fermentation is considered to be responsible in part for the increase in total phenolic compounds in ginseng seeds.
3.4. Phenolic compound component
Ten kinds of phenolic compounds are found in ginseng: maltol, p-coumaric acid, trans-cinnamic acid, salicylic acid, vanillic acid, syringic acid, ferulic acid, gentisic acid, caffeic acid, and p-hydroxy benzoic acid. Table 2 shows the phenolic compounds of fermented ginseng seed extracts, which were found in significantly higher amounts than those in the nonfermented control group (6.9 μg/100 g). The amount of p-coumaric acid in fermented ginseng seeds was approximately 5–6 times higher than that of the nonfermented control group, and P. pentosaceus LY 011 yielded the highest amount (44.8 μg/100 g). These results were similar to those reported by Jung et al [29], i.e., p-coumaric acid, which was not detected in fresh ginseng, was produced at a ratio of 59.0 μg/100 g in steamed red ginseng; this is considered to be effected by the sterilization pretreatment process of ginseng seed prior to the inoculation of the strains.
Table 2.
Phenolic compound of fermented ginseng seed on the different strains
| Phenolic compound (μg/100 g) | Fermentation strain |
||||
|---|---|---|---|---|---|
| Control |
Bacillus subtilis KFRI 1124 |
Bacillus subtilis KFRI 1127 |
Pediococcus pentosaceus LY 011 |
Lactobacillus gasseri KCTC 3162 |
|
| Maltol | ND | ND | ND | ND | ND |
| p-Hydroxy benzoic acid | ND | ND | ND | ND | ND |
| Gentisic acid | ND | ND | ND | ND | ND |
| Vanillic acid + caffeic acid | ND | ND | ND | ND | ND |
| Syringic acid | ND | ND | ND | ND | ND |
| p-Coumaric acid | 6.9 | 44.4 | 36.9 | 44.8 | 38.1 |
| Ferulic acid | ND | ND | ND | ND | ND |
| Salicylic acid | ND | ND | ND | ND | ND |
| trans-Cinnamic acid | ND | ND | ND | ND | ND |
ND, not detected
3.5. ABTS radical scavenging activity
The ABTS radical scavenging activity of fermented ginseng seed extracts was measured at 1 ppm, 10 ppm, and 100 ppm. Fig. 1 shows that the activity was not significantly different between treatment groups at 1 ppm and 10 ppm but was significantly different at 100 ppm compared to the nonfermented control group. The ABTS radical scavenging activity in samples fermented by B. subtilis KFRI 1124 (31.6%) and KFRI 1127 (32.7%), in P. pentosaceus LY 011 (27.9%) and L. gasseri KCTC 3162 (25.5%) were all higher than that in the control group (15.2%), showing that fermentation increased ABTS radical scavenging activity. In particular, fermentation by B. subtilis strains led to higher ABTS radical scavenging activity than fermentation by Pediococcus or Lactobacillus strains; especially, fermentation by B. subtilis KFRI 1127 led to the highest ABTS radical scavenging activity observed. These results suggest that extracts may act as free radical scavengers and contribute hydrogen from the phenolic hydroxyl groups themselves, thereby forming stable free radicals that do not initiate or spread further oxidation of lipids [30,31]. Similar results were reported by Pyo et al [32] for lactic acid bacteria and bifidobacteria, wherein a 47% increase in antioxidative activity and a 38% increase in lactic acid bacteria and bifidobacteria, respectively, were noted compared to a control.
Fig. 1.
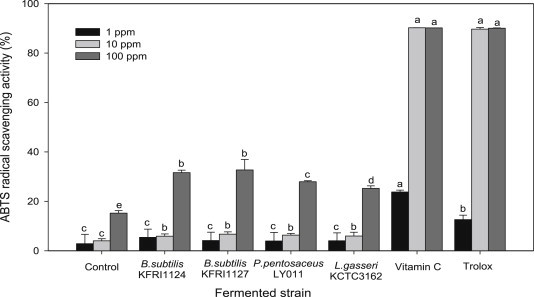
ABTS radical scavenging activity of fermentation ginseng seed on the different strains. ABTS, 2,2′-azine-bis-(3-ethylbenzothiazoline-6-sulfonic acid).
3.6. Antioxidant enzyme activity
The SOD enzyme activity of fermented ginseng seed extracts was measured at concentrations of 1 ppm, 10 ppm, 100 ppm, and 1,000 ppm, and the results are presented in Fig. 2. The SOD enzyme activity of fermented ginseng seeds was higher at all concentrations compared to that of nonfermented ginseng seed extracts. The activity was significantly different at 1,000 ppm depending on the fermentation strain. The SOD enzyme activity of ginseng seed extracts fermented by B. subtilis KFRI 1124 (39.9%) and KFRI 1127 (34.2%), P. pentosaceus LY 011 (41.7%), and L. gasseri KCTC 3162 (41.8%) were all higher than that of the control group (22.2%). Fermentation by Lactobacillus and Pediococcus strains led to higher SOD enzyme activity than that of the Bacillus strains. Jeon et al [4] reported that the SOD enzyme activity was greatly increased when ginseng fruit extract was fermented with lactic acid bacteria; this was similar to our results with fermented ginseng seed, which showed that fermentation treatment effectively inhibited superoxide (O2−).
Fig. 2.
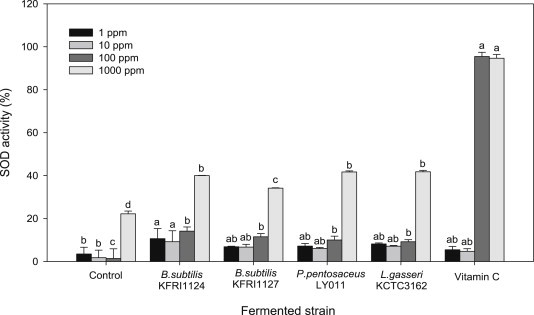
SOD enzyme activity of fermentation ginseng seed on the different strains.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Lee N.R., Han J.S., Kim J.S., Choi J.E. Effects of extraction temperature and time on ginsenoside content and quality in ginseng (Panax ginseng) flower water extract. Korean J Med Crop Sci. 2011;19:271–275. [Google Scholar]
- 2.Nam K.Y. The comparative understanding between red ginseng and white ginsengs, processed ginsengs (Panax ginseng Meyer) J Ginseng Res. 2005;29:1–18. [Google Scholar]
- 3.Natarajan K., Rajendan A. Effect of fermentation parameters on extra cellular tannase production by Lactobacillus plantarum MTCC 1407. E J Chem. 2009;6:979–984. [Google Scholar]
- 4.Jeon J.M., Choi S.K., Kim Y.J., Jang S.J., Cheon J.W., Lee H.S. Antioxidant and antiaging effect of ginseng berry extract fermented by lactic acid bacteria. J Soc Cosmet Sci Korea. 2011;37:75–81. [Google Scholar]
- 5.Kang B.H., Lee K.J., Hur S.S., Lee D.S., Lee S.H., Shin K.S. Ginsenoside derivatives and quality characteristics of fermented ginseng using lactic acid bacteria. Korean Soc Food Preserv. 2013;20:573–582. [Google Scholar]
- 6.Jung H.W., Kim J.E., Seo J.H., Lee S.P. Physicochemical and antioxidant properties of red ginseng marc fermented by Bacillus subtilis HA with mugwort powder addition. J Korean Soc Food Sci Nutr. 2010;39:1391–1398. [Google Scholar]
- 7.Kim J.E., Lee S.P. Evaluation of radical scavenging activity and physical properties of textured vegetable protein fermented by solid culture with Bacillus subtilis HA according to fermentation time. J Korean Soc Food Sci Nutr. 2010;39:872–879. [Google Scholar]
- 8.Goldin B.R. Health benefits of probiotics. Br J Nutr. 1998;80:203–207. [PubMed] [Google Scholar]
- 9.Quan L.H., Cheng L.Q., Kim H.B., Kim J.H., Son N.R., Kim S.Y. Bioconversion of ginsenoside Rd into compound K by Lactobacillus pentosus DC101 isolated from Kimchi. J Ginseng Res. 2010;34:288–295. [Google Scholar]
- 10.Kim H.J., Chae I.G., Lee S.G., Jeong H.J., Lee E.J., Lee I.S. Effects of fermented red ginseng extracts on hyperglycemia in streptozotocin-induced diabetic rats. J Ginseng Res. 2010;34:104–112. doi: 10.5142/jgr.2011.35.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H.J., Lee S.G., Chae I.G., Kim M.J., Im N.K., Yu M.H. Antioxidants effects of fermented red ginseng extracts in streptozotocin-induced rats. J Ginseng Res. 2011;35:129–137. doi: 10.5142/jgr.2011.35.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doh E.S., Chang J.P., Lee K.H., Seong N.S. Ginsenoside change and antioxidation of fermented ginseng. Korean J Med Crop Sci. 2010;18:255–265. [Google Scholar]
- 13.Kim J.K., Kim B.S., Park C.W., Seo D.B., Yoo H.R., Lee S.J. Effect of ginseng-berry extract on the improvement of blood microcirculation and skin brightness. Korean J Oriental Physiol Pathol. 2010;24:85–90. [Google Scholar]
- 14.Yeom M.H., Lee J.Y., Kim J.S., Park C.W., Kim D.H., Kim H.K. The anti-aging effects of Korean ginseng berry in the skin. Korean J Pharmacogn. 2010;41:26–30. [Google Scholar]
- 15.Park Q.H., Lee M.K., Park H. Change of ginsenosides and free sugars in seeds during stratification and seedling during early growth stage of Panax ginseng. Korean J Crop Sci. 1986;31:286–292. [Google Scholar]
- 16.Hu J.N., Lee J.H., Shin J.A., Choi J.E., Lee K.T. Determination of ginsenosides content in Korean ginseng seeds and roots by high performance liquid chromatography. Food Sci Biotechnol. 2008;17:430–433. [Google Scholar]
- 17.Choi J.E., Li X., Han Y.H., Lee K.T. Changes of saponin contents of leaves, stems and flower-buds of Panax ginseng C.A. Meyer by harvesting days. Korean J Medicinal Crop Sci. 2009;17:251–256. [Google Scholar]
- 18.Ko S.K., Bae H.M., Cho O.S., Im B.O., Chung S.H., Lee B.Y. Analysis of ginsenoside composition of ginseng berry and seed. Food Sci Biotechnol. 2008;17:1379–1382. [Google Scholar]
- 19.Beveridge T.H.J., Li T.S.C., Drover J.C.G. Phytosterol content in American ginseng seed oil. J Agric Food Chem. 2002;50:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.C. Changes in contents of ginsenosides, free sugars and fatty acids in developing ginseng seed. Korean J Crop Sci. 1988;33:134–137. [Google Scholar]
- 21.Zhu X.M., Hu J.N., Shin J.A., Lee J.H., Hong S.T., Lee K.T. Comparison of seed oil characteristics from Korean ginseng, Chinese ginseng (Panax ginseng Meyer) and American ginseng (Panax quinquefolium L.) J Food Sci Nutr. 2010;15:275–281. [Google Scholar]
- 22.Dubois M., Gilles K.A., Hamilton J.K., Robers P.A., Smith F. Colorimetric method for determination of sugar and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 23.Do J.H., Lee H.O., Lee S.K., Jang J.K., Lee S.D., Sung H.S. Colorimetric determination of acidic polysaccharide from panax ginseng, its extraction condition and stability. Korean J Ginseng Sci. 1993;17:139–144. [Google Scholar]
- 24.Singleton V.L., Rossi J.A., Jr. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 25.van den Berg R., Haenen G.R.M.M., van den Berg H., Bast A. Applicability of and improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999;66:511–517. [Google Scholar]
- 26.Gui Y., Ryu G.H. Effects of extrusion cooking on physicochemical properties of white and red ginseng (powder) J Ginseng Res. 2014;38:146–153. doi: 10.1016/j.jgr.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang I.G., Woo K.S., Kim T.M., Kim D.J., Yang M.H., Jeong H.S. Change of physicochemical characteristics of Korean pear juice with heat treatment conditions. Korean J Food Sci Technol. 2006;38:342–347. [Google Scholar]
- 28.Kim Y.C., Hong H.D., Rho J.H., Cho C.W., Rhee Y.K., Rim J.H. Changes of phenolic acid contents and radical scavenging activities of ginseng according to steaming times. J Ginseng Res. 2007;31:230–236. [Google Scholar]
- 29.Jung K.H., Hong H.D., Cho C.W., Lee M.Y., Choi U.K., Kim Y.C. Phenolic acid composition and antioxidative activity of red ginseng prepared by high temperature and high pressure process. Korean J Food Nutr. 2012;25:827–832. [Google Scholar]
- 30.Sherwin E.R. Oxidation and antioxidants in fat and oil procession. J Am Oil Chem Soc. 1978;55:809–814. [Google Scholar]
- 31.Dziezak J.D. Antioxidants. Food Technol. 1986;40:94–102. [Google Scholar]
- 32.Pyo Y.H., Lee T.C., Lee Y.C. Enrichment of bioactive isoflavones in soymilk fermented with β-glucosidase-producing lactic acid bacteria. Food Res Int. 2005;38:551–559. [Google Scholar]


