Abstract
Background
Gut microflora play a crucial role in the biotransformation of ginsenosides to compound K (CK), which may affect the pharmacological effects of ginseng. Prebiotics, such as NUTRIOSE, could enhance the formation and consequent absorption of CK through the modulation of gut microbial metabolic activities. In this study, the effect of a prebiotic fiber (NUTRIOSE) on the pharmacokinetics of ginsenoside CK, a bioactive metabolite of ginsenosides, and its mechanism of action were investigated.
Methods
Male Sprague–Dawley rats were given control or NUTRIOSE-containing diets (control diet + NUTRIOSE) for 2 wk, and ginseng extract or vehicle was then orally administered. Blood samples were collected to investigate the pharmacokinetics of CK using liquid chromatography–tandem mass spectrometry. Fecal activities that metabolize ginsenoside Rb1 to CK were assayed with fecal specimens or bacteria cultures.
Results
When ginseng extract was orally administered to rats fed with 2.5%, 5%, or 10% NUTRIOSE containing diets, the maximum plasma concentration (Cmax) and area under the plasma concentration–time curve values of CK significantly increased in a NUTRIOSE content-dependent manner. NUTRIOSE intake increased glycosidase activity and CK formation in rat intestinal contents. The CK-forming activities of intestinal microbiota cultured in vitro were significantly induced by NUTRIOSE.
Conclusion
These results show that prebiotic diets, such as NUTRIOSE, may promote the metabolic conversion of ginsenosides to CK and the subsequent absorption of CK in the gastrointestinal tract and may potentiate the pharmacological effects of ginseng.
Keywords: compound K, ginsenoside Rb, NUTRIOSE, Panax ginseng, pharmacokinetic
1. Introduction
Ginseng (the root of Panax ginseng Meyer, Araliaceae), which contains ginsenosides as major bioactive ingredients, is popularly used as functional food or dietary supplement in Asian countries [1]. Ginsenosides exhibit different biological activities, such as anti-inflammatory and antitumor activities [2,3]. To exert these pharmacological actions, ginsenosides are metabolized by human intestinal microbes after oral administration [4,5]. The major ginsenosides contained in ginseng (ginsenosides Rb1, Rb2, and Rc) are metabolized to 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol [compound K (CK); Fig. 1], a deglycosylated metabolite of ginsenosides, by human intestinal microbiota prior to being absorbed into the blood [6–10]. CK exhibits antitumor, anti-inflammatory, and antiallergic activities more potently than the parental ginsenoside Rb1 and is considered a major bioactive metabolite responsible for the pharmacological actions of orally administered ginseng [3,4,11]. Therefore, intestinal microbes involved in CK formation play an important role in exerting the pharmacological effects of ginseng.
Fig. 1.
Chemical structures of ginsenoside Rb1 and compound K.
Prebiotics are defined as nondigestible food ingredients that stimulate the growth and/or activity of the gastrointestinal microflora and promote host well-being and health [12]. Dietary sources such as acacia gums, beans, inulin sources, raw oats, unrefined wheat, unrefined barley, and yacon contain prebiotics. NUTRIOSE, a nonviscous, soluble fiber derived from wheat and corn, is produced through a digestion-like process and thermal treatment and is considered a prebiotic. This prebiotic fiber is resistant to hydrolysis in the small intestine owing to a structure of linear and branched glucosidic linkages so that it may be available for bacterial fermentation in the gut [13]. According to previous reports, NUTRIOSE increased the number of intestinal Lactobacillus spp. and Bacteroides [14–16]. Short- and long-term NUTRIOSE supplementation in human volunteers significantly increased fecal α/β-glucosidase activities and improved intestinal bowel disease through a protective immune effect [14]. NUTRIOSE has beneficial effects on colonic ecology, which may lead to improvements in insulin resistance, energy intake, and body composition [17].
In this study, the pharmacokinetics of CK following oral administration of ginseng extract was investigated in rats treated with NUTRIOSE as prebiotics. In addition, the effects of NUTRIOSE on intestinal bacterial metabolic activities, in particular CK-forming activities, were investigated to characterize its mechanism of action on ginseng pharmacokinetics and pharmacodynamics.
2. Materials and methods
2.1. Materials
Ginseng extract was prepared, and ginsenoside Rb1 (purity >92%) was isolated according to the method that we had previously reported [8]. The extracted powder contained 8.9% ginsenoside Rb1, 8.5% ginsenoside Rg1, and 1.4% ginsenoside Rd, but CK was not detected. NUTRIOSE was kindly donated by Roquette (Lestrem, France). CK was purchased from Fleton Natural Products Co., Ltd. (Chengdu, China).
2.2. Human participants
The participants consisted of three healthy Korean men (average age, 73 ± 2 years). Exclusion criteria included smoking and current medication, especially regular or current use of antibiotics. The recruitment of participants and the consent procedure as well as the collection of their stools were approved by the Ethics Committee for the Care and Use of Clinical Study in the Medical School, Kyung Hee University, Seoul, Korea (KHS-IRB-12-011-1). The participants provided their written informed consent to participate in the study.
2.3. Animals
Male Sprague–Dawley rats (210–240 g) were supplied by the Orient Experimental Animal Breeding Center (Gyunggi-do, Korea). All animals were housed in wire cages (2 rats per cage) maintained at a temperature range of 20–22°C and 50 ± 10% humidity, fed standard laboratory chow (Samyang Co., Seoul, Korea), and allowed water ad libitum. All experiments were performed in accordance with the National Institutes of Health and Kyung Hee University Guides for Laboratory Animals Care and Use and approved by the Committee for the Care and Use of Laboratory Animals in the College of Pharmacy, Kyung Hee University (KHP-2012-04-06-R1).
2.4. Pharmacokinetic study
Male Sprague–Dawley rats were orally administered ginseng extract or vehicle after receiving control or NUTRIOSE-containing diets (control diet + NUTRIOSE) for 2 wk. Blood samples (200 μL) were collected from the tail vein at 0 h, 1 h, 2 h, 4 h, 8 h, 12 h, 16 h, 20 h, and 24 h after administration of ginseng extract and centrifuged for 10 min at 4,000 × g to prepare the plasma samples. The samples were analyzed using liquid chromatography–tandem mass spectrometry analyses according to a previously reported method [18]. Plasma concentration data for individual rats were analyzed with a noncompartmental method by using WinNonlin Professional 3.1 software (Scientific Consulting, Inc., Lexington, KY, USA). The area under the plasma concentration–time curve (AUC) was calculated by using the trapezoidal rule. The maximum plasma concentration (Cmax) and the time to reach Cmax (Tmax) were estimated directly from the plasma concentration–time profiles.
2.5. Metabolic activity measurement
Fecal α-d-glucosidase, β-d-glucosidase, β-d-xylosidase, and α-l-rhamnosidase activities were tested with rat gastrointestinal contents [19]. Fecal activities that metabolize ginsenoside Rb1 to CK were assayed with fecal specimens or bacteria cultures [20]. For fecal bacteria culture, rat or human fecal specimens (approximately 1 g) were collected in plastic cups and then carefully mixed with a spatula and suspended in 9 mL cold saline. Fecal bacterial suspension was centrifuged at 500 × g for 5 min. The resulting supernatant is inoculated in control or 1% NUTRIOSE-containing Gifu anaerobic medium (GAM; glucose-free broth) and cultured for 24 h. The culture media were collected by centrifugation at 10,000 × g for 20 min. The precipitate was used as a crude enzyme preparation to assay the ginsenoside Rb1 to CK metabolizing activity [20].
3. Results
3.1. Formation of CK from ginsenoside Rb1 or ginseng extracts in rat and human fecal suspension
Prior to investigation of prebiotic effects on CK formation mediated by gut microbacterial enzymes, the metabolic activity for CK formation from ginsenoside Rb1 or ginseng extracts were measured in rat and human fecal suspensions (Fig. 2). The incubation of ginsenoside Rb1 or ginseng extracts in fecal suspension resulted in CK formation. The metabolic rates with ginsenoside Rb1 were 28.0 ± 2.9 nmol/h/g and 34.1 ± 18.9 nmol/h/g, respectively, in rat and human feces. In ginseng extract samples, the metabolic rates of rat and human feces were 38.4 ± 16.9 nmol/h/g and 96.3 ± 80.7 nmol/h/g, respectively. As for ginsenoside Rb1 samples, CK formation rates in human and rat fecal suspensions were comparable, and the variation between individuals was relatively low. However, in ginseng extract samples, a higher variation was observed, particularly in human samples and the difference between humans and rats was greater. Ginseng extract contains various protopanaxadiol ginsenosides that can be transformed to CK, and thus individual differences may be greater. Accordingly, the subsequent experiments in rats were carried out with ginsenoside Rb1 as a substrate to minimize the variations between individuals and species.
Fig. 2.
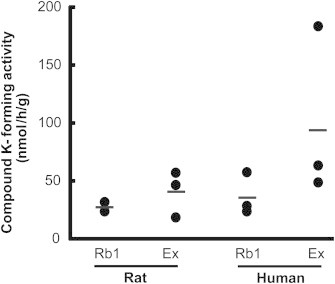
Compound K-forming activities from ginsenoside Rb1 or ginseng extract in rat and human feces.
3.2. Effect of NUTRIOSE on plasma pharmacokinetics of CK
To understand the effect of NUTRIOSE on CK pharmacokinetics, plasma concentrations of CK after oral administration of ginseng extract (2,000 mg/kg) were determined in rats fed a control or NUTRIOSE-containing diet for 2 wk. The mean plasma concentration–time profiles of CK are shown in Fig. 3. The pharmacokinetic parameters are provided in Table 1. As shown in Table 1, CK Cmax values increased with increasing NUTRIOSE content; the Cmax value of CK in the 10% NUTRIOSE-treated group (N10-G; 54.4 ± 26.2 ng/mL) was >2 times higher than that of the control group (G; 24.1 ± 5.5 ng/mL), and the difference was statistically significant (p = 0.04). The AUC values also increased in a NUTRIOSE content-dependent manner. The AUC of the N10-G group was 2.8 times greater than that of the control group (153.1 ± 30.6 ng h/mL vs. 429.9 ± 160.6 ng h/mL, p = 0.006). Thus, CK systemic exposure was significantly increased in NUTRIOSE-fed rats. In addition, CK Tmax was reached earlier in NUTRIOSE-fed rats as compared to controls (12.0 ± 0.0 h vs. 15.2 ± 1.8 h, p = 0.004).
Fig. 3.
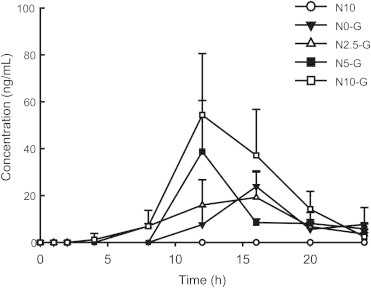
Plasma concentrations of compound K after oral administration of ginseng extract (2,000 mg/kg) in rats fed with or without NUTRIOSE. Rats were orally administered ginseng extracts at a dose of 2,000 mg/kg after being fed control or NUTRIOSE-containing diet for 2 wk. Group fed 10% NUTRIOSE-containing diet (N10); group administered ginseng extract after being fed control diet (N0-G) and 2.5% (N2.5-G), 5% (N5-G), or 10% (N10-G) NUTRIOSE-containing diet. All values are indicated as the mean ± standard deviation (n = 5). *p < 0.05 compared with N0-G.
Table 1.
Pharmacokinetic parameters of compound K after oral administration of ginseng extract in rats treated with NUTRIOSE
| Group | Tmax (h) | Cmax (ng/mL) | AUC (ng h/mL) |
|---|---|---|---|
| N0-G | 15.2 ± 1.8 | 24.1 ± 5.5 | 153.1 ± 30.6 |
| N2.5-G | 12.8 ± 3.3 | 24.0 ± 9.3 | 187.2 ± 24.0 |
| N5-G | 12.0 ± 0.0* | 38.8 ± 21.8 | 218.5 ± 60.7 |
| N10-G | 12.0 ± 0.0* | 54.4 ± 26.2* | 429.9 ± 160.8* |
Tmax, maximum drug concentration time; Cmax, maximum plasma concentration; AUC, area under the blood concentration curve. Group administered ginseng extract after being fed control diet (N0-G) and 2.5% (N2.5-G), 5% (N5-G), or 10% (N10-G) NUTRIOSE®-containing diet. All values are indicated as the mean ± SD (n = 5).
*p < 0.05 compared with N0-G.
3.3. Effect of NUTRIOSE on glycosidase activities of intestinal microbiota
Ginsenosides have different sugar moieties such as glucose, rhamnose, and xylose. Thus, biotransformation of ginsenosides to CK involves glycosidic bond cleavage by gut microbial hydrolysis. To investigate the effects of NUTRIOSE on ginsenoside-metabolizing enzyme activities of intestinal microbiota, the glucosidase activities of intestinal contents were measured in rats treated with NUTRIOSE for 2 wk (the intestinal contents were collected from the rats used for the pharmacokinetic study described above). As shown in Fig. 4, α/β-d-glucosidase activities were increased by NUTRIOSE treatment. This result is accordance with previously reported data [14]. α-l-Rhamnosidase activity was also increased by treatment with NUTRIOSE. The mean β-xylosidase activity increased as well, although this result was not statistically significant. The effect of NUTRIOSE appeared to be content-dependent based on the data obtained for the ginseng dosed groups (N0-G, N2.5-G, N5-G, and N10-G).
Fig. 4.
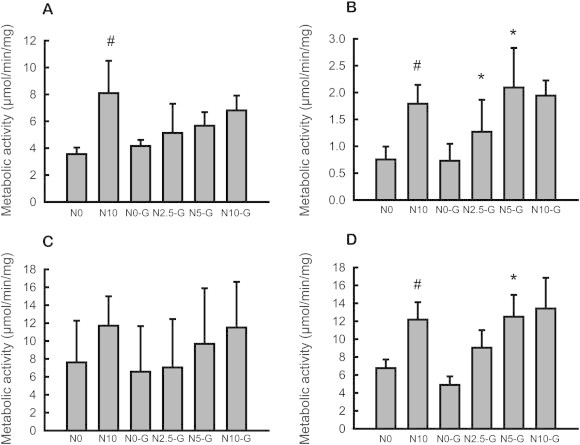
Effects of NUTRIOSE on glycosidase activities of rat intestinal contents. (A) α-d-Glucosidase activity; (B) β-d-glucosidase activity; (C) β-d-xylosidase activity; and (D) α-l-rhamnosidase activity. N0, control. Other sample codes are as indicated in Fig. 3. All values are presented mean ± standard deviation (n = 4). #p < 0.05 compared with N0. *p < 0.05 compared with N0-G.
3.4. Effect of NUTRIOSE on CK formation by intestinal microbiota
To investigate the effects of NUTRIOSE on CK formation by gut bacterial metabolism, CK-forming enzyme activities of rat intestinal contents were measured following NUTRIOSE treatment. Ginsenoside Rb1 was used as a substrate to determine CK-forming enzyme activities. Ginsenoside Rb1 is a representative protopanaxadiol ginsenoside and the most abundant ingredient of ginseng extract, which is extensively metabolized by gut microflora to yield CK. The resulting data showed biotransformation of ginsenoside Rb1 to CK by fecal bacteria increased with increasing NUTRIOSE content (Fig. 5A). The metabolic rates in the 5% and 10% NUTRIOSE-treated groups (289 ± 84 nmol/h/g and 311 ± 86 nmol/h/g, respectively) were >10 times higher than that in the control group (26 ± 14 nmol/h/g).
Fig. 5.

Effect of NUTRIOSE on the biotransformation of Rb1 to compound K (A) in rat intestinal contents and (B) in rat and human fecal microbiota cultures. (A) Rats were treated with NUTRIOSE-containing diets for 2 wk and the metabolic activity of the intestinal contents was measured. (B) Rat and human fecal bacteria were cultured in 1% dextrose (control) or 1% NUTRIOSE-containing GAM broth for 24 h and the metabolic activity was measured. Ginsenoside Rb1 was used as a substrate at a concentration of 0.1mM. *p < 0.05 compared with dextrose. **p < 0.01 compared with control diet group (n = 4).
3.5. Effect of NUTRIOSE on CK-forming activities of rat and human fecal microflora cultured in GAM
To confirm whether NUTRIOSE could induce the CK-forming activity of intestinal microbiota, fecal microbiota of rats and humans were cultured in vitro in GAM broth with or without NUTRIOSE for 24 h, and formation rates of CK from ginsenoside Rb1 were measured (Fig. 5B). The addition of NUTRIOSE significantly induced the metabolic activity of cultured fecal microbiota in both rats and humans. The CK-forming activities of NUTRIOSE-supplemented fecal microbiota cultures were 4.5-fold (4.5 ± 2.0, p = 0.01) and 5.5-fold (5.5 ± 2.3, p = 0.03) higher in rats and humans, respectively, compared with those in dextrose-supplemented cultures.
4. Discussion
Dietary food or herbal supplements or medicines are orally ingested and their ingredients inevitably come in contact with the microbiota in the gastrointestinal tract. The gastrointestinal tract harbors > 1,000 microbial species, which is about 10 times the number of body cells [1,21]. The intestinal microbiota metabolizes exogenous xenobiotics, including dietary compounds and phytochemicals, as well as endogenous compounds secreted into the gastrointestinal tract [22–25]. Thus, intestinal microbiota may transform a considerable proportion of orally administered herbal supplement ingredients to their corresponding metabolites, and in numerous cases to bioactive metabolites, before the ingredients are absorbed from the gastrointestinal tract [1,6,7]. Therefore, intestinal microbiota is considered to play an important role in regulating the biological activities of functional foods or herbal supplements such as ginseng.
This study demonstrated that the intake of NUTRIOSE elevated the plasma concentration level of CK after the oral administration of ginseng. This is thought to result from the increase in the formation and consequent absorption of CK in the gastrointestinal tract by NUTRIOSE. Prebiotics are known to modulate and generally enhance the metabolic activities of gut microflora [26]. This is consistent with the result of this study; NUTRIOSE treatment increased the glycosidase activities of intestinal or fecal microbiota and promoted the biotransformation of ginsenoside Rb1 to CK. Thus, NUTRIOSE intake enhanced the uptake of CK by increasing its formation through induction of ginsenoside-metabolizing enzyme activity of gastrointestinal microbiota.
CK is a ginsenoside with potent chemoprevention and anticancer activities. The CK content of raw ginseng extract is minimal or negligible; however, CK is a major metabolite after the oral administration of protopanaxadiol ginsenosides and could be a major bioactive metabolite responsible for the pharmacological effects of ginseng [23,27]. As ginsenosides are converted to CK mainly by gut microflora, the metabolic activity of the intestinal microbiota would be closely related to the pharmacological activity of ginseng.
However, the intestinal CK forming activities seem to be significantly different among individuals. According to the report by Lee et al [10], in the CK formation assay with the fecal suspension collected from 32 human participants, the highest metabolic activity was found to be >20 times of the lowest metabolic activity. This result is in accordance with our data (Fig. 2). In addition, such an individual variation was reflected to the plasma concentration profiles; the area under the concentration curve (AUC) values calculated after the oral administration of ginseng extract powder also showed a large variation (221.98 ± 221.42 ng h/mL). Even CK was not detected in the plasma samples of some participants. Thus, the effects of ginseng intake might not be minimal to those individuals. In these cases, it would be helpful to activate the metabolic activities of gut microbiota to enhance the biotransformation of ginsenosides to active metabolites such as CK. Based on our results, prebiotics may be useful to maximize the pharmacological effects of ginseng.
Many papers have reported the involvement of the gut microflora in the biotransformation of ginsenosides [7,27]. However, to our knowledge, there has been no study showing that the pharmacokinetics of ginsenosides is affected by alterations in gut microflora metabolic activities. The present study experimentally demonstrated this by using a prebiotic-treated rat model. Furthermore, the results suggested that the intake of prebiotics may enhance the pharmacological effects of functional foods or herbal supplements that are likely to be metabolized by gut microflora.
Conflicts of interest
H.-L. C, K.Y., and L.G.-D. are employees of Roquette.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Kim D.-H. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joh E.-H., Lee I.-A., Jung I.-H., Kim D.-H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation — the key step of inflammation. Biochem Pharmacol. 2011;82:278–286. doi: 10.1016/j.bcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Choo M.-K., Sakurai H., Kim D.-H., Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-α-promoted metastasis by suppressing nuclear factor-κB signaling in murine colon cancer cells. Oncol Rep. 2008;19:595–600. [PubMed] [Google Scholar]
- 4.Wakabayashi C., Murakami K., Hasegawa H., Murata J., Saiki I. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998;246:725–730. doi: 10.1006/bbrc.1998.8690. [DOI] [PubMed] [Google Scholar]
- 5.Shin Y.-W., Kim D.-H. Antipruritic effect of ginsenoside Rb1 and compound K in scratching behavior mouse models. J Pharmacol Sci. 2005;99:83–88. doi: 10.1254/jphs.fp0050260. [DOI] [PubMed] [Google Scholar]
- 6.Akao T., Kanaoka M., Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration—measurement of compound K by enzyme immunoassay. Biol Pharm Bull. 1998;21:245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- 7.Akao T., Kida H., Kanaoka M., Hattori M., Kobashi K. Drug metabolism: intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 8.Bae E.A., Park S.Y., Kim D.H. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 9.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 10.Lee J., Lee E., Kim D., Lee J., Yoo J., Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Bae E.A., Choo M.K., Park E.K., Park S.Y., Shin H.Y., Kim D.H. Metabolism of ginsenoside R(c) by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25:743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 12.Petschow B., Doré J., Hibberd P., Dinan T., Reid G., Blaser M., Cani P.D., Degnan F.H., Foster J., Gibson G. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann N Y Acad Sci. 2013;1306:1–17. doi: 10.1111/nyas.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musilova S., Rada V., Vlkova E., Bunesova V. Beneficial effects of human milk oligosaccharides on gut microbiota. Benef Microbes. 2014;5:273–283. doi: 10.3920/BM2013.0080. [DOI] [PubMed] [Google Scholar]
- 14.van den Heuvel E.G., Wils D., Pasman W.J., Saniez M.-H., Kardinaal A.F. Dietary supplementation of different doses of NUTRIOSE® FB, a fermentable dextrin, alters the activity of faecal enzymes in healthy men. Eur J Nutr. 2005;44:445–451. doi: 10.1007/s00394-005-0552-0. [DOI] [PubMed] [Google Scholar]
- 15.Lefranc-Millot C. NUTRIOSE® 06: a useful soluble dietary fibre for added nutritional value. Nutr Bull. 2008;33:234–239. [Google Scholar]
- 16.Lefranc-Millot C., Guerin-Deremaux L., Wils D., Neut C., Miller L., Saniez-Degrave M.H. Impact of a resistant dextrin on intestinal ecology: how altering the digestive ecosystem with NUTRIOSE®, a soluble fibre with prebiotic properties, may be beneficial for health. J Int Med Res. 2012;40:211–224. doi: 10.1177/147323001204000122. [DOI] [PubMed] [Google Scholar]
- 17.Guerin-Deremaux L., Li S., Pochat M., Wils D., Mubasher M., Reifer C., Miller L.E. Effects of NUTRIOSE® dietary fiber supplementation on body weight, body composition, energy intake, and hunger in overweight men. Int J Food Sci Nutr. 2011;62:628–635. doi: 10.3109/09637486.2011.569492. [DOI] [PubMed] [Google Scholar]
- 18.Kim J.S., Kim Y., Han S.-H., Jeon J.-Y., Hwang M., Im Y.J., Kim J.H., Lee S.Y., Chae S.W., Kim M.G. Development and validation of an LC-MS/MS method for determination of compound K in human plasma and clinical application. J Ginseng Res. 2013;37:135–141. doi: 10.5142/jgr.2013.37.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee D.-S., Kim Y.-S., Ko C.-N., Cho K.-H., Bae H.-S., Lee K.S., Kim J.J., Park E.K., Kim D.H. Fecal metabolic activities of herbal components to bioactive compounds. Arch Pharm Res. 2002;25:165–169. doi: 10.1007/BF02976558. [DOI] [PubMed] [Google Scholar]
- 20.Choi J.-R., Hong S.-W., Kim Y., Jang S.-E., Kim N.-J., Han M.J., Kim D.H. Metabolic activities of ginseng and its constituents, ginsenoside Rb1 and Rg1, by human intestinal microflora. J Ginseng Res. 2011;35:301–307. doi: 10.5142/jgr.2011.35.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crow J.M. Microbiome: that healthy gut feeling. Nature. 2011;480:S88–S89. doi: 10.1038/480S88a. [DOI] [PubMed] [Google Scholar]
- 22.Scheline R.R. Metabolism of foreign compounds by gastrointestinal microorganisms. Pharmacol Rev. 1973;25:451–523. [PubMed] [Google Scholar]
- 23.Mikov M. The metabolism of drugs by the gut flora. Eur J Drug Metab Pharmacokinet. 1994;19:201–207. doi: 10.1007/BF03188922. [DOI] [PubMed] [Google Scholar]
- 24.Sousa T., Paterson R., Moore V., Carlsson A., Abrahamsson B., Basit A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008;363:1–25. doi: 10.1016/j.ijpharm.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Jin M.J., Kim U., Kim I.S., Kim Y., Kim D.-H., Han S.B., Kim D.H., Kwon O.S., Yoo H.H. Effects of gut microflora on pharmacokinetics of hesperidin: a study on non-antibiotic and pseudo-germ-free rats. J Toxicol Environ Health A. 2010;73:1441–1450. doi: 10.1080/15287394.2010.511549. [DOI] [PubMed] [Google Scholar]
- 26.Hijová E., Bomba A., Bertková I., Strojný L., Szabadosová V., Šoltésová A. Prebiotics and bioactive natural substances induce changes of composition and metabolic activities of the colonic microflora in cancerous rats. Acta Biochim Pol. 2012;59:271–274. [PubMed] [Google Scholar]
- 27.Kim U., Park M.H., Kim D.H., Yoo H.H. Metabolite profiling of ginsenoside Re in rat urine and faeces after oral administration. Food Chem. 2013;136:1364–1369. doi: 10.1016/j.foodchem.2012.09.050. [DOI] [PubMed] [Google Scholar]


