Abstract
Parvovirus B19 (PVB19) is a DNA virus which causes clinically relevant infection in renal transplant recipients (RTR) leading to significant morbidity. Manifestations include erythropoietin resistant anemia, proteinuria, and glomerulosclerosis in the allograft. Severe infection may require administration of intravenous immunoglobulin, reduction in immunosuppression and transfusions. The major challenge in managing and preventing the infection in RTR involves the act of balancing the decreased level of immunosuppression and the risk of rejection. The objective of this article is to understand the importance of PVB19 infection and its outcome in RTR. We reviewed the medical records of three RTR with confirmed PVB19 infection and recorded patient information including demographics, clinical and laboratory data, management, and outcome. The average time of occurrence of PVB19 infection as transplant was 8.6 weeks and they presented with symptomatic anemia. Elevated creatinine values were noted in two of them. Following treatment, anemia improved and creatinine values returned to baseline. One of them developed an early relapse and had to be treated once again similarly. We emphasize the importance of maintaining a high index of suspicion for PVB19 infection in patients with anemia in the posttransplant phase, especially in patients on higher doses of immunosuppressants. Early and proper treatment can prevent worsening clinical condition and possible effects on the allograft.
Keywords: allograft, anemia, immunosuppression, intravenous immunoglobulin, parvovirus B19, renal transplant
Parvovirus B19 (PVB19) is a nonenveloped DNA virus which is a ubiquitous human pathogen, primarily spread via respiratory droplets. The infection can be symptomatic or asymptomatic according to the host's age and immunologic status. The natural course of the infection in immunosuppressed individuals such as renal transplant recipients (RTR) are varied.1 Although anemia is the most common manifestation,2 PVB19 can also cause pancytopenia, hepatitis, myocarditis, neurological disease,1 3 4 and allograft dysfunction.5 Transmission can occur to the recipient from the allograft itself, as the virus may persist for years in seropositive individuals.6 Therefore, it may be relevant to screen selected donors for PVB19 and monitor for possible manifestations such as resistant anemia in RTR. The major challenge in managing and preventing PVB19 infection in RTR involves the act of balancing the decreased level of immunosuppression and the risk of rejection.
Patients and Methods
We retrospectively reviewed the medical records of 144 RTR who were followed up at a tertiary care hospital affiliated transplant program from January 2007 to June 2013 and selected three patients with confirmed PVB19 infection. The maintenance immunosuppression protocol that was followed consisted of tacrolimus, mycophenolate mofetil, and prednisone. These patients presented with anemia in the posttransplant period, and were subsequently diagnosed with PVB19 infection. We collected patient information including demographics, clinical, and laboratory data. The detection of infection was done by polymerase chain reaction (PCR) or immunoglobulin M (IgM) serology of blood samples or bone marrow study indicative of PVB19. The management and outcome was also recorded. The study has been approved by the Institutional Review Board at the hospital.
Results
Of 144 RTR, there were 3 cases (2%) of PVB19 infection. All recipients had normal baseline creatinine posttransplant. The average time for detection of PVB19 infection since transplant was 8.6 weeks and they presented with symptomatic anemia. The lowest hemoglobin (Hb) value noted was 6 mg/dL. Epstein-Barr virus and cytomegalovirus were the associated coinfections. Elevated creatinine values were noted in two of them. Following treatment, anemia improved and creatinine values returned to baseline. Sustained improvement in Hb was seen after 4 weeks in two of them and after 12 weeks in the third patient. One of them developed an episode of recurrence.
Refer to Tables 1 to 3 for details regarding patient characteristics, presentation and investigations, treatment and outcome, respectively.
Table 1. General information of the recipients and donors.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age (y) | 58 | 44 | 30 |
| Gender | M | F | M |
| Comorbidities | DM, HTN | HTN | HTN |
| Cause of ESRD | DM, HTN | Obstructive uropathy | Chronic glomerulonephritis |
| Year of transplant | 2013 | 2010 | 2008 |
| Age of donor (y) | 23 | 41 | 22 |
| Type of donor | Live | Live | Live |
| HLA mismatches | 5/6 | 6/6 | 5/6 |
| Induction protocol | Basiliximab, steroid | Basiliximab, steroid | Basiliximab, steroid |
| Immunosuppression protocol | Tacrolimus, MMF, prednisone | Tacrolimus, MMF, prednisone | Tacrolimus, MMF, prednisone |
| Acute rejection | Acute cellular rejection at 2 weeks posttransplant, confirmed with biopsy, treated with pulse IV Solumedrol | No | No |
| Baseline creatinine (mg/dL) | 1.3 | 1.12 | 1.2 |
Abbreviations: DM, diabetes mellitus; ESRD, end-stage renal disease; F, female; HLA, human leukocyte antigen; HTN, hypertension; M, male; MMF, mycophenolate mofetil.
Table 3. Treatment and outcome of the patients.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Treatment | IVIG infusion, decreased immunosuppression, 3 units PRBC transfusion | IVIG infusion, decreased immunosuppression, 4 units PRBC transfusion | IVIG infusion, decreased immunosuppression, 2 units PRBC transfusion |
| Hb posttreatment (g/dL) | 12.1 | 13.1 | 11.5 |
| Cr posttreatment (mg/dL) | 1.19 | 1.15 | 1.3 |
| Sustained Hb improvement | After 4 wk | After 4 wk | After 12 wk |
| Recurrences | No | No | Yes, one recurrence. treated similarly |
| Allograft dysfunction | Transient | Transient | No |
| Outcome | Anemia resolved, no allograft dysfunction, viral titers decreased on follow-up, patient is still on routine immunosuppression protocol because of his history of acute rejection | Anemia resolved, no allograft dysfunction, viral serology became negative on follow-up. Patient is on a reduced dose of immunosuppression as the infection | Anemia resolved, no allograft dysfunction, viral IgM serology became negative. Patient is on a reduced dose of immunosuppression since the infection |
Abbreviations: Cr, creatinine; Hb, hemoglobin; IgM, immunoglobulin M; IVIG, intravenous immunoglobulin; PRBC, packed red blood cells.
Table 2. Parvovirus B19 infection presentation and investigations.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Time of parvovirus infection posttransplant | 8 wk | 7 wk | 11 wk |
| Presentation | Dizziness, dyspnea on exertion, dark stools | Dizziness, dark stools | Dizziness, dyspnea on exertion, palpitation |
| Physical exam | HR, 89; BP, 134/74; RR, 18; Temp, 98, SpO2, 96% | HR, 100; BP, 130/54; RR, 18, Temp, 98.6; SpO2, 98% | HR, 86; BP,110/68; RR,18; Temp, 98.4; SpO2, 98% |
| Investigations: blood | Hb, 6.6; Hct, 19.6; RBC count, 2.34; TC, 4,600; Plt, 295, RBC indices, normal | Hb, 7.1; Hct, 21.2; RBC count, 2.31; TC, 6,300; Plt, 419; RBC indices, normal | Hb, 6.3; Hct, 18.4; RBC count, 2.22; TC, 4,600; Plt, 320; RBC indices, normal |
| Reticulocyte count, 0.3% | Reticulocyte count, 0.2% | Reticulocyte count, 0.2% | |
| RBS, 230; Na, 140; K, 4.1; Cl, 106; HCO3, 21 | RBS, 86; Na, 137; K,4.5; Cl, 105; HCO3, 20 | RBS, 113; Na, 130; K,4.7; Cl, 100; HCO3, 21 | |
| BUN/Cr, 31/1.51 | BUN/Cr, 20/1.27 | BUN/Cr, 13/1.1 | |
| LDH, 134 | LDH, 188 | LDH, 144 | |
| Iron studies-increased iron and ferritin, decreased TIBC | Iron studies-normal iron, increased ferritin, decreased TIBC | Iron studies-increased iron and ferritin, decreased TIBC | |
| Haptoglobin, normal; B12 and folate, normal | Haptoglobin, normal; B12 and folate, normal | Haptoglobin, normal; B12 and folate, normal | |
| Urinalysis, no proteinuria | Urinalysis, no proteinuria | Urinalysis, no proteinuria | |
| Others | FOB, negative | FOB, positive | Not indicated |
| CT abdomen-no evidence of bleeding | CT abdomen-no evidence of bleeding | CT abdomen-no evidence of bleeding | |
| OGD-erosive gastropathy | OGD-normal | Not indicated | |
| Colonoscopy- normal | Colonoscopy-pan colitis | Not indicated | |
| Initial management | Aspirin stopped and patient monitored for bleeding. In spite of no active bleeding, Hb continued to drop rapidly and Parvovirus infection was suspected | Treated conservatively for colitis, no further bleeding but Hb continued to drop, BMA done and showed erythroid aplasia. Renal biopsy was done due to rise in creatinine and it showed hydropic changes in tubular epithelial cells | Hb continued to drop, BMA done which showed erythroid aplasia. |
| Detection of parvovirus | Positive PCR | Bone marrow study, positive PCR | Positive PCR, positive IgM serology, bone marrow study |
| Co infections | CMV PCR positive, EBV antigen positive | EBV antigen positive | CMV PCR positive |
| Lowest Hb level after diagnosis | 6 | 6.2 | 6 |
| Highest Cr level after diagnosis | 1.74 | 2.25 | 1.3 |
Abbreviations: BP, blood pressure (mm Hg); BMA, bone marrow aspiration; BUN, blood urea nitrogen (mg/dL); Cl, chloride (mEq/L); CMV, cytomegalovirus; Cr, creatinine (mg/dL); EBV, Epstein-Barr virus; FOB, fecal occult blood; Hb, hemoglobin (g/dL); HCO3, bicarbonate (mEq/L); Hct, hematocrit (%); HR, heart rate (no of beats/min); K, potassium (mEq/L); LDH, lactate dehydrogenase (IU/L); Na, sodium (mEq/L); OGD, esophagoduodenoscopy; PCR, polymerase chain reaction; Plt, platelet; RBS, random blood sugar (mg/dL); RBC, red blood cells; RR, respiratory rate (per min); TC, total count; Temp, temperature (°F); TIBC, total iron binding capacity.
Refer to Fig. 1 for graph showing variations in Hb of the three patients and Fig. 2 showing variations in creatinine values of the three patients.
Fig. 1.
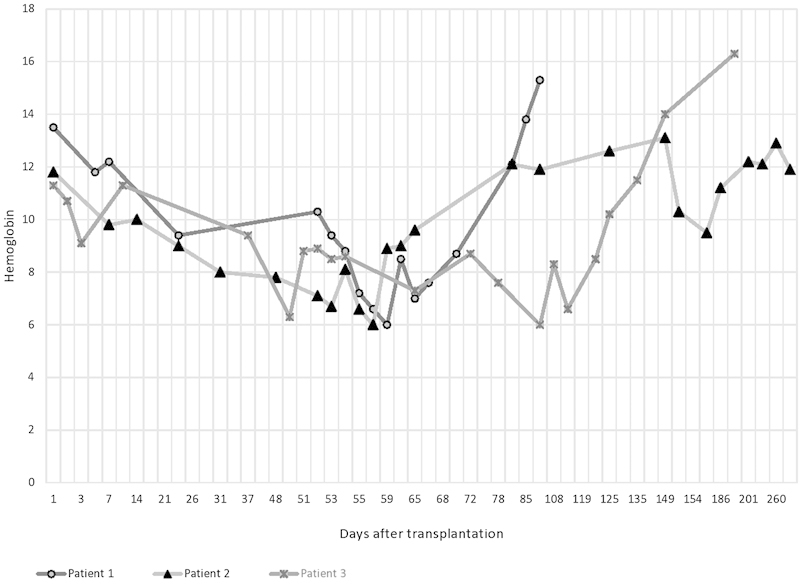
Graph showing variations in hemoglobin of the three patients.
Fig. 2.
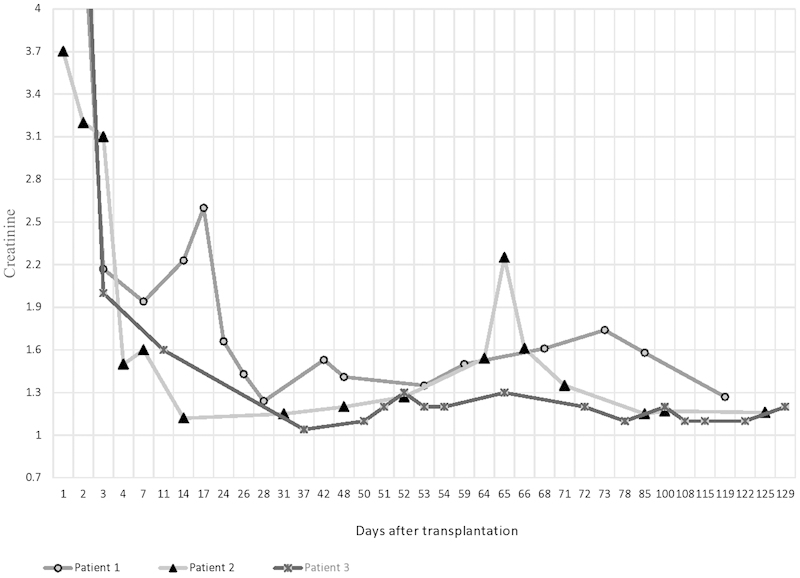
Graph showing variations in creatinine values of the three patients.
Discussion
RTR are highly susceptible to infections such as PVB197 because of the increased use of induction therapy to prevent early acute rejection and because of the effect of sustained long-term immunosuppression to prevent chronic rejection.
A study in 2006 identified that the median time for occurrence of PVB19 infection after transplantation was around 1.75 months, with majority of the cases occurring within 3 months posttransplant.5
Chronic anemia is well-recognized complication in approximately 39% of RTR, with erythropoietin-resistant anemia occurring in almost 9% of them.8 PVB19 is capable of targeting erythroid progenitor cells in the bone marrow leading to a cessation in erythropoiesis. This usually reverts back to normal by the production of antibodies against the virus in immunocompetent individuals. However in RTR, such infections often lead to persistent pure red cell aplasia with normal white cell and platelet counts.8 Studies suggest that nearly 23% of RTR with persistent anemia had positive PCR values for PVB19.8
Immunosuppression is the major risk factor for the infection in RTR, indicated by improvement in anemia when immunosuppression is decreased.9 Induction with antithymocyte globulin is found to have a higher risk for the infection compared with basiliximab.10 Substitution of tacrolimus with cyclosporine was also followed by viral clearance and resolution of anemia.9 11 Vigilance should be observed during high-risk periods such as early after transplantation and after treatment for rejection, when the dose of immunosuppressants are high.4
Coinfection of PBV19 and other viruses such as cytomegalovirus and with human herpes virus 6 have also been reported.12
Detection of PVB19 can be done via molecular techniques or by measurement of viral antibodies. IgM assays will detect a recent infection,13 but it is less reliable due to delayed or inadequate humoral response.14 Viral DNA can be detected in blood, bone marrow, and infected organs using PCR15 and is also detected in asymptomatic individuals.16 However, the detection of viral DNA with clinical findings is likely to represent active infection. If serology and PCR are negative but the clinical suspicion is high, examination of bone marrow specimen using immunohistochemical staining or in situ hybridization is helpful in establishing the diagnosis.5
The main histopathological pictures of PVB19 infection of allograft kidney are thrombotic microangiopathy and collapsing glomerulopathy.17 18 19 Reports have shown an elevation of plasma creatinine and proteinuria.20
The treatment of PBV19 is reduction of immunosuppression and intravenous immunoglobulin (IVIG) administration.21 It is also important to avoid the use of erythropoietin to combat the anemia while treating PVB19, as this can lead to a resistance of the virus to proven treatment.22
Conclusion
PVB19 is an important treatable cause of anemia in RTR. A high index of suspicion should be maintained in patients with anemia in the posttransplant phase, especially those who have received higher doses of immunosuppressants. It may be relevant to screen-selected donors for asymptomatic PVB19 infection and to keep an eye out for anemia in RTR if the donor has the infection. Further studies on a larger sample are required to determine whether recommendations for routine donor screening need to be made. Early diagnosis and appropriate intervention can minimize the negative impacts of the infection.
Acknowledgment
We thank all the staff members at North Shore University Hospital transplant program for their wholehearted support.
References
- 1.Lee P C, Hung C J, Lin Y J, Wang J R, Jan M S, Lei H Y. A role for chronic parvovirus B19 infection in liver dysfunction in renal transplant recipients? Transplantation. 2002;73(10):1635–1639. doi: 10.1097/00007890-200205270-00019. [DOI] [PubMed] [Google Scholar]
- 2.Eid A J Chen S F; AST Infectious Diseases Community of Practice. Human parvovirus B19 in solid organ transplantation Am J Transplant 20131304201–205. [DOI] [PubMed] [Google Scholar]
- 3.Liefeldt L, Plentz A, Klempa B. et al. Recurrent high level parvovirus B19/genotype 2 viremia in a renal transplant recipient analyzed by real-time PCR for simultaneous detection of genotypes 1 to 3. J Med Virol. 2005;75(1):161–169. doi: 10.1002/jmv.20251. [DOI] [PubMed] [Google Scholar]
- 4.Waldman M, Kopp J B. Parvovirus B19 and the kidney. Clin J Am Soc Nephrol. 2007;2 01:S47–S56. doi: 10.2215/CJN.01060307. [DOI] [PubMed] [Google Scholar]
- 5.Eid A J, Brown R A, Patel R, Razonable R R. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis. 2006;43(1):40–48. doi: 10.1086/504812. [DOI] [PubMed] [Google Scholar]
- 6.Waldman M, Kopp J B. Parvovirus-B19-associated complications in renal transplant recipients. Nat Clin Pract Nephrol. 2007;3(10):540–550. doi: 10.1038/ncpneph0609. [DOI] [PubMed] [Google Scholar]
- 7.Sturm I, Watschinger B, Geissler K. et al. Chronic parvovirus B19 infection-associated pure red cell anaemia in a kidney transplant recipient. Nephrol Dial Transplant. 1996;11(7):1367–1370. [PubMed] [Google Scholar]
- 8.Egbuna O, Zand M S, Arbini A, Menegus M, Taylor J. A cluster of parvovirus B19 infections in renal transplant recipients: a prospective case series and review of the literature. Am J Transplant. 2006;6(1):225–231. doi: 10.1111/j.1600-6143.2005.01139.x. [DOI] [PubMed] [Google Scholar]
- 9.Geetha D, Zachary J B, Baldado H M, Kronz J D, Kraus E S. Pure red cell aplasia caused by Parvovirus B19 infection in solid organ transplant recipients: a case report and review of literature. Clin Transplant. 2000;14(6):586–591. doi: 10.1034/j.1399-0012.2000.140612.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim J M, Jang H R, Kwon C H. et al. Rabbit antithymocyte globulin compared with basiliximab in kidney transplantation: a single-center study. Transplant Proc. 2012;44(1):167–170. doi: 10.1016/j.transproceed.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 11.Wong T Y, Chan P K, Leung C B, Szeto C C, Tam J S, Li P K. Parvovirus B19 infection causing red cell aplasia in renal transplantation on tacrolimus. Am J Kidney Dis. 1999;34(6):1132–1136. doi: 10.1016/S0272-6386(99)70021-1. [DOI] [PubMed] [Google Scholar]
- 12.Barzon L, Murer L, Pacenti M. et al. Investigation of intrarenal viral infections in kidney transplant recipients unveils an association between parvovirus B19 and chronic allograft injury. J Infect Dis. 2009;199(3):372–380. doi: 10.1086/596053. [DOI] [PubMed] [Google Scholar]
- 13.Bruu A L, Nordbø S A. Evaluation of five commercial tests for detection of immunoglobulin M antibodies to human parvovirus B19. J Clin Microbiol. 1995;33(5):1363–1365. doi: 10.1128/jcm.33.5.1363-1365.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzman G J, Ozawa K, Cohen B, Hanson G, Oseas R, Young N S. Chronic bone marrow failure due to persistent B19 parvovirus infection. N Engl J Med. 1987;317(5):287–294. doi: 10.1056/NEJM198707303170506. [DOI] [PubMed] [Google Scholar]
- 15.Manaresi E, Gallinella G, Zuffi E, Bonvicini F, Zerbini M, Musiani M. Diagnosis and quantitative evaluation of parvovirus B19 infections by real-time PCR in the clinical laboratory. J Med Virol. 2002;67(2):275–281. doi: 10.1002/jmv.2218. [DOI] [PubMed] [Google Scholar]
- 16.Cassinotti P, Siegl G. Quantitative evidence for persistence of human parvovirus B19 DNA in an immunocompetent individual. Eur J Clin Microbiol Infect Dis. 2000;19(11):886–887. doi: 10.1007/s100960000384. [DOI] [PubMed] [Google Scholar]
- 17.Ardalan M R, Shoja M M, Tubbs R S, Esmaili H, Keyvani H. Postrenal transplant hemophagocytic lymphohistiocytosis and thrombotic microangiopathy associated with parvovirus b19 infection. Am J Transplant. 2008;8(6):1340–1344. doi: 10.1111/j.1600-6143.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- 18.Barsoum N R, Bunnapradist S, Mougdil A, Toyoda M, Vo A, Jordan S C. Treatment of parvovirus B-19 (PV B-19) infection allows for successful kidney transplantation without disease recurrence. Am J Transplant. 2002;2(5):425–428. doi: 10.1034/j.1600-6143.2002.20505.x. [DOI] [PubMed] [Google Scholar]
- 19.Zolnourian Z R, Curran M D, Rima B K, Coyle P V, O'Neill H J, Middleton D. Parvovirus B19 in kidney transplant patients. Transplantation. 2000;69(10):2198–2202. doi: 10.1097/00007890-200005270-00043. [DOI] [PubMed] [Google Scholar]
- 20.Cavallo R, Merlino C, Re D. et al. B19 virus infection in renal transplant recipients. J Clin Virol. 2003;26(3):361–368. doi: 10.1016/s1386-6532(02)00104-x. [DOI] [PubMed] [Google Scholar]
- 21.Liefeldt L, Buhl M, Schweickert B. et al. Eradication of parvovirus B19 infection after renal transplantation requires reduction of immunosuppression and high-dose immunoglobulin therapy. Nephrol Dial Transplant. 2002;17(10):1840–1842. doi: 10.1093/ndt/17.10.1840. [DOI] [PubMed] [Google Scholar]
- 22.Eid A J Posfay-Barbe K M; AST Infectious Diseases Community of Practice. Parvovirus B19 in solid organ transplant recipients Am J Transplant 20099404S147–S150. [DOI] [PubMed] [Google Scholar]


