Abstract
Background
This prospective, randomized controlled study was undertaken to compare stress hormone response to open thoracotomy for lung resection at different anesthetic depths, as determined by bispectral index (BIS) monitoring, in patients under propofol-remifentanil anesthesia.
Methods
Forty-eight adult patients scheduled for lung resection surgery using one-lung ventilation were randomly assigned to either a deep anesthesia group (BIS score of 40 ± 5, n = 24) or a light anesthesia group (BIS score of 60 ± 5, n = 24) by adjusting propofol infusion rates. Blood norepinephrine, epinephrine, adrenocorticotropic hormone, and cortisol levels were measured before the induction of anesthesia, at the end of surgery, and at 2 hours postoperatively. Blood glucose, hemodynamic, and oxygenation-ventilation variables, and postoperative outcomes were also measured.
Results
Norepinephrine and epinephrine levels remained unchanged over time in the deep group, but norepinephrine levels significantly increased in the light group at 2 h after surgery than at baseline (P = 0.007 and 0.004, respectively). Temporal changes in norepinephrine, but not in epinephrine, were significantly different between the two groups (P = 0.036). Plasma glucose levels in the light group increased with time and were significantly higher than in the deep group at the end of surgery (P = 0.002).
Conclusions
A deep level of anesthesia achieved using high propofol infusion rates during lung surgery provided lower perioperative norepinephrine and glucose responses than light level of anesthesia during the early postoperative period but failed to affect immediate postoperative outcomes.
Keywords: Bispectral index monitor, Catecholamines, Propofol, Pulmonary surgical procedures, Stress
Introduction
Neuroendocrine response to surgical stress increases secretions of adrenocorticotrophic hormone (ACTH), cortisol, aldosterone, and catecholamines, such as, norepinephrine (NE) and epinephrine, to blood from the hypothalamus-pituitary-adrenal (HPA) axis and sympathetic nervous system, and consequently increases catabolism [1]. The magnitude of stress response generally depends on the severity of surgical injury and is linked to adverse short-term outcomes after surgery [2,3]. In lung resection surgery open thoracotomy provokes much greater inflammatory and cytokine responses than video-assisted thoracoscopic surgery due to the greater severity of surgical trauma [4]. Thus, the suppression of stress response may offer a means of improving postoperative outcomes in patients, especially in those with substantial co-morbidities after open lung resection.
Although no gold standard has been established yet to monitor stress response to surgery directly, increased sympathetic activity reflected by tachycardia and hypertension is often regarded to reflect stress response during surgery and is managed by deepening anesthesia and increasing supplemental analgesia in clinical practice. However, studies on the effects of different anesthetic depths achieved using different anesthetic doses on surgical stress response have reported conflicting results. Baldini et al. [5] reported that depth of desflurane anesthesia did not influence endocrine-metabolic response to adult pelvic surgery. On the other hand, total intravenous anesthesia (TIVA) using propofol and opioid was suggested to reduce stress response compared with inhalational anesthetic-based anesthesia during pulmonary resection [6], and deep propofol anesthesia, guided by BIS values in the range of 25-35, was found to offer more effective stress response reduction in children undergoing adenoidectomy and tonsillectomy [7]. However, it remains to be clarified whether different anesthetic depths achieved by adjusting propofol infusion rates affect neuroendocrine response to major thoracic surgery involving a high degree of surgical stimulation.
In the present study, we compared deep and light levels of anesthesia, as determined by BIS monitoring, under TIVA using propofol and remifentanil with respect to stress hormone response to thoracotomy for lung resection. The primary endpoints were changes in plasma catecholamine levels and the secondary end-points were metabolic and inflammatory responses, hemodynamic variables, and postoperative outcomes in the immediate perioperative period.
Materials and Methods
This prospective, randomized, single-blinded study was approved by the Institutional Ethics Committee. Written informed consent was obtained from all patients. Forty-eight patients, aged between 18 and 75, scheduled for lung resection surgery under TIVA using propofol and remifentanil were initially enrolled. The exclusion criteria were empyema, lung abscess, a recent history of medication known to affect sympathetic response or hormonal secretions, allergy to the medications used, drug or alcohol abuse, diabetes mellitus, an endocrine or neuropsychiatric disease, cardiac disease categorized as NYHA class II-IV, severe respiratory dysfunction (vital capacity of < 50% or a forced expiratory volume in 1 sec of < 50% of predicted), renal insufficiency (creatinine > 1.5 mg/dl), or hepatic dysfunction (aspartate aminotransferase > 40 IU/L or alanine aminotransferase > 40 IU/L).
Intramuscular glycopyrrolate 0.2 mg was given 30 minutes before surgery. On arrival in the operating room, patients were continuously monitored by Electrocardiography and pulse oximetry. A 20 gauge intravenous catheter was inserted into the radial artery under local anesthesia to measure invasive arterial pressure and to collect blood samples for arterial blood gas and biochemical analysis. BIS electrodes were placed on the forehead to monitor depth of anesthesia using a BIS monitor (Model DSC-XP, DX5800; Aspect Medical Systems, Norwood, MA, USA). After achieving a baseline recording at 10 minutes after arterial cannulation, general anesthesia was induced by target controlled infusion (TCI) of propofol (5 µ/ml) and remifentanil (2 ng/ml) using the Marsh and Minto pharmacokinetic model, respectively. After loss of consciousness, vecuronium 0.1 mg/kg was administered to facilitate orotracheal intubation. After inserting double-lumen endobronchial tube (DLT, Silbroncho®, 35-37 Fr, Fuji systems Co., Tokyo, Japan), both lungs were ventilated with a mixture of 50% oxygen in air to maintain eucapnea.
Immediately after the induction of general anesthesia, patients were allocated to one of the two study groups, the light anesthesia group (Light group, n = 24) or the deep anesthesia group (Deep group, n = 24) using a computer-generated randomization schedule. By adjusting the TCI of propofol, BIS score was maintained at 60 ± 5 in the light group and at 40 ± 5 in the deep group. Remifentanil was maintained at a target effect-site concentration of 2 ng/ml until the end of surgery in both groups. Vecuronium was continuously administered to less than two responses to train-of-four stimulation to minimize the influence of muscle movements on BIS values until the completion of lung resection. In each patient, a double-lumen central venous catheter was inserted via the right internal jugular vein to monitor central venous pressure and for the rapid infusion of intravenous volume, as clinically indicated. A forced-air warming blanket was positioned over the exposed part of the body and a fluid warmer (Ranger® Blood and Fluid Warming Systems, Arizant Healthcare Inc. Eden Prairie, MN, USA) was used to maintain normothermia during surgery.
In all patients, surgery was started between 8:30 and 9:00 am to avoid bias caused by circadian rhythms of circulating stress hormones (cortisol). After placing the patient in the lateral decubitus position, the position of the DLT was confirmed using a fiberoptic bronchoscope. One-lung ventilation (OLV) was started with pressure controlled ventilation at a tidal volume of 6-8 ml/kg and a PEEP of 5 cmH2O to maintain EtCO2 between 35 and 45 mmHg, oxygen saturation at > 95%, and peak airway pressure at < 30 cmH2O. Intra-operative mean arterial pressure (MAP) was kept within 30% of baseline and hypotension or hypertension lasting > 5 min was treated with intermittent doses of phenylephrine (20 µg/ml) or nicardipine (500 µg/ml), respectively.
After completing the surgical procedure, the collapsed lung was re-inflated using a peak airway pressure of 20 cmH2O and then both lungs were ventilated until skin suture closure. At the end of surgery, propofol and remifentanil TCI were discontinued simultaneously and residual neuromuscular block was antagonized using pyridostigmine 0.2 mg/kg and glycopyrrolate 0.008 mg/kg. For postoperative pain management, continuous infiltration of 0.375% ropivacaine into the subcutaneous space was started at 4 ml/h using an On-Q painbuster (I-Flow Co, Lake Forest, CA, USA) by the surgeon 10 min before the discontinuation of anesthetic agents, and then maintained for 48 hours. All patients were extubated in the operating room after recovery of consciousness and spontaneous ventilation, and then transferred to a post-anesthetic care unit (PACU). Intravenous fentanyl 1 ug/kg was administered when the visual analogue scale (VAS) were higher than 4 at rest during stay in the PACU.
Arterial blood samples were collected for arterial blood gas analysis and for determining concentrations of epinephrine, NE, cortisol, ACTH, glucose, and C-reactive protein (CRP), blood cell counts at three time points, before the administration of anesthetics (T0; baseline), at the end of surgery (T1), and at 2 hours after surgery (T2). Blood samples for catecholamine and hormone analyses were immediately centrifuged and analyzed within 24 hours of collection. Plasma epinephrine and NE levels were measured by high-performance liquid chromatography, and plasma ACTH and serum cortisol levels were determined using radioimmunoassay kits (Neodin Medical Institute, Seoul, Korea). Pain levels were assessed at 2 hours postoperatively using a VAS at rest (0 = no pain and 10 = worst imaginable pain). All patients were asked whether they could recall intraoperative events at 2 hours postoperatively. Anesthesia and surgery-related outcomes were also recorded.
Power analysis was conducted using G*Power 3.1.5. Estimates of effective sizes were made using preliminary data. An effect size of 0.93 was calculated using a mean NE level of 153 with a SD of 125 in the deep group and 287 with a SD of 160 in the light group. A sample size of 21 participants per group was found to provide 80% power to detect the effect size with a set α of 0.05 for a two-sided design. Taking into account a potential drop-out rate of 15%, 24 patients were allocated per group. Statistical analyses were performed using SPSS version 19 (SPSS Inc, Chicago, IL, USA). The Kolmogorov-Smironov test was used to verify normal data distributions. Results are expressed as means ± SDs for continuous variables and as percentages for categorical variables. Continuous variables were analyzed using the student's t test or the Mann-Whitney U test for parametric or nonparametric data, respectively. Categorical variables were analyzed using the Chi-Square test. Repeated measures ANOVA and Friedman's repeated measures ANOVA on ranks were used to compare normally and non-normally distributed hemodynamic and biochemical variables in the two groups at different time points. Inter-variable correlation analysis was performed using Pearson's coefficients or Spearman's rank coefficients for normally and non-normally distributed variables, respectively, and was followed by post hoc Bonferroni's correction when an inter-group difference was detected. Statistical significance was accepted for P values of < 0.05.
Results
The data of 42 patients were analyzed (Fig. 1). No significant intergroup differences in demographics, anesthetic, or surgical data were found, except for a higher propofol infusion rate in the deep group (P < 0.001, Table 1). Changes in MAP throughout the study period, that is, from the commencement of surgery until 2 hours postoperatively, were maintained within 15% of baseline value in both groups, despite a significant lower MAP in the deep group at T1 (P < 0.05, Table 2). Changes in heart rate over time were also comparable in the two groups despite a significantly higher baseline value in the deep group (P < 0.05). No significant intergroup differences were observed in requirements for intravenous fluid, transfusion, or vasopressor use (Table 1). Furthermore, changes in ventilation and oxygenation variables from T0 to T2 were similar in the two groups (Table 2).
Fig. 1. Flow diagram showing patient flow according to the study protocol.
Table 1. Demographic and Clinical Characteristics of Patients.
| Deep group (n = 21) | Light group (n = 21) | P value | |
|---|---|---|---|
| Age (yr) | 57.8 ± 14.7 | 63.3 ± 11.9 | 0.188 |
| Gender (M/F) | 17/4 | 15/6 | 0.469 |
| Weight (kg) | 56.9 ± 6.1 | 58.9 ± 8.5 | 0.403 |
| Height (cm) | 164.4 ± 8.7 | 161.3 ± 11.3 | 0.326 |
| ASA physical status (1/2/3) | 4/15/2 | 2/15/4 | 0.561 |
| Type of Surgery (n) | 1.000 | ||
| Wedge resection | 4 (19.0) | 3 (14.3) | |
| Segmentectomy | 1 (4.8) | 2 (9.5) | |
| Lobectomy | 14 (66.7) | 15 (71.4) | |
| Pneumonectomy | 2 (9.5) | 1 (4.8) | |
| Propofol Dose (mg/kg/h) | 8.2 ± 2.4 | 4.2 ± 1.0 | < 0.001 |
| Remifentanil Dose (µg/kg/h) | 3.2 ± 1.2 | 3.1 ± 1.3 | 0.838 |
| BIS score at end of surgery | 39.3 ± 2.5 | 62.6 ± 5.9 | < 0.001 |
| Intraoperative fluid (ml) | 1795 ± 857 | 2019 ± 841 | 0.396 |
| EBL (ml) | 394 ± 244 | 305 ± 179 | 0.194 |
| Nicardipine requirement (n) | 7 (33.3) | 9 (42.9) | 0.525 |
| Phenylephrine requirement (n) | 11 (52.4) | 5 (23.8) | 0.057 |
| Duration of OLV (min) | 136.8 ± 60.9 | 147.4 ± 69.1 | 0.600 |
| Duration of Surgery (min) | 192.4 ± 74.5 | 197.8 ± 64.9 | 0.804 |
| Duration of Anesthesia (min) | 262.3 ± 79.7 | 271.3 ± 80.6 | 0.719 |
| Postoperative VAS (cm) | 4.6 ± 2.4 | 4.8 ± 2.9 | 0.790 |
| Fentanyl requirement in postoperative 2 h (n) | 7 (35.0) | 8 (40.0) | 0.744 |
Data are presented as means ± SDs or numbers (%) of patients. ASA: American Society of Anesthesiologists, BIS: bispectral index, EBL: estimated blood loss during surgery, OLV: one-lung ventilation, VAS: visual analogue scale.
Table 2. Changes in Hemodynamic and Arterial Blood Gas Variables.
| Deep group (n = 21) | Light group (n = 21) | ||
|---|---|---|---|
| MAP (mmHg) | T0 | 102.8 ± 14.2 | 103.4 ± 14.3 |
| T1 | 89.7 ± 9.2*,† | 96.4 ± 10.6 | |
| T2 | 98.8 ± 11.9§ | 91.3 ± 12.3† | |
| Heart rate (beats/min) | T0 | 85.3 ± 16.7* | 74.0 ± 8.7 |
| T1 | 73.3 ± 14.3† | 75.5 ± 15.9 | |
| T2 | 83.2 ± 15.2‡ | 84.9 ± 13.9†,‡ | |
| PaCO2 (mmHg) | T0 | 39.6 ± 4.2 | 39.2 ± 2.5 |
| T1 | 41.6 ± 5.8 | 42.0 ± 6.4 | |
| T2 | 40.2 ± 4.5 | 39.6 ± 6.5 | |
| PaO2/FIO2 (mmHg) | T0 | 464.4 ± 76.4 | 453.6 ± 117.3 |
| T1 | 185.8 ± 64.3† | 181.4 ± 46.9† | |
| T2 | 404.2 ± 175.2‡ | 418.6 ± 180.1‡ |
Data are presented as means ± SDs. MAP: Mean arterial pressure, T0: before induction of anesthesia, T1: end of surgery, T2: 2 hours after surgery. *P < 0.05 between the two groups, †P < 0.01 compared with T0 within group, ‡,§P < 0.01 and < 0.05, respectively, compared with T1 within group.
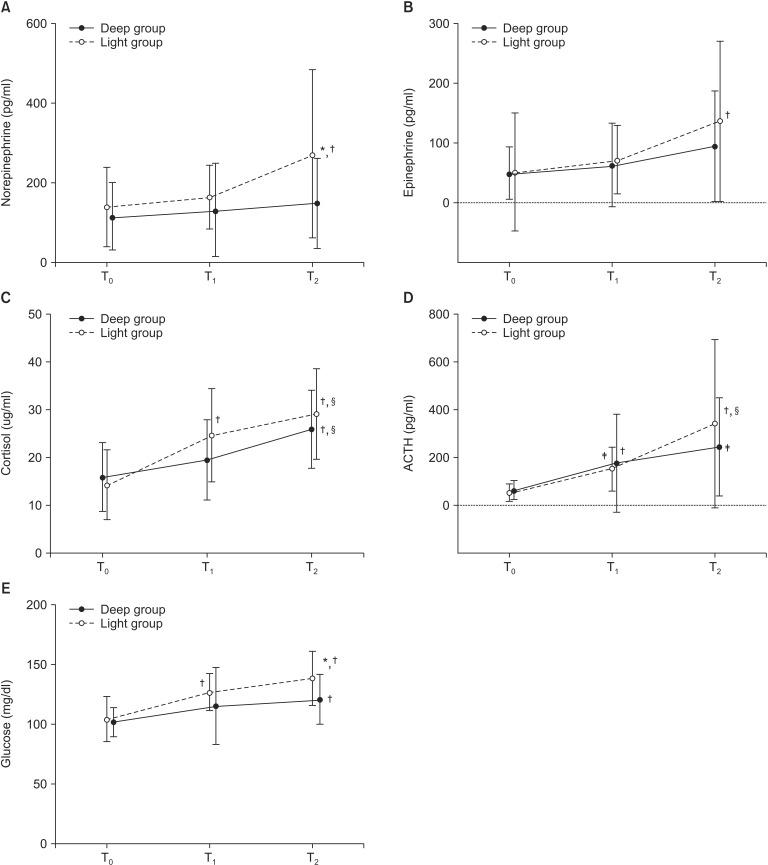
Baseline concentrations of NE, epinephrine, ACTH, cortisol, and glucose in the two groups were comparable (Fig. 2). Both groups showed a moderate negative correlation between age and plasma cortisol before induction of anesthesia (ρ = -0.468 and -0.469 in the deep and light groups, respectively, P < 0.05). Plasma NE and epinephrine levels in the two groups remained unchanged from baseline until the end of surgery. However, at 2h after surgery both catecholamine levels remained unchanged in the deep group but had increased significantly in the light group (P < 0.01), but only NE levels were significantly different between two groups at T2 (P < 0.05). Plasma glucose levels increased versus baseline in both groups but were significantly higher in the light group at T2 (P < 0.01). CRP levels were significantly higher at T2 than at T1 in the light group (Table 3, P < 0.05), but no significant intergroup difference was evident. Increases in leukocyte and segmented neutrophil counts, and decreases in blood lymphocyte counts were similar in the two groups at all time-points.
Fig. 2. Changes in neuroendocrine hormone and glucose levels during the perioperative period. (A) Changes in plasma norepinephrine levels. (B) Changes in plasma epinephrine levels. (C) Changes in serum cortisol levels. (D) Changes in plasma ACTH levels. (E) Changes in plasma glucose levels. ACTH: adrenocorticotropic hormone, T0: before induction of anesthesia, T1: end of surgery, T2: 2 hours after surgery. *P < 0.01 between two groups. †,‡P < 0.01 and < 0.05, respectively, compared with T0 within group. §P < 0.05 compared with T1 within group.
Table 3. Changes in C-reactive Protein levels and Leukocyte Counts.
| Deep group (n = 21) | Light group (n = 21) | ||
|---|---|---|---|
| CRP (mg/ml) | T0 | 3.3 ± 4.4 | 3.3 ± 5.8 |
| T1 | 3.2 ± 5.3 | 3.0 ± 4.9 | |
| T2 | 3.6 ± 6.1 | 4.0 ± 6.0‡ | |
| Leukocyte count (103/µl) | T0 | 5,590 ± 1,853 | 5,806 ± 1,925 |
| T1 | 9,134 ± 3,504* | 10,363 ± 5,160* | |
| T2 | 13,033 ± 2,584*,† | 13,647 ± 4,816*,† | |
| Neutrophil (%) | T0 | 58.9 ± 11.1 | 55.7 ± 12.7 |
| T1 | 82.8 ± 8.4* | 81.3 ± 12.0* | |
| T2 | 86.3 ± 4.5* | 85.6 ± 7.2*,‡ | |
| Lymphocyte (%) | T0 | 28.7 ± 10.0 | 30.7 ± 12.3 |
| T1 | 10.3 ± 6.9* | 12.1 ± 10.5* | |
| T2 | 7.8 ± 3.3* | 8.4 ± 5.7* |
Data are presented as means ± SDs. CRP: C-reactive protein, T0: before induction of anesthesia, T1: end of surgery, T2: 2 hours after surgery. *P < 0.01 compared with T0 within group,. †,‡P < 0.01 and < 0.05, respectively, compared with T1 within group.
Patients in the deep group awoke at lower BIS (P < 0.05) and took more time to extubate (P < 0.01) after discontinuation of propofol infusion (Table 4). However, pain scores, incidences of postoperative adverse events, and hospital stays were similar in the two groups. No patient reported intraoperative recall. Furthermore, catecholamine and cortisol levels showed no significant correlation with pain scores in the PACU, and no correlation was found between stress hormone levels and hospital stays in either group. In contrast, leukocyte counts (ρ = 0.695 and ρ = 0.636 in the deep and the light groups, respectively, P < 0.05) and neutrophil percentages (P < 0.05, ρ = 0.641 in both groups) at T1 were found to be positively correlated with length of hospital stays in both groups.
Table 4. Postoperative Outcomes.
| Deep group (n = 21) | Light group (n = 21) | P value | |
|---|---|---|---|
| Time to extubation (min) | 24.9 ± 12.8 | 13.3 ± 5.1 | 0.001 |
| BIS score at ROC | 75.6 ± 4.7 | 79.4 ± 5.3 | 0.021 |
| Hypertension (n) | 3 (14.3) | 3 (14.3) | 0.669 |
| Hypotension (n) | 0 | 1 (4.8) | 0.500 |
| Chest pain (n) | 1 (4.8) | 0 | 0.500 |
| Pulmonary infection (n) | 0 | 1 (4.8) | 0.500 |
| Hospital stay (D) | 9.9 ± 3.9 | 10.0 ± 4.7 | 0.964 |
| 30 day mortality (n) | 0 | 0 | 1.000 |
Data are presented as means ± SDs or numbers (%) of patients. BIS: Bispectral index, ROC: Recovery of consciousness.
Discussion
The present study shows that a deep level of propofol and remifentanil anesthesia, achieved by adjusting the infusion rate of propofol, more effectively attenuates norepinephrine and glucose response, but not ACTH-cortisol or inflammatory response, immediately after lung surgery than a light level of anesthesia. Stress response to surgery is characterized by activation of the HPA axis, which stimulates ACTH release from the anterior pituitary gland, and subsequently, cortisol release from the adrenal cortex and sympathetic nervous system (SNS) activation, which in turn, augments NE release from presynaptic sympathetic fibers and NE and epinephrine release from the adrenal medulla. Thus, our findings suggest that propofol-induced anesthetic depth during lung surgery impacts stress response by inhibiting SNS activation rather than activation of the HPA axis.
Previous investigations have shown greater suppression of catecholamine response by propofol than sevoflurane during minor and major surgery [6,8,9,10]. However, the effect of propofol dose (and thus of anesthetic depth) in a clinically attainable range on stress response to surgery has not been previously explored in major thoracic surgery involving a high degree of stress. In the present study, catecholamine responses to lung surgery at two levels of propofol anesthesia were similarly attenuated during anesthesia, but diverged after surgery. The greatest change in circulating catecholamine levels in patients undergoing surgery occurs during the postoperative period due to perception of surgical pain, airway reflex stimulation, and tapering of general anesthetic-induced sympathetic inactivation. In the present study, the extent and duration of surgical injury, remifentanil doses used for analgesia during surgery, pain scores, and analgesic requirements after surgery were comparable in the two study groups. Our findings suggest that intraoperative propofol dose contributed to observed intergroup catecholamine response divergence.
Changes in individual catecholamine response to surgery probably depend on the type of surgery and the anesthetic used [9,11,12]. In the present study, changes in NE levels were significantly different, whereas epinephrine levels increased similarly in both groups. We propose two explanations for this observed difference between perioperative changes in epinephrine and NE levels. First, propofol may have a dose-dependent suppressive effect on SNS via adrenomedullary inhibition at lower concentration and a peripheral neural inhibitory effect at higher concentration, because plasma NE primarily originates from presynaptic sympathetic nerve endings and epinephrine from the adrenal medulla. Second, epinephrine and NE may exhibit different responses according to intraoperative and postoperative stresses in lung surgery patients because of OLV, irrespective of anesthetic depth. In a previous study, lung surgery was accompanied by a distinct pattern of catecholamine response, a peak increase during OLV followed by a gradual decrease in epinephrine release and increase in NE release in the postoperative period [11]. These two proposed explanations indicate the importance of determining individual catecholamine response to surgical nociceptive stimulation, to pulmonary injury by OLV during surgery, and to postoperative pain perception after surgery in lung surgery patients.
In the present study, increased adrenocortical response to lung surgery was not prevented by the high propofol infusion rate (8 mg/kg/h), which contrasts to the successful attenuation of cortisol release at lower propofol infusion rates (4-6 mg/kg/h) reported in previous studies [8,9,13]. Adrenocortical response is known to be directly proportional to the severity of surgical trauma and lung surgery evokes a high stress response because it involves major surgical injury and OLV-associated pulmonary inflammation. Thus, despite successful attenuation of adrenocortical response to gynecological surgery [8,9], propofol anesthesia at infusion rates of 4-12 mg/kg/h did not attenuate adrenocortical response to thoracic surgery [14,15]. These reports indicate that clinically relevant concentrations of propofol are unlikely to attenuate HPA axis activation to major thoracic surgery.
Neuroendocrine and inflammatory responses to surgical stress result in stress-induced hyperglycemia due to insulin resistance and the promotion of glucose production by catabolic hormones [1]. In the present study, deep propofol anesthesia attenuated increase in plasma glucose after surgery more effectively than light anesthesia. We are unable to clarify the cause of these different glucose responses because we did not measure the levels of any other glucose-related hormones or demonstrate a consistent correlation between plasma glucose and catecholamine levels at each time point. However, it appears that dose-dependent inhibition of sympathetic activity under propofol anesthesia contributed to the intergroup plasma glucose level differences based on the concomitant change observed in catecholamine response.
Inflammatory response to surgery is known to be related to increases in circulating cortisol and catecholamine levels [1]. However, the effect of anesthetic per se on inflammatory response to surgery is not clearly understood because of the complexities of interactions between inflammatory cytokines and neuroendocrine hormones and the relation between inflammatory response and degree of surgical trauma. In the present study, light and deep propofol anesthesia failed to blunt leukocytosis, granulocytosis, or lymphopenia in response to lung surgery, and we were unable to clarify the nature of the interaction between inflammatory and neuroendocrine response because plasma cytokine levels were not measured.
Furthermore, we found the two study groups to be similar in terms of clinical outcomes and hospital stays, and we encountered no case of intraoperative awareness or 30-day mortality. Additional larger-scale randomized studies are needed to determine the relationship between long-term outcomes and changes in the levels of neuroendocrine hormones at different depths of propofol anesthesia in high-risk patients.
Some comments are necessary concerning the limitations of this study. First, we confined the measurement of stress response to the early postoperative period. Because stress response occurs over a considerably longer period after surgery [11], we did not explore the whole course of stress response to lung surgery or long-term outcomes. Second, the contribution made by remifentanil to neuroendocrine stress response was not determined. Opioids have been reported to suppress stress response dose-dependently and may act synergistically with propofol [16,17]. However, the remifentanil infusion rate (0.05 µg/kg/min) used was not enough to suppress whole stress response to minor surgery [18] and identical infusion rates were used in the two groups. Thus, we believe the contribution made by remifentanil to stress response to lung surgery can be disregarded. Third, we did not measure plasma propofol concentrations and stress hormone levels simultaneously, and chose to use BIS, which is a reliable surrogate of plasma propofol concentration during anesthesia [19,20]. However, BIS scores at 2 hours postoperatively were not significantly different in the two groups. Nevertheless, measurements of plasma propofol concentrations, especially after surgery, might have provided important clues regarding different catecholamine and glucose responses.
In conclusion, a deep level of anesthesia achieved using high propofol infusion rates during lung surgery were found to lower perioperative norepinephrine and glucose responses more so than light level of anesthesia, but failed to affect immediate postoperative outcome. Further research is required to clarify the impact of anesthetic depth on the course of postoperative stress response and long-term outcome in high-risk patients.
Acknowledgments
This work was supported by Konyang University Myunggok Research Fund of 2010.
References
- 1.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 2.Hadimioglu N, Ulugol H, Akbas H, Coskunfirat N, Ertug Z, Dinckan A. Combination of epidural anesthesia and general anesthesia attenuates stress response to renal transplantation surgery. Transplant Proc. 2012;44:2949–2954. doi: 10.1016/j.transproceed.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257:8–14. doi: 10.1097/SLA.0b013e31827b6bbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yim AP, Wan S, Lee TW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg. 2000;70:243–247. doi: 10.1016/s0003-4975(00)01258-3. [DOI] [PubMed] [Google Scholar]
- 5.Baldini G, Bagry H, Carli F. Depth of anesthesia with desflurane does not influence the endocrine-metabolic response to pelvic surgery. Acta Anaesthesiol Scand. 2008;52:99–105. doi: 10.1111/j.1399-6576.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 6.Satani M, Hamada T, Nakada K, Umemoto Y, Fujii T, Takaki O. Comparison of total intravenous anesthesia and inhalation anesthesia regarding hormonal responses during lung lobectomy. Masui. 2005;54:1109–1115. [PubMed] [Google Scholar]
- 7.Yang LQ, Li JJ, Chen SQ, Wang YW. Effect of different depths of anesthesia on perioperative stress response in children undergoing adenoidectomy and tonsillectomy. CNS Neurosci Ther. 2013;19:134–135. doi: 10.1111/cns.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marana E, Colicci S, Meo F, Marana R, Proietti R. Neuroendocrine stress response in gynecological laparoscopy: TIVA with propofol versus sevoflurane anesthesia. J Clin Anesth. 2010;22:250–255. doi: 10.1016/j.jclinane.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Ihn CH, Joo JD, Choi JW, Kim DW, Jeon YS, Kim YS, et al. Comparison of stress hormone response, interleukin-6 and anaesthetic characteristics of two anaesthetic techniques: volatile induction and maintenance of anaesthesia using sevoflurane versus total intravenous anaesthesia using propofol and remifentanil. J Int Med Res. 2009;37:1760–1771. doi: 10.1177/147323000903700612. [DOI] [PubMed] [Google Scholar]
- 10.Adams HA, Schmitz CS, Baltes-Götz B. Endocrine stress reaction, hemodynamics and recovery in total intravenous and inhalation anesthesia. Propofol versus isoflurane. Anaesthesist. 1994;43:730–737. doi: 10.1007/s001010050115. [DOI] [PubMed] [Google Scholar]
- 11.Tønnesen E, Höhndorf K, Lerbjerg G, Christensen NJ, Hüttel MS, Andersen K. Immunological and hormonal responses to lung surgery during one-lung ventilation. Eur J Anaesthesiol. 1993;10:189–195. [PubMed] [Google Scholar]
- 12.Madsen SN, Fog-Møller F, Christiansen C, Vester-Andersen T, Engquist A. Cyclic AMP, adrenaline and noradrenaline in plasma during surgery. Br J Surg. 1978;65:191–193. doi: 10.1002/bjs.1800650315. [DOI] [PubMed] [Google Scholar]
- 13.Ledowski T, Bein B, Hanss R, Paris A, Fudickar W, Scholz J, et al. Neuroendocrine stress response and heart rate variability: a comparison of total intravenous versus balanced anesthesia. Anesth Analg. 2005;101:1700–1705. doi: 10.1213/01.ane.0000184041.32175.14. [DOI] [PubMed] [Google Scholar]
- 14.Novak-Jankoviĉ V, Paver-Eržen V, Bovill JG, Ihan A, Osredkar J. Effect of epidural and intravenous clonidine on the neuro-endocrine and immune stress response in patients undergoing lung surgery. Eur J Anaesthesiol. 2000;17:50–56. doi: 10.1046/j.1365-2346.2000.00602.x. [DOI] [PubMed] [Google Scholar]
- 15.Tacconi F, Pompeo E, Sellitri F, Mineo TC. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg. 2010;10:666–671. doi: 10.1510/icvts.2009.224139. [DOI] [PubMed] [Google Scholar]
- 16.Kern SE, Xie G, White JL, Egan TD. A response surface analysis of propofol-remifentanil pharmacodynamic interaction in volunteers. Anesthesiology. 2004;100:1373–1381. doi: 10.1097/00000542-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Mertens MJ, Engbers FH, Burm AG, Vuyk J. Predictive performance of computer-controlled infusion of remifentanil during propofol/remifentanil anaesthesia. Br J Anaesth. 2003;90:132–141. doi: 10.1093/bja/aeg046. [DOI] [PubMed] [Google Scholar]
- 18.Shinoda T, Murakami W, Takamichi Y, Iizuka H, Tanaka M, Kuwasako Y. Effect of remifentanil infusion rate on stress response in orthopedic surgery using a tourniquet application. BMC Anesthesiol. 2013;13:14. doi: 10.1186/1471-2253-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeganeh N, Roshani B, Almasi A, Jamshidi N. Correlation between bispectral index and predicted effect-site concentration of propofol in different levels of target-controlled, propofol induced sedation in healthy volunteers. Arch Iran Med. 2010;13:126–134. [PubMed] [Google Scholar]
- 20.Schultz A, Siedenberg M, Grouven U, Kneif T, Schultz B. Comparison of Narcotrend Index, Bispectral Index, spectral and entropy parameters during induction of propofol-remifentanil anaesthesia. J Clin Monit Comput. 2008;22:103–111. doi: 10.1007/s10877-008-9111-6. [DOI] [PubMed] [Google Scholar]