Abstract
Background
The early detection of coagulopathy helps guide decisions regarding optimal transfusion management during cardiac surgery. This study aimed to determine whether rotational thromboelastometry (ROTEM) analysis during cardiopulmonary bypass (CPB) could predict thrombocytopenia and hypofibrinogenemia after CPB.
Methods
We analyzed 138 cardiac surgical patients for whom ROTEM tests and conventional laboratory tests were performed simultaneously both during and after CPB. An extrinsically activated ROTEM test (EXTEM), a fibrin-specific ROTEM test (FIBTEM) and PLTEM calculated by subtracting FIBTEM from EXTEM were evaluated. Correlations between clot amplitude at 10 min (A10), maximal clot firmness, platelet count, and fibrinogen concentrations at each time point were calculated. A receiver operating characteristic analysis with area under the curve (AUC) was used to assess the thresholds of EXTEM, PLTEM and FIBTEM parameters during CPB and for predicting thrombocytopenia and hypofibrinogenemia after weaning of CPB.
Results
The A10 on EXTEM, PLTEM, and FIBTEM during CPB showed a good correlation with platelet counts (r = 0.622 on EXTEM and r = 0.637 on PLTEM; P < 0.0001 for each value) and fibrinogen levels (r = 0.780; P < 0.0001) after CPB. A10 on a FIBTEM threshold of 8 mm during the CPB predicted a fibrinogen concentration < 150 mg/dl (AUC = 0.853) after CPB. Additionally, the threshold level of A10 on EXTEM during CPB for predicting platelet counts < 100,000 /µl after CPB was 42 mm (AUC = 0.768).
Conclusions
EXTEM, PLTEM, and FIBTEM parameters during CPB may be useful for predicting thrombocytopenia and hypofibrinogenemia after weaning of CPB.
Keywords: Cardiac surgery, Cardiopulmonary bypass, Coagulopathy, Thromboelastometry
Introduction
Coagulopathy is one of the most frequent postoperative complications in patients who undergo cardiac surgery with cardiopulmonary bypass (CPB). Coagulopathy after CPB is caused by multiple complex factors, which include the activation and consumption of coagulation factors, hemodilution, platelet dysfunction, fibrinolysis, and inappropriate heparin reversal [1]. Excessive bleeding can be associated with a prolonged intensive care unit stay and both increased morbidity and mortality [2,3], and this clinically important event can be minimized by performing a proper examination and determining the cause of the coagulation abnormality, followed by rapid and proper treatment [4].
Rotational thromboelastometry (ROTEM) is a point-of-care device that assesses the viscoelastic properties of blood samples under low shear conditions and enables the evaluation of the processes of clot initiation, formation, and stability using whole blood. A ROTEM device can conduct several tests, including the extrinsically activated (EXTEM) and intrinsically activated (INTEM) tests, and the fibrin-specific clot formation test (FIBTEM). Identifying the need for specific blood components should be possible using these tests [5,6]. Moreover, ROTEM, unlike conventional laboratory tests, can provide prompt results within 10 to 15 min, which enables the early detection and treatment of coagulopathy in various clinical practices [7]. Several studies have shown the usefulness of ROTEM for coagulation monitoring in several different settings of major blood loss [4,8,9,10].
During cardiac surgery several authors have suggested that there may be a potential benefit of using ROTEM during CPB (i.e., before heparin reversal) for the early detection of coagulopathies and the use of more effective hemostatic therapies after weaning of CPB [11,12,13]. However, the high heparin concentrations during CPB could influence ROTEM tests like EXTEM and FIBTEM [13,14,15]. Accordingly, validation of these tests performed during CPB is necessary for guiding appropriate hemostatic therapies after CPB. To the best of our knowledge, no previous study has evaluated the predictive value of ROTEM during CPB to detect coagulopathies to guide appropriate blood component therapies after CPB weaning.
In our present study, we evaluated whether EXTEM and FIBTEM parameters during CPB could predict the hemostatic status, such as thrombocytopenia or hypofibrinogenemia after weaning of CPB. Additionally, we assessed whether PLTEM, which was calculated by subtracting FIBTEM from EXTEM, could be useful to predict thrombocytopenia. We also evaluated the effect of hematocrit on the relationships between the ROTEM values and conventional laboratory tests.
Materials and Methods
Study population
After obtaining approval from the Institutional Review Board of our institution (2013-0857), we retrospectively investigated a total of 262 patients who were at least 20 years old and had undergone elective cardiovascular surgery using CPB at a single academic medical center between February 2012 and December 2012. Additionally, the patients who we selected had undergone simultaneous ROTEM and conventional laboratory tests both during and after CPB. We excluded individuals who had incomplete ROTEM (including inadequate run time or technical irregularities in the traces) or laboratory tests (n = 105), who showed signs of hyperfibrinolysis (n = 7), or who restarted CPB for revision or bleeding control after the ROTEM test during CPB (n = 12). The remaining 138 patients were included in this analysis. Clinical and anesthetic data were obtained from computerized databases.
Intraoperative management
All patients were subjected to our institutional standard anesthesia protocol for open heart surgery. To adjust the preload, we used 0.9% saline or balanced crystalloid and 6% hydroxyethyl starch 130/0.4 (Voluven or Volulyte, FreseniusKabi, Bad Homburg, Germany). Before the CPB, a bolus of heparin (300 IU/kg) was administered intravenously to achieve an activated clotting time of > 480 s. The activated clotting time was checked every 30 min and heparin was added to maintain an adequate activated clotting time. Priming solutions included 20% mannitol, 20% albumin, and crystalloid solution. Packed red blood cells were added to the priming solution of patients with a preoperative hematocrit value < 30% to prevent excessive hemodilution when CPB was initiated. During the CPB the hematocrit was maintained between 23 and 27%. Typically, patients were rewarmed to a nasopharyngeal or rectal temperature of 37℃ prior to separation from bypass. After weaning from the CPB, heparin was antagonized by protamine sulfate (3 mg/kg).
Blood sampling and thromoboelastometric measurements
Both conventional coagulation assays and ROTEM tests were performed using blood samples drawn from the radial artery at two pre-determined time points: during CPB rewarming (32-33℃) and after weaning from the CPB (i.e., 5 min after protamine administration). Thromboelastometric measurements were conducted using a ROTEM device (ROTEM® delta, TEM International GmbH, Munich, Germany) in accordance with the manufacturer's instructions using citrated whole blood. Briefly, ROTEM were conducted at 37℃ using 300 µl of whole blood after recalcification with 20 µl of 0.2 mol/L CaCl2 and the addition of different reagents to trigger coagulation and to differentiate between hemostatic disorders. EXTEM was performed to monitor the extrinsic coagulation system by tissue factor activation using 20 µl of thromboplastin reagent. FIBTEM was performed for qualitative analysis of fibin clot stability by the combination of tissue factor activation with platelet inhibition using cytochalasin D. The ROTEM device was placed in the operating room and all tests were performed by an anesthesia nurse trained to conduct ROTEM tests. The ROTEM variables analyzed in EXTEM and FIBTEM included clot amplitude at 10 min (A10) and maximum clot firmness (MCF). Additionally, PLTEM, which was calculated by subtracting FIBTEM from EXTEM, was also analyzed [16]. Hematocrit, platelets, and fibrinogen levels were measured at each time point. Hematocrit and platelet counts were measured by RBC cumulative pulse height detection or flow cytometry, respectively, using Sysmex XE 2100 (Sysmex Corp., Kobe, Japan). Fibrinogen was measured by the Clauss method using Sysmex CA 7000 (Sysmex Corp., Kobe, Japan).
Statistical analysis
All values are expressed as mean ± SD, median and interquartile range, or number and percentage. Correlations among ROTEM parameters and between ROTEM parameters and conventional laboratory tests were assessed using a Pearson's or Spearman's rank order correlation coefficient as appropriate. We calculated the area under the curve (AUC) values of the receiver operating characteristic (ROC) curves of the ROTEM parameters during CPB rewarming that predict thrombocytopenia (< 100,000 /µl) and hypofibrinogenemia (< 150 mg/dl) after weaning of CPB. Sensitivity, specificity, and positive and negative predictive values (PPV and NPV) were also calculated to assess the clinical significance of ROTEM tests and to evaluate the value of ROTEM thresholds to predict thrombocytopenia and hypofibrinogenemia. The AUC values were compared with each other using a method described by Hanley and McNeil [17]. Subgroup analyses were performed according to hematocrit levels (≥ 25% or < 25%) during CPB rewarming. This cut-off value was chosen according to the results of a previous study by Ogawa et al. [18] in which lower hematocrit levels (< 25%) affected the correlation between plasma fibrinogen concentrations and ROTEM values. A threshold of P < 0.05 was used to identify statistically significant differences. All statistical analyses were performed using MedCalc 13.1.1.0 (Mariakerke, Belgium) and SPSS 20.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
The characteristics and procedural data for the 138 study patients are shown in Table 1. The median interval between two blood samples was 45 (range, 31-55) min. Conventional laboratory results and ROTEM measurements during CPB rewarming and after weaning of CPB are presented in Table 2. The A10 of EXTEM, PLTEM, and FIBTEM showed an excellent linear correlation with the MCF of EXTEM, PLTEM, and FIBTEM during CPB rewarming (r = 0.899, r = 0.902, and r = 0.987, respectively; P < 0.0001 for each value) and after weaning of CPB (r = 0.953, r = 0.915, and r = 0.954, respectively; P < 0.0001 for each value). The A10 and MCF of EXTEM, PLTEM, and FIBTEM during CPB rewarming also exhibited a linear correlation with A10 (r = 0.703, r = 0.669, and r = 0.822, respectively; P < 0.0001 for each value) and MCF (r = 0.715, r = 0.606, and r = 0.828, respectively; P < 0.0001 for each value) after weaning of CPB. The platelet count and fibrinogen levels during CPB rewarming were strongly correlated with those after weaning of CPB (r = 0.814 and r = 0.900, respectively; P < 0.0001 for both values). Hematocrit levels during CPB rewarming were also moderately correlated with those after weaning of CPB (r = 0.414; P < 0.0001).
Table 1. Baseline Characteristics of the Study Patients and Procedural Data.
| Number of patients (n) | 138 |
| Age (yr) | 61.9 ± 11.1 |
| Sex (M/F) | 66/72 |
| Body mass index (kg/m2) | 23.4 ± 3.3 |
| Logistic EuroSCORE | 2.2 ± 2.4 |
| Hematocrit (%) | 37.6 ± 5.4 |
| Platelets (×103 /µl) | 209.8 ± 80.9 |
| Prothrombin Time (INR) | 1.1 ± 0.2 |
| Activated Partial Thromboplastin Time (s) | 31.5 ± 8.2 |
| Type of surgery | |
| Valve | 85 |
| Aorta | 6 |
| Combined | 47 |
| Operative time (min) | 319.2 ± 121.8 |
| CPB time (min) | 158.1 ± 67.1 |
Data are expressed as number of patients or mean ± SD. EuroSCORE: European System for Cardiac Operative Risk Evaluation, CPB: cardiopulmonary bypass.
Table 2. Intraoperative ROTEM and Conventional Laboratory Parameters during CPB Rewarming and after CPB Weaning.
| During CPB rewarming | After CPB weaning | |
|---|---|---|
| EXTEM A10 (mm) | 41 (36-46) | 41 (36-46) |
| EXTEM MCF (mm) | 50 (44-55) | 51 (44-55) |
| FIBTEM A10 (mm) | 9 (8-13) | 9 (7-12) |
| FIBTEM MCF (mm) | 10 (9-14) | 11 (8-14) |
| PLTEM A10 (mm) | 31 (27-35) | 32 (28-37) |
| PLTEM MCF (mm) | 39 (34-43) | 40 (35-44) |
| Hematocrit (%) | 26.6 ± 2.7 | 29.3 ± 3.2 |
| Platelets (×103 /µl) | 97.5 (74.0-125.0) | 82.0 (66.8-107.3) |
| Fibrinogen (mg/dl) | 156.0 (130.3-196.5) | 155.0 (128.9-196.3) |
Data are expressed as median and interquartile range or mean ± SD. CPB: cardiopulmonary bypass, A10: clot amplitude at 10 min, MCF: maximum clot firmness.
The A10 and MCF on both EXTEM and PLTEM were correlated with platelet counts during CPB rewarming (r = 0.649 and r = 0.575 on EXTEM, and r = 0.690 and r = 0.563 on PLTEM, respectively; P < 0.0001 for each value) and after weaning of CPB (r = 0.717 and r = 0.663 on EXTEM, and r = 0.750 and r = 0.630 on PLTEM, respectively; P < 0.0001 for each value). The A10 and MCF on FIBTEM were also well correlated with fibrinogen levels during CPB rewarming (r = 0.767 and r = 0.766, respectively; P < 0.0001 for both values) and after weaning of CPB (r = 0.817 and r = 0.803, respectively; P < 0.0001 for both values). The A10 and MCF on EXTEM, PLTEM, and FIBTEM during CPB rewarming showed moderate to good correlations with platelet counts (r = 0.622 and r = 0.572 on EXTEM, and r = 0.637 and r = 0.509 on PLTEM, respectively; P < 0.0001 for each value) and fibrinogen levels (r = 0.780 and r = 0.771 on FIBTEM, respectively; P < 0.0001 for both values) after weaning of CPB.
The results of all ROC curve analyses to assess the predictive value of ROTEM tests during CPB rewarming for thrombocytopenia and hypofibrinogenemia after weaning of CPB are shown in Table 3. The AUCs of PLTEM A10/MCF were slightly higher than those of EXTEM A10/MCF at a threshold of 100,000 /µl, but these differences were not statistically significant (P = 0.309 on A10 and P = 0.630 on MCF).
Table 3. Cut-off Values of ROTEM Parameters during CPB Rewarming to Predict Thrombocytopenia and Hypofibrinogenemia after CPB Weaning.
| ROTEM | Cutoff | Sensitivity | Specificity | PPV | NPV | AUC | SE | 95% CI | z statistic | P* | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelets < 100,000 /µl (n = 98) | EXTEM A10 | ≤ 42 | 74.2 | 73.7 | 87.8 | 52.8 | 0.768 | 0.0486 | 0.688-0.836 | 5.522 | < 0.0001 |
| EXTEM MCF | ≤ 53 | 77.6 | 59.0 | 82.6 | 51.1 | 0.723 | 0.0508 | 0.640-0.796 | 4.396 | < 0.0001 | |
| PLTEM A10 | ≤ 33 | 80.4 | 71.1 | 87.6 | 58.7 | 0.795 | 0.0484 | 0.717-0.860 | 6.094 | < 0.0001 | |
| PLTEM MCF | ≤ 44 | 90.8 | 48.7 | 81.7 | 67.9 | 0.738 | 0.0495 | 0.656-0.809 | 4.811 | < 0.0001 | |
| Fibrinogen < 150 mg/dl (n = 62) | FIBTEM A10 | ≤ 8 | 62.3 | 88.2 | 80.9 | 74.4 | 0.853 | 0.0310 | 0.783-0.908 | 11.403 | < 0.0001 |
| FIBTEM MCF | ≤ 9 | 67.7 | 85.5 | 79.2 | 76.5 | 0.849 | 0.0317 | 0.779-0.905 | 11.034 | < 0.0001 |
CPB: cardiopulmonary bypass, PPV: positive predictive value, NPV: negative predictive value, AUC: area under the curve, SE: standard error, CI: confidence interval. *Comparison to AUC of 0.5.
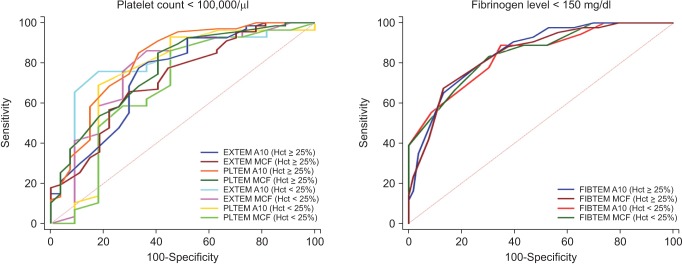
In the first ROTEM test (i.e. during CPB rewarming), 41 patients had low hematocrit levels (< 25%). In the two subgroups by hematocrit level the mean levels of those with a low (< 25%) and high (≥ 25%) hematocrit were 23.6 ± 1.0% and 27.9 ± 2.1%, respectively. In patients with low hematocrit levels during CPB, A10 and MCF on EXTEM, PLTEM, and FIBTEM during CPB showed moderate to good correlations with platelet counts (r = 0.651 and r = 0.655 on EXTEM, and r = 0.527 and r = 0.457 on PLTEM, respectively; P < 0.0001 for all values) and fibrinogen levels (r = 0.808 and r = 0.833, respectively; P < 0.0001 for both values) after weaning of CPB. In patients with high hematocrit levels during CPB, A10, and MCF on EXTEM, PLTEM, and FIBTEM also showed moderate to good correlations with platelet counts (r = 0.670 and r = 0.549 on EXTEM, and r = 0.688 and r = 0.532 on PLTEM, respectively; P < 0.0001 for all values) and fibrinogen levels (r = 0.770 and r = 0.749, respectively; P < 0.0001 for both values) after weaning of CPB. The results for all ROC curve analyses to assess the predictive value of ROTEM tests during CPB rewarming for thrombocytopenia and hypofibrinogenemia after weaning of CPB in the two subgroups are shown in Table 4. Discriminative abilities of ROTEM parameters between the high and low hematocrit level groups are shown in Fig. 1. The AUCs of EXTEM A10/MCF and PLTEM A10/MCF for thrombocytopenia did not differ in the subgroup of patients with a hematocrit level < 25% (A10 = 0.80 and MCF = 0.77 on EXTEM, and A10 = 0.75 and MCF = 0.69 on PLTEM) or ≥ 25% (A10 = 0.75 and MCF = 0.71 on EXTEM, and A10 = 0.82 and MCF = 0.76 on PLTEM). The AUCs of FIBTEM A10/MCF for hypofibrinogenemia were also comparable in the subgroup of patients with a hematocrit level < 25% (A10 = 0.84 and MCF = 0.85) or ≥ 25% (A10 = 0.85 and MCF = 0.85).
Table 4. Cut-off Values of ROTEM Parameters Classified by Hematocrit during CPB Rewarming to Predict Thrombocytopenia and Hypofibrinogenemia after CPB Weaning.
| ROTEM | Hematocrit | Cutoff | Sensitivity | Specificity | PPV | NPV | AUC | SE | 95% CI | z statistic | P* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelets < 100,000 /µl | EXTEM A10 | < 25% | ≤ 43 | 76.7 | 81.8 | 92.0 | 56.3 | 0.798 | 0.0936 | 0.644-0.907 | 3.190 | 0.0014 |
| ≥ 25% | ≤ 42 | 77.6 | 66.7 | 85.2 | 54.5 | 0.745 | 0.0601 | 0.645-0.829 | 4.079 | <0.0001 | ||
| EXTEM MCF | < 25% | ≤ 54 | 86.7 | 63.6 | 86.7 | 63.6 | 0.774 | 0.0980 | 0.617-0.890 | 2.798 | 0.0051 | |
| ≥ 25% | ≤ 50 | 66.2 | 67.9 | 83.3 | 45.2 | 0.705 | 0.0602 | 0.603-0.793 | 3.396 | 0.0007 | ||
| PLTEM A10 | < 25% | ≤ 33 | 70.0 | 81.8 | 91.3 | 50.0 | 0.752 | 0.108 | 0.592-0.873 | 2.325 | 0.0201 | |
| ≥ 25% | ≤ 33 | 85.1 | 66.7 | 86.4 | 64.3 | 0.815 | 0.0535 | 0.721-0.887 | 5.876 | <0.0001 | ||
| PLTEM MCF | < 25% | ≤ 44 | 86.7 | 54.6 | 83.9 | 60.0 | 0.686 | 0.112 | 0.523-0.822 | 1.661 | 0.0967 | |
| ≥ 25% | ≤ 42 | 85.3 | 57.1 | 82.9 | 61.5 | 0.757 | 0.0558 | 0.658-0.838 | 4.597 | <0.0001 | ||
| Fibrinogen < 150 mg/dl | FIBTEM A10 | < 25% | ≤ 10 | 88.9 | 65.2 | 66.7 | 88.2 | 0.842 | 0.0609 | 0.694-0.937 | 5.614 | <0.0001 |
| ≥ 25% | ≤ 9 | 79.1 | 73.6 | 70.8 | 81.2 | 0.854 | 0.0372 | 0.767-0.918 | 9.514 | <0.0001 | ||
| FIBTEM MCF | < 25% | ≤ 10 | 83.3 | 69.6 | 68.2 | 84.2 | 0.848 | 0.0590 | 0.701-0.941 | 5.899 | <0.0001 | |
| ≥ 25% | ≤ 9 | 68.2 | 86.8 | 81.1 | 76.7 | 0.848 | 0.0384 | 0.761-0.913 | 9.078 | <0.0001 |
CPB: cardiopulmonary bypass, PPV: positive predictive value, NPV: negative predictive value, AUC: area under the curve, SE: standard error, CI: confidence interval. *Comparison to AUC of 0.5.
Fig. 1. Receiver-operating characteristic (ROC) curves of EXTEM and PLTEM for thrombocytopenia and FIBTEM for hypofibrinogenemia when the hematocrit levels were < 25% or ≥ 25%.
Discussion
We have here demonstrated that the A10 and MCF of EXTEM, PLTEM, and FIBTEM during CPB rewarming correlates well with platelet counts and fibrinogen levels after CPB weaning. Hence, these measurements could be useful for predicting clinically relevant thrombocytopenia and hypofibrinogenemia events after CPB weaning. Furthermore, the ability of ROTEM parameters to predict thrombocytopenia and hypofibrinogenemia after CPB weaning was comparable, regardless of the hematocrit concentrations during CPB rewarming.
Bleeding is a major complication in cardiac surgery with CPB, and excessive bleeding after CPB weaning and inadequate blood transfusion is associated with poor outcomes [2,3,19,20]. Thus, earlier detection and correction of coagulopathies may be important for improving patient outcomes [21]. Although few data are available [11,12,13,22,23], we speculate that coagulation monitoring during CPB could provide an estimation of coagulopathies after CPB, and could help guide hemostatic interventions after CPB. Indeed, because of the time required for preparing hemostatic blood products, including platelet concentrates or cryoprecipitates, early detection and prediction of coagulopathy before CPB weaning can spare a considerable amount of time and result in the more effective use of hemostatic blood products. However, the need for heparinization during the CPB can affect the results of conventional laboratory tests and ROTEM tests [13,14,15]. Gertler et al. [14] examined the in vitro effects of heparin levels on conventional laboratory tests and ROTEM. They found that high heparin concentrations can significantly alter fibrinogen values as well as the results of EXTEM and FIBTEM test. However, another recent study has shown that HEPTEM and EXTEM are valid in the presence of high heparin levels and can be performed to evaluate hemostasis before protamine administration in patients undergoing cardiac surgery with CPB [13]. Thus, even though the use of these tests during CPB is recommended, the reliability of ROTEM in the presence of a high concentration of heparin is still controversial.
In our current study, the A10 and MCF of EXTEM and FIBTEM correlated with platelet counts and fibrinogen levels both during CPB rewarming and after weaning of CPB. Furthermore, our findings show that EXTEM, PLTEM, and FIBTEM during CPB rewarming also correlated well with platelet counts and fibrinogen levels after CPB weaning, and these tests during the CPB are useful for predicting coagulopathies after CPB weaning. Our data are in agreement with previous ROTEM studies in cardiac surgery with CPB. In a previous study of cardiac surgery with CPB, A10 and MCF on EXTEM and FIBTEM showed robust correlations with platelet counts and fibrinogen levels during the procedure [22,23]. Other studies have also suggested that A10 and MCF on EXTEM and FIBTEM during CPB could be used to guide the transfusion of platelets and fibrinogen concentrates [11,12]. These findings strongly support the notion that the use of ROTEM during CPB could allow for the earlier detection of coagulopathies after CPB and could thus help guide the more effective and prompt use of hemostatic blood products, such as platelet concentrates or cryoprecipitates, after CPB weaning.
The relationships between ROTEM parameters and conventional laboratory tests, including platelet counts and fibrinogen levels, can be affected by changing hematocrit levels altered by hemorrhage and hemodilution during CPB. Previous studies have reported that MCF values on EXTEM and FIBTEM were higher with decreasing hematocrit levels, and a lower hematocrit showed a better correlation between MCF on FIBTEM and plasma fibrinogen levels [18,24,25]. Similarly, our present results show that the A10 and MCF values on FIBTEM during CPB that predict hypofibrinogenemia after CPB in the low hematocrit group were higher than those in the high hematocrit group. Additionally, a stronger correlation was shown between FIBTEM values during the CPB and fibrinogen levels after the CPB in the low hematocrit group. Finally, our present findings also show that the A10 and MCF values on EXTEM and PLTEM during CPB the predict thrombocytopenia after CPB in the low hematocrit group were also higher than those in the high hematocrit group. However, in contrast with FIBTEM, A10 and MCF on EXTEM and PLTEM showed a better correlation with platelets in the high hematocrit group than in the low hematocrit group. Although we do not have a clear explanation for this finding, it may be a consequence of the heparinization status or the different sample times between EXTEM and platelets [15]. Further studies are warranted to better investigate this potential association.
We here used the PLTEM value as a calculating parameter by subtracting FIBTEM from EXTEM to distinguish thrombocytopenia from hypofibrinogenemia. The EXTEM test, which has been performed to guide the transfusion of platelet concentrates, can be influenced by at least two substrates, platelet count and fibrinogen level [11,12]. Therefore, when the values of A10 and MCF on EXTEM are reduced in both thrombocytopenia and hypofibrinogenemia, distinguishing them clearly and preparing adequate blood components is difficult. With PLTEM, the influence of fibrinogen on EXTEM can be eliminated, so PLTEM has appeared to be more accurate in estimating platelet counts and thrombocytopenia than EXTEM alone [16]. Along with FIBTEM, which provides an acceptable guide for the transfusion of fibrinogen-rich products [22], the implementation of PLTEM may be an important new parameter in cardiac surgery with CPB. Similar to the findings of a previous study [16], we found in our current that A10 and MCF on PLTEM were well correlated with platelet count, both during and after CPB, and the AUCs of PLTEM were higher than the AUCs of EXTEM at a threshold of 100,000/µl.
We further found in our present investigation that the threshold levels of A10 and MCF on FIBTEM values during the CPB were 8 and 9 mm, respectively, for predicting a fibrinogen concentration < 150 mg/dl after CPB. Additionally, the threshold levels of A10 and MCF on both EXTEM and PLTEM during CPB for predicting platelet counts < 100,000 /µl after CPB were 42 and 53 mm, and 33 and 44 mm, respectively. Although platelet counts of < 100,000 /µl and a fibrinogen concentration of < 150 to 200 mg/dl were used as thresholds of thrombocytopenia and hypofibrinogenemia in our current study, and have been generally used as transfusion thresholds by others [11,12,26], there is currently no consensus regarding the thresholds of thrombocytopenia and hypofibrinogenemia for transfusion decisions in cardiac surgery. Thus, our ROTEM cut-off values for transfusion should be adjusted to the desired transfusion thresholds before applying them to transfusion management strategies in patients undergoing cardiac surgery with CPB.
Our study had some limitations. First, it was a retrospective and single-center study, so there might have been some observation and selection biases. Second, during surgery we used synthetic colloids, such as 6% hydroxyethyl starch 130/0.4, which are known to affect ROTEM parameters [27]. Thus, our data should be interpreted with caution. Third, because platelet dysfunction during cardiac surgery cannot be detected by ROTEM tests, performing platelet function tests in addition to ROTEM tests would be helpful. Finally, heparin concentrations during CPB were not measured in our current analyses. Although it has been reported that the average heparin concentration during CPB usually is maintained between 1.3 and 7.8 IU/ml in adults [13,28], we cannot exclude the possibility of the presence of very high heparin levels. Thus, the use of high doses of heparin during CPB should be taken into consideration when interpreting our findings.
In conclusion, our current findings show that A10 and MCF of EXTEM, PLTEM, and FIBTEM during CPB rewarming may be useful for predicting thrombocytopenia and hypofibrinogenemia after CPB weaning. Further studies to assess both the threshold levels of ROTEM parameters during CPB rewarming to predict clinical bleeding and the effect on clinical outcomes of the transfusion protocol using ROTEM parameters during CPB rewarming are warranted.
Acknowledgments
This work was supported solely from institutional and departmental sources.
The authors thank Sung-Cheol Yun, Ph.D. in the Department of Clinical Epidemiology and Biostatistics at Asan Medical Centre (Seoul, Korea) for assistance with the statistical analysis.
References
- 1.Despotis GJ, Avidan MS, Hogue CW., Jr Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg. 2001;72:S1821–S1831. doi: 10.1016/s0003-4975(01)03211-8. [DOI] [PubMed] [Google Scholar]
- 2.Christensen MC, Dziewior F, Kempel A, von Heymann C. Increased chest tube drainage is independently associated with adverse outcome after cardiac surgery. J Cardiothorac Vasc Anesth. 2012;26:46–51. doi: 10.1053/j.jvca.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Görlinger K, Shore-Lesserson L, Dirkmann D, Hanke AA, Rahe-Meyer N, Tanaka KA. Management of hemorrhage in cardiothoracic surgery. J Cardiothorac Vasc Anesth. 2013;27(4 Suppl):S20–S34. doi: 10.1053/j.jvca.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–295. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 5.Reinhöfer M, Brauer M, Franke U, Barz D, Marx G, Lösche W. The value of rotation thromboelastometry to monitor disturbed perioperative haemostasis and bleeding risk in patients with cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2008;19:212–219. doi: 10.1097/MBC.0b013e3282f3f9d4. [DOI] [PubMed] [Google Scholar]
- 6.Nuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology. 2001;94:773–781. doi: 10.1097/00000542-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Toulon P, Ozier Y, Ankri A, Fleron MH, Leroux G, Samama CM. Point-of-care versus central laboratory coagulation testing during haemorrhagic surgery. A multicenter study. Thromb Haemost. 2009;101:394–401. [PubMed] [Google Scholar]
- 8.Roullet S, Pillot J, Freyburger G, Biais M, Quinart A, Rault A, et al. Rotation thromboelastometry detects thrombocytopenia and hypofibrinogenaemia during orthotopic liver transplantation. Br J Anaesth. 2010;104:422–428. doi: 10.1093/bja/aeq022. [DOI] [PubMed] [Google Scholar]
- 9.Rahe-Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138:694–702. doi: 10.1016/j.jtcvs.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Lee SM, Kim CS, Cho HS, Kim GS, Gwak MS, et al. Use of fibrin-based thromboelastometry for cryoprecipitate transfusion in cardiac surgery involving deep hypothermic circulatory arrest during cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2010;21:687–691. doi: 10.1097/MBC.0b013e32833e4228. [DOI] [PubMed] [Google Scholar]
- 11.Girdauskas E, Kempfert J, Kuntze T, Borger MA, Enders J, Fassl J, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2010;140:1117–1124.e2. doi: 10.1016/j.jtcvs.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 12.Gorlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115:1179–1191. doi: 10.1097/ALN.0b013e31823497dd. [DOI] [PubMed] [Google Scholar]
- 13.Gronchi F, Perret A, Ferrari E, Marcucci CM, Fleche J, Crosset M, et al. Validation of rotational thromboelastometry during cardiopulmonary bypass: A prospective, observational in-vivo study. Eur J Anaesthesiol. 2014;31:68–75. doi: 10.1097/EJA.0b013e328363171a. [DOI] [PubMed] [Google Scholar]
- 14.Gertler R, Wiesner G, Tassani-Prell P, Braun SL, Martin K. Are the point-of-care diagnostics MULTIPLATE and ROTEM valid in the setting of high concentrations of heparin and its reversal with protamine? J Cardiothorac Vasc Anesth. 2011;25:981–986. doi: 10.1053/j.jvca.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Dirkmann D, Gorlinger K, Dusse F, Kottenberg E, Peters J. Early thromboelastometric variables reliably predict maximum clot firmness in patients undergoing cardiac surgery: a step towards earlier decision making. Acta Anaesthesiol Scand. 2013;57:594–603. doi: 10.1111/aas.12040. [DOI] [PubMed] [Google Scholar]
- 16.Olde Engberink RH, Kuiper GJ, Wetzels RJ, Nelemans PJ, Lance MD, Beckers EA, et al. Rapid and correct prediction of thrombocytopenia and hypofibrinogenemia with rotational thromboelastometry in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28:210–216. doi: 10.1053/j.jvca.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa S, Szlam F, Bolliger D, Nishimura T, Chen EP, Tanaka KA. The impact of hematocrit on fibrin clot formation assessed by rotational thromboelastometry. Anesth Analg. 2012;115:16–21. doi: 10.1213/ANE.0b013e31824d523b. [DOI] [PubMed] [Google Scholar]
- 19.Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med. 2004;30:1873–1881. doi: 10.1007/s00134-004-2388-0. [DOI] [PubMed] [Google Scholar]
- 20.Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg. 2011;113:1319–1333. doi: 10.1213/ANE.0b013e3182354b7e. [DOI] [PubMed] [Google Scholar]
- 21.Weber CF, Gorlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–547. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa S, Szlam F, Chen EP, Nishimura T, Kim H, Roback JD, et al. A comparative evaluation of rotation thromboelastometry and standard coagulation tests in hemodilution-induced coagulation changes after cardiac surgery. Transfusion. 2012;52:14–22. doi: 10.1111/j.1537-2995.2011.03241.x. [DOI] [PubMed] [Google Scholar]
- 23.Solomon C, Hagl C, Rahe-Meyer N. Time course of haemostatic effects of fibrinogen concentrate administration in aortic surgery. Br J Anaesth. 2013;110:947–956. doi: 10.1093/bja/aes576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiezia L, Radu C, Marchioro P, Bertini D, Rossetto V, Castelli M, et al. Peculiar whole blood rotation thromboelastometry (Rotem) profile in 40 sideropenic anaemia patients. Thromb Haemost. 2008;100:1106–1110. [PubMed] [Google Scholar]
- 25.Nagler M, Kathriner S, Bachmann LM, Wuillemin WA. Impact of changes in haematocrit level and platelet count on thromboelastometry parameters. Thromb Res. 2013;131:249–253. doi: 10.1016/j.thromres.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss G, Lison S, Spannagl M, Heindl B. Expressiveness of global coagulation parameters in dilutional coagulopathy. Br J Anaesth. 2010;105:429–436. doi: 10.1093/bja/aeq199. [DOI] [PubMed] [Google Scholar]
- 28.Culliford AT, Gitel SN, Starr N, Thomas ST, Baumann FG, Wessler S, et al. Lack of correlation between activated clotting time and plasma heparin during cardiopulmonary bypass. Ann Surg. 1981;193:105–111. doi: 10.1097/00000658-198101000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]


