Abstract
Objective
To evaluate the association between chronic opioid use for non-cancer pain and fracture risk by conducting a meta-analysis of cohort studies.
Methods
Cohort studies were identified by searching PubMed and EMBASE from their inception to July 2014. A fracture was considered an endpoint. The information was extracted by two authors independently. When the heterogeneity was significant, a random-effects model was used to calculate the overall pooled risk estimates.
Results
Eight cohort studies were included in the final meta-analysis. On the basis of the Newcastle-Ottawa Scale (NOS), six studies were considered to be of high quality. The overall combined relative risk for the use of opioids and fractures was 1.88 (95% confidence interval [CI] 1.51-2.34). A subgroup analysis revealed the sources of heterogeneity. The sensitivity analysis indicated stable results, and no publication bias was observed.
Conclusions
This meta-analysis of cohort studies demonstrates that opioids significantly increase the risk of fractures.
Introduction
The World Health Organization estimates that at least 20% of individuals worldwide have varying degrees of chronic pain [1]. Opioids, which provide effective pain relief in a range of persistent non-cancer pain conditions, are widely and increasingly used for their analgesic and psychotropic effects [2]. An epidemiological study of chronic, non-malignant pain in Denmark revealed that nearly 3% of the Danish population used opioids regularly [3]. Chronic exposure to opioids is frequently encountered in clinical practice. Chen’s study [4] showed a lack of correlation between changes in opioid dose and clinical pain scores in a group of chronic pain patients regardless of the clinical pain conditions for which opioid therapy was intended. However, a large increase in opioid use has occurred in the USA, with more than 3% of persons 70 years and older in the U.S. estimated to be regular users of opioids [5]. In addition, the misuse of opioids is the fastest growing form of drug misuse and is the leading cause of accidental overdose and mortality [6]. Because pain is the fifth vital sign in the USA, there has been increasing attention paid to the use and effects of opioids. We know that approximately 80% of patients taking opioid therapy will experience an adverse effect, such as constipation, hypogonadism or the suppression of the innate and acquired immune systems; thus, considerable controversy remains regarding the use of opioids to treat persistent non-cancer pain [2]. Some previous studies have reported an association between opioids and fracture risk [2,7–9], although these studies have failed to demonstrate a significant increase in fracture risk when using opioids. However, a trend toward a higher fracture risk with the use of opioids was found [4,10]. A previous meta-analysis [11] demonstrated that a relative fracture risk was associated with several classes of psychotropic drugs, including opioids. However, only six studies on opioids were included in this analysis, which did not allow firm conclusions to be drawn because of the potential of heterogeneity and publication bias.
Opioids are widely used for non-cancer pain, and to our knowledge, no specific meta-analysis of the association between fracture risk and opioid use has been conducted to date. Therefore, we performed a meta-analysis with the purpose of assessing the fracture risk among opioid users. In this study, we followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [12].
Materials and Methods
Search strategy and data sources
We searched MEDLINE (PubMed) and EMBASE (1947 to 2014 July 21) for cohort studies describing the association between opioid use and fracture risk without restrictions. We also searched the bibliographies of relevant articles to identify any additional studies. We used the following search terms: (i) fracture*[Title/Abstract] OR “Fractures, Bone”[Mesh]; (ii) opioid*[Title/Abstract] OR “Analgesics, Opioid”[Mesh]; and (iii) cohort study OR "Cohort Studies"[Mesh].
Study selection
Studies were considered eligible if they met all of the following criteria: (i) presented original data from a cohort study; (ii) evaluated the association of opioid use with fracture incidence; (iii) had opioids as the exposure of interest; and (iv) provided hazard ratios (HRs) or the adjusted relative risks (RRs) and the corresponding 95% confidence intervals (CIs). If the data were duplicated or the population was studied in more than one study, we included the study with the largest sample size and the most comprehensive outcome evaluation.
Data extraction and quality assessment
Two investigators (ZWT, YZ) independently evaluated the eligibility of the studies retrieved from the databases based on the pre-determined selection criteria. In addition, a cross-reference search of eligible articles was conducted to identify studies not found in the computerized search. These two authors independently extracted the following data: the first author’s name; year of publication, patients’ ages, cohort size, study regions, years of follow-up, study design, HR or RR and the 95% CIs, and statistical adjustments for confounding factors. Any disagreements were resolved either by discussion or in consultation with the co-corresponding author (XGZ). The methodological quality assessment was based on the Newcastle-Ottawa Scale (NOS) [13]. The maximum NOS score was 9. We defined low quality as a Newcastle-Ottawa Scale score < 7.0 and high quality as a score ≥ 7.0.
Statistical analyses
We investigated the association between the use of opioids and the risk of fracture by using adjusted data for the main analyses. We computed a pooled RR and 95% CI from the adjusted RRs or HRs and 95% CIs reported in the studies. The HRs were considered to correspond to RRs. The Cochran Q and I2 statistics were used to evaluate the statistical heterogeneity [14]. When the P value was < 0.1 and the I2 value was > 50%, the data were considered to be heterogeneous, and a random-effects model (DerSimonian and Laird method) [15] was applied because it represents a more conservative approach to the calculation of a weighted estimate effect using an RR. Otherwise, a fixed-effects model [16] was used to estimate the overall summary effect sizes when no heterogeneity was present in the included studies. To further explore the origin of heterogeneity, we also performed subgroup analyses by study design, study region and fracture type (any fracture, with all fracture types combined, and hip fractures).
To assess the stability of our results, a sensitivity analysis (by excluding each single study in turn) was conducted to estimate the influence of individual studies on the pooled result.
We used Egger’s test (linear regression method) [17] and Begg’s test (rank correlation method) [18] to assess the potential publication bias.
Results
Literature search and study characteristics
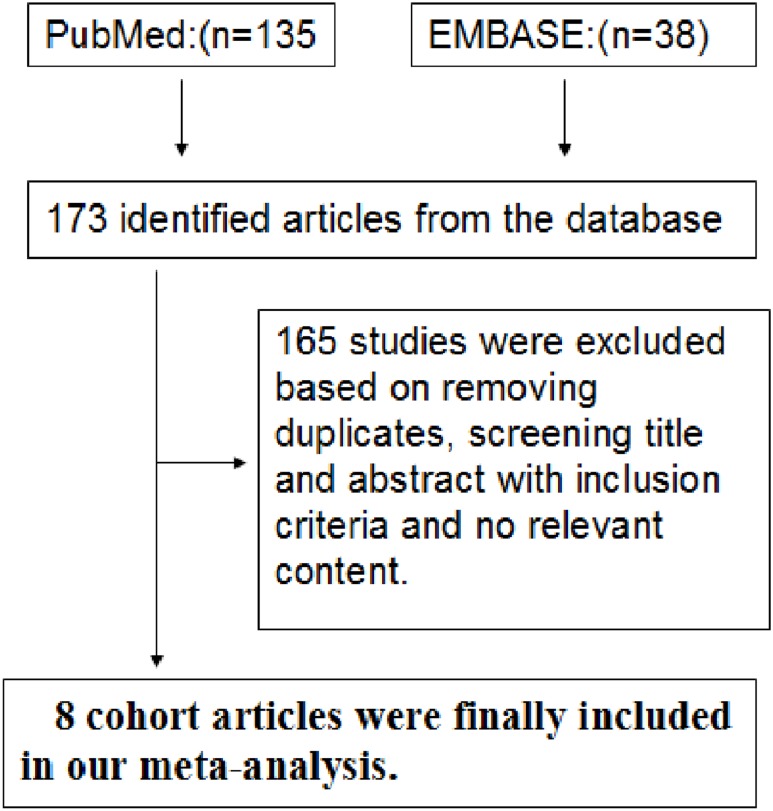
A total of 173 articles were identified in the initial search. Of these articles, 165 were excluded after reviewing the titles and abstracts, removing duplicates, and thoroughly reading the full text. As a result, we included eight cohort studies in our final analysis (Fig 1) [10,19–25]. Five [10,21,23–25] of the 8 cohort studies were from the United States, and 3 studies were from other countries, namely, Sweden, England, and Denmark. The general characteristics of the eight studies and the quality scores for the studies are summarized in Table 1. Of the 8 studies, six were prospective studies and two were retrospective studies. Six were high-quality studies (scores ≥ 7.0) (Table 1).
Fig 1. Flow Chart Illustrating the Literature Search for Cohort Studies on Opioids in Relation to Fractures.
Table 1. Characteristics of the 8 Cohort Studies included in the Final Analysis of Opioids and Fracture Risk.
| Author, year; location | Age of the participants, years | Fracture type/assessment | Study design | Cohort size | Follow-up time | Models | Adjustment for covariates | NOS |
|---|---|---|---|---|---|---|---|---|
| Guo, 1998, Swedish | ≥75 | Hip/ICD-9 | Prospective cohort | 1608 | 4.4 years | Cox proportional hazards models | Age, gender, education, residence, ADL limitation, cognitive impairment, history of stroke and tumors | 8 |
| Kristine, 2003; USA | ≥65 | Fractures/radiology reports | Prospective cohort | 8127 | 4.8 years | Cox proportional hazards models | Age, gender, race, health status, smoking, walking exercise, functional impairment, cognitive function, depression, weight change | 9 |
| Card et al., 2004; UK | NA | Hip/NA | Prospective cohort | 99467 | 7.3 per 10000 person-years | Cox regression models | Age, gender, corticosteroid use | 6 |
| Sachin, 2006; USA | ≥65 | Hip/ICD-9 | Prospective cohort | 362503 | 464 days | Cox regression models | Age, gender, antidepressant use, antipsychotic use, anxiolytic /hypnotic use | 7 |
| Kathleen, 2010; USA | ≥60 | Fractures/ICD-9 | Prospective cohort | 2341 | 32.7 months | Cox proportional hazards models | Age, gender, smoking, depression, substance abuse, dementia, comorbidities, prior fracture, pain site, antidepressant use, sedative use, HRT /bisphosphonate use | 9 |
| Miller, 2011; USA | ≥65 | Fractures/ICD-9 | Retrospective cohort | 17310 | 451 per 1000 person years | Cox proportional hazards models | Age, gender, diabetes stroke, osteoarthritis, comorbidity index, stroke, diabetes | 6 |
| Vestergaard 2012; Denmark | 45–58 | Fractures/X-ray | Prospective cohort | 2016 | 10 years | Cox proportional hazards models | Age, HT, BMI, baseline spine BMD, family or prior fracture, serum 25-hydroxy-vitamin D and smoking | 9 |
| Laura, 2013; USA | 58.73±13.43 | Lower extremity/ICD-9 | Retrospective cohort | 7447 | 3–8 years | Cox proportional hazards models | Age, race, completeness of SCI level and duration of SCI | 7 |
Note: fractures indicate overall fractures; hip indicates hip fracture; NOS: Newcastle-Ottawa Scale
Main analysis
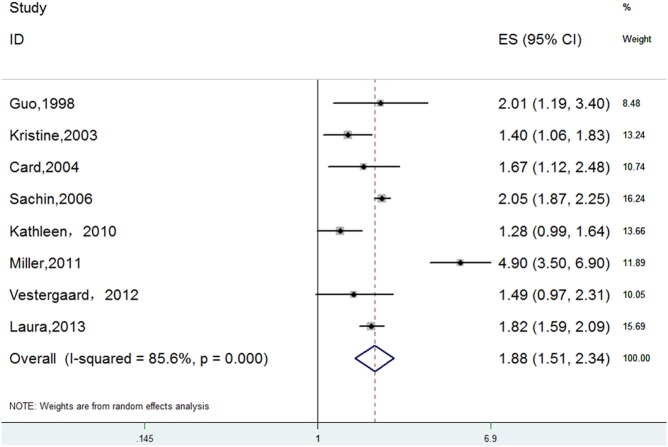
The meta-analysis of the 8 cohort studies, which included 500819 individuals, indicated significant, positive associations between opioid use and overall fracture risk (RR 1.88, 95% CI 1.51–2.34). Substantial heterogeneity was observed (P = 0.000, I2 = 85.6%) (Fig 2).
Fig 2. Forest Plot of RR with CI for Opioid Use and Fracture Risk.
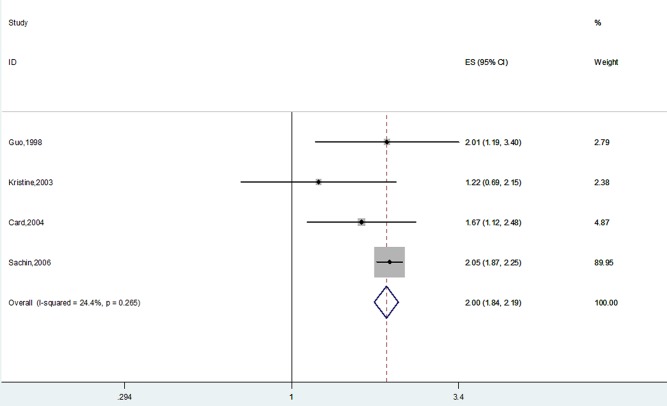
Data on opioid use and hip fracture risk were available for 4 studies [20–23]. The pooled results indicated that opioids contribute significantly to the risk of fracture (RR 2.00, 95% CI 1.84–2.19). No statistical heterogeneity was observed (P = 0.266, I2 = 24.4%) (Fig 3).
Fig 3. Forest Plot of RR with CI for Opioid Use and Hip Fracture Risk.
Subgroup meta-analysis
A subgroup meta-analysis was performed according to the study design type. We observed a significant, positive association between opioid use and fracture risk in both prospective and retrospective cohort studies (Table 2).
Table 2. Subgroup Analyses of the Association between Opioid Use and Fracture Risk.
| Factor | No. of studies | RR (95% CI) | Heterogeneity P (I2%) | |
|---|---|---|---|---|
| Study design | Retrospective cohort | 2 | 2.95 (1.12–7.78) | 0.000 (96.4) |
| Prospective cohort | 6 | 1.62 (1.31–2.02) | 0.003 (72.5) | |
| Region | Europe | 3 | 1.68 (1.30–2.17) | 0.690 (0.0) |
| USA | 5 | 1.97 (1.49–2.61) | 0.000 (91.4) | |
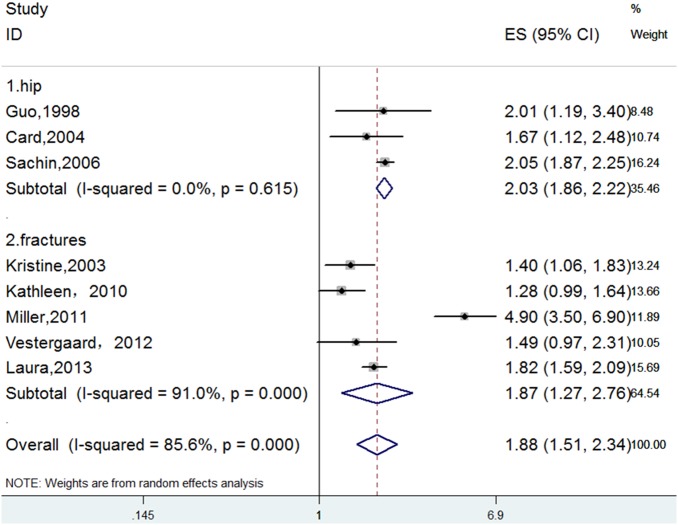
| Fracture type | hip fracture | 3 | 2.03 (1.86–2.22) | 0.615 (0.0) |
| any fracture | 5 | 1.88 (1.51–2.34) | 0.000 (91.0) | |
The subgroup analysis by region indicated a significant tendency toward increased fracture risk with the use of opioids. No statistical heterogeneity was observed in the European group (Table 2).
A subgroup analysis was performed according to the anatomical fracture site. We assigned studies that presented data on hip fracture to one group, and the other studies were assigned to another group. We also observed significant, positive associations between opioid use and the risk of hip fractures. No statistical heterogeneity was observed in the hip group (Fig 4, Table 2).
Fig 4. Forest Plot for a Subgroup Meta-analysis by Anatomical Fracture Site.
To identify the influence of the retrospective studies, we performed further analyses without two studies [21,25]. We found that when we eliminated the Miller [21] study, the pooled outcome was stable, and the heterogeneity was significantly decreased (RR = 1.52, 95% CI 1.25–1.85, I2 = 59.3%, P = 0.061). After we also eliminated the Laura [25] study, no heterogeneity was discovered (RR = 1.36, 95% CI 1.14–1.61, I2 = 0.0%, P = 0.805).
Sensitivity analysis
To evaluate the robustness of our analysis, we conducted a sensitivity analysis by recalculating the pooled results of the primary analysis by excluding one study per iteration. The outcome revealed that the exclusion of any single study did not alter the overall combined result (Table 3).
Table 3. Sensitivity Analysis of the Association between Opioid Use and Fracture Risk.
| Excluded study | RR (95% CI) | P (I2%) |
|---|---|---|
| Guo et al., 1998 | 1.87 (1.48–2.36) | 0.000 (87.7) |
| Kristine, 2003 | 1.97 (1.56–2.49) | 0.000 (86.1) |
| Card et al., 2004 | 1.91 (1.51–2.42) | 0.000 (87.5) |
| Sachin, 2006 | 1.86 (1.37–2.52) | 0.000 (86.6) |
| Kathleen, 2010 | 2.00 (1.60–2.50) | 0.000 (84.2) |
| Miller et al., 2011 | 1.68 (1.44–1.96) | 0.006 (67.2) |
| Vestergaard et al., 2012 | 1.93 (1.53–2.44) | 0.000 (87.3) |
| Laura, 2013 | 1.90 (1.41–2.54) | 0.000 (87.5) |
Note: The values of P and I2 represent the heterogeneity.
Publication bias
The Begg rank correlation test and Egger linear regression test indicated no evidence of publication bias among the studies [Begg, P > |z| = 0.902; Egger, P = 0.809, 95% CI -4.98–4.05] (Figs 5 and 6).
Fig 5. Egger’s Publication Bias Plot.
Fig 6. Begg’s Funnel Plot of the 8 Cohort Studies.
Discussion
To the best of our knowledge, this is the first meta-analysis performed to describe the relationship between opioid use and fracture risk based on cohort studies. The primary findings from our study consistently suggest that opioids are associated with an 88% increase in fracture risk. When the fractures were restricted to the hip, the risk of fracture increased 2-fold. The findings are comparable to those of both a meta-analysis [11] and recent clinical trials [26,27] that demonstrated a significantly increased fracture risk associated with opioid use. In addition, our results are comparable to meta-analyses that analyzed other pharmaceutical agents and fracture risk. For instance, Eom’s study [28] showed that a significant positive association was observed between the use of selective serotonin reuptake inhibitors and the risk of fracture (OR 1.69, 95% CI 1.51–1.90), Bazelier et al. [29], using an individual patient data meta-analysis, reported a 1.2 to 1.5-fold increased risk of fractures for women using thiazolidinediones. Takkouche’s study [11] found that psychotropic medications may play an important modifiable role in the development of fractures. Studies on opioid medications have suggested that opioids are associated with a 38% increase in fracture risk. However, in Takkouche’s study [11], only six trials were included, and only three were cohort studies. Heterogeneity was present among the included studies, which may make the results unreliable. In the present study, although heterogeneity was also observed, the findings were stable and robust based on our sensitivity analysis. There are several plausible mechanisms by which opioids may increase fracture risk [30,31]. For example, adverse opioid effects, such as sedation and dizziness, can increase the propensity to fall due to central nervous system effects [32]. Opioids may decrease bone mineral density by impairing the production of endogenous sex steroids, and the effect on bone metabolism may directly weaken bone structure [32]. As elderly persons are at increased risk of developing osteoporosis and pain, the opioids used to treat pain in this population may increase the risk of subsequent fractures.
Although the relationship between opioid analgesics and fractures is well known [10,24,33,34], no convincing meta-analysis of this relationship has been conducted to date. For example, a previous meta-analysis [11] of the associations between opioid use and fracture risk did not obtain robust conclusions because of publication bias and study heterogeneity. However, in our meta-analysis, the combined result was stable and robust according to the sensitivity analysis, and publication bias was not observed.
Observational studies cannot prove causality [35]. However, a major strength of our study is that all of the included studies adopted a cohort design, and no other epidemiological observational studies were included. Six studies were prospective cohort studies that tracked patients for a long period of time. Most of the studies were of good quality and had large sample sizes and accurate outcome assessments. In addition, although a high I2 (85.6%) was present for overall fracture risk, we identified heterogeneity sources by performing subgroup analyses. In the subgroup analysis by study design type, the heterogeneity of prospective cohort studies was significantly reduced, which indicated that retrospective cohort studies may introduce heterogeneity. By performing a subgroup analysis based on study region, we found that studies conducted in the USA made major contributions to heterogeneity. Therefore, one possible reason for the heterogeneity may be the inclusion of studies conducted in different regions. In the subgroup analysis by overall fractures and hip fractures, we found that no heterogeneity was observed in the hip fracture studies, and all of the heterogeneity was derived from the overall fracture group. Two studies [21,25] played a key role in the heterogeneity. Therefore, study design and region increased the heterogeneity. Of course, many other factors may or may not have had additional contributions. We will evaluate these factors in the future when the necessary data are available.
Despite the advantages of this study, some limitations should be mentioned. First, although we searched all cohort studies describing the association of opioids with fractures, the eligible studies were restricted to the English language. The number of relevant studies was still relatively small, which implies that some studies may have been missed due to their publication in non-English language journals or publication in a book or a journal that is not included in the computer databases. Second, studies with nonsignificant results, especially those that show an absence of effect, may not be published because they are rejected by the journals or because the investigators are unwilling to submit them for publication [36]. Although we controlled for publication bias using statistical methods, publication bias could not be completely ruled out. Thus, the pooled effect measure may be overestimated. Third, the degree of control for confounding variables, such as age, gender, body mass index and comorbidity, also varied between studies. Fourth, in this meta-analysis, we were not able to investigate the effect of different opioid doses because relevant data were available in only a few studies. Fifth, it was unfortunate that we were not able to define contributing factors because all of the included papers were from Western countries and all of th pe participants were Caucasian. Therefore, our results cannot be generalized to worldwide populations, especially non-Western populations. As a result, more investigations of contributing factors, such as ethnicity, participant education level and socioeconomic class, especially in non-Western populations, are required. Sixth, we were not able to determine the fracture timing, whether medication sedation effects would be more likely shortly after starting opioid therapy, or whether metabolic biomechanical reasons would be more likely the longer the medication is taken. Thus, further research into fracture timing is needed. Finally, our meta-analysis is of good quality but is not the most comprehensive study because only cohort studies were included. Thus, higher quality and more comprehensive analyses are still needed as more data are published in the future.
Conclusions
In summary, our meta-analysis of cohort studies demonstrates that opioids may play an important role in the development of fractures and that opioid use may significantly increase fracture risk. Further studies, including studies that are well-designed, international trials (especially prospective, non-Western studies), studies that examine the mechanisms responsible for the fractures due to opioid use and studies that aim to prevent these fractures from occurring are required to provide more convincing evidence for clinical practice and fracture prevention.
Acknowledgments
No sources of funding were used in the preparation of this manuscript. All authors declare no conflicts of interest. All authors participated in the study. ZWT and YZ were responsible for the initial plan, study design, data collection, data extraction, data interpretation, manuscript drafting, statistical analysis, and performance of the study. ZWT and YZ were responsible for data collection, data extraction, and critical revision of the manuscript. FHW, YHZ, SNW, LZ and CLZ were responsible for data interpretation, manuscript drafting, supervision, and critical revision of the manuscript. XGZ and ZWT act as the guarantors for this article and take full responsibility for this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
These authors have no support or funding to report.
References
- 1. Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization study in primary care. JAMA. 1998;280: 147–151. [DOI] [PubMed] [Google Scholar]
- 2. Stewart G, Owen M. Opioids in the management of persistent non-cancer pain. Anaesth Intens Care Med. 2013;14: 533–535. [Google Scholar]
- 3. Eriksen J, Jensen MK, Sjøgren P, Ekholm O, Rasmussen NK. Epidemiology of chronic non-malignant pain in Denmark. Pain. 2003;106: 221–228. [DOI] [PubMed] [Google Scholar]
- 4. Chen L, Vo T, Seefeld L, Malarick C, Houghton M, Ahmed S, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14: 384–392. 10.1016/j.jpain.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138: 507–513. 10.1016/j.pain.2008.01.027 [DOI] [PubMed] [Google Scholar]
- 6. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81: 103–107. [DOI] [PubMed] [Google Scholar]
- 7. Shorr RI, Griffin MR, Daugherty JR, Ray WA. Opioid analgesics and the risk of hip fracture in the elderly: codeine and propoxyphene. J Gerontol. 1992;47: M111–M115. [DOI] [PubMed] [Google Scholar]
- 8. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260: 76–87. [DOI] [PubMed] [Google Scholar]
- 9. Spector W, Shaffer T, Potter DE, Correa-de-Araujo R, Rhona Limcangco M. Risk factors associated with the occurrence of fractures in U.S. nursing homes: resident and facility characteristics and prescription medications. J Am Geriatr Soc. 2007;55: 327–333. [DOI] [PubMed] [Google Scholar]
- 10. Saunders KW, Dunn KM, Merrill JO, Sullivan M, Weisner C, Braden JB, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25: 310–315. 10.1007/s11606-009-1218-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takkouche B, Montes-Martínez A, Gill SS, Etminan M. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30: 171–184. [DOI] [PubMed] [Google Scholar]
- 12. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 13. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute; Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 25 October 2011. 10.1371/journal.pntd.0002195 [DOI] [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7: 177–188. [DOI] [PubMed] [Google Scholar]
- 16. Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19: 251–253. [DOI] [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50: 1088–1101. [PubMed] [Google Scholar]
- 19. Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish osteoporosis prevention study (DOPS). Osteoporos Int. 2012;23: 1255–1265. 10.1007/s00198-011-1692-0 [DOI] [PubMed] [Google Scholar]
- 20. Guo Z, Wills P, Viitanen M, Fastbom J, Winblad B. Cognitive impairment, drug use, and the risk of hip fracture in persons over 75 years old: a community-based prospective study. Am J Epidemiol. 1998;148: 887–892. [DOI] [PubMed] [Google Scholar]
- 21. Ensrud KE, Blackwell T, Mangione CM, Bowman PJ, Bauer DC, Schwartz A, et al. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163: 949–957. [DOI] [PubMed] [Google Scholar]
- 22. Card T, West J, Hubbard R, Logan RF. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut. 2004;53: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamal-Bahl SJ, Stuart BC, Beers MH. Propoxyphene use and risk for hip fractures in older adults. Am J Geriatr Pharmacother. 2006;4: 219–226. [DOI] [PubMed] [Google Scholar]
- 24. Miller M, Stürmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59: 430–438. 10.1111/j.1532-5415.2011.03318.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carbone LD, Chin AS, Lee TA, Burns SP, Svircev JN, Hoenig HM, et al. The association of opioid use with incident lower extremity fractures in spinal cord injury. J Spinal Cord Med. 2013;36: 91–96. 10.1179/2045772312Y.0000000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of fracture in adults: A nested case-control study using the general practice research database. Am J Epidemiol. 2013;178: 559–569 178: 559–569. 10.1093/aje/kwt013 [DOI] [PubMed] [Google Scholar]
- 27. Spector WT, Shaffer T, Potter DE, Correa-de-Araujo R, Rhona Limcangco M. Risk factors associated with the occurrence of fractures in US nursing homes: resident and facility characteristics and prescription medications. J Am Geriatr Soc. 2007;55: 327–333. [DOI] [PubMed] [Google Scholar]
- 28. Eom CS, Lee HK, Ye S, Park SM, Cho KH. Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis. J Bone Miner Res. 2012;27: 1186–1195. 10.1002/jbmr.1554 [DOI] [PubMed] [Google Scholar]
- 29. Bazelier MT, De Vries F, Vestergaard P, Herings RM, Gallagher AM, Leufkens HG, et al. Risk of fracture with thiazolidinediones: an individual patient data meta-analysis. Front Endocrinol. 2013;4: 11 10.3389/fendo.2013.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain. 2002;3: 377–384. [DOI] [PubMed] [Google Scholar]
- 31. Ali II, Schuh L, Barkley GL, Gates JR. Antiepileptic drugs and reduced bone mineral density. Epilepsy Behav. 2004;5: 296–300. [DOI] [PubMed] [Google Scholar]
- 32. Daniell HW. Opioid osteoporosis. Arch Intern Med. 2004;164: 338 [DOI] [PubMed] [Google Scholar]
- 33. Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170: 1968–1978. 10.1001/archinternmed.2010.391 [DOI] [PubMed] [Google Scholar]
- 34. Solomon DH, Rassen JA, Glynn RJ, Garneau K, Levin R, Lee J, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170: 1979–1986. 10.1001/archinternmed.2010.450 [DOI] [PubMed] [Google Scholar]
- 35. Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta-analysis of cohort studies. Br J Dermatol. 2012;167: 1345–1350. 10.1111/bjd.12002 [DOI] [PubMed] [Google Scholar]
- 36. Coursol A, Wagner EE. Effect of positive findings on submission and acceptance rates: a note on meta-analysis bias. Prof Psychol Res Pre. 1986;17: 136–137. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.