Abstract
Cytochrome P-450 epoxygenase (EPOX)-derived epoxyeicosatrienoic acids (EETs), 5-lipoxygenase (5-LO), and leukotriene B4 (LTB4), the product of 5-LO, all play a pivotal role in the vascular inflammatory process. We have previously shown that EETs can alleviate oxidized low-density lipoprotein (ox-LDL)-induced endothelial inflammation in primary rat pulmonary artery endothelial cells (RPAECs). Here, we investigated whether ox-LDL can promote LTB4 production through the 5-LO pathway. We further explored how exogenous EETs influence ox-LDL-induced LTB4 production and activity. We found that treatment with ox-LDL increased the production of LTB4 and further led to the expression and release of both monocyte chemoattractant protein-1 (MCP-1/CCL2) and intercellular adhesion molecule-1 (ICAM-1). All of the above ox-LDL-induced changes were attenuated by the presence of 11,12-EET and 14,15-EET, as these molecules inhibited the 5-LO pathway. Furthermore, the LTB4 receptor 1 (BLT1 receptor) antagonist U75302 attenuated ox-LDL-induced ICAM-1 and MCP-1/CCL2 expression and production, whereas LY255283, a LTB4 receptor 2 (BLT2 receptor) antagonist, produced no such effects. Moreover, in RPAECs, we demonstrated that the increased expression of 5-LO and BLT1 following ox-LDL treatment resulted from the activation of nuclear factor-κB (NF-κB) via the p38 mitogen-activated protein kinase (MAPK) pathway. Our results indicated that EETs suppress ox-LDL-induced LTB4 production and subsequent inflammatory responses by downregulating the 5-LO/BLT1 receptor pathway, in which p38 MAPK phosphorylation activates NF-κB. These results suggest that the metabolism of arachidonic acid via the 5-LO and EPOX pathways may present a mutual constraint on the physiological regulation of vascular endothelial cells.
Introduction
The biological features of cyclooxygenases (COXs) and lipoxygenases (LOXs) have been extensively studied, as their eicosanoid products play central roles in inflammatory processes. The LOX pathway is involved in the biosynthesis of hydroxyeicosatetraenoic acids (HETEs), lipoxins (LXs), and leukotrienes (LTs). These metabolites have been implicated in vasoregulatory and inflammatory events, such as asthma, allergic rhinitis, and atherosclerosis [1–3]. A growing body of evidence has shown that the LT pathway is critical to the development and progression of atherosclerotic lesions [4, 5]. LTs are potent lipid mediators that are derived from arachidonic acid (AA). The 5-lipoxygenase (5-LO) pathway is responsible for the production of leukotriene B4 (LTB4) and cysteinyl LTs (cysLTs). LTB4 is an extremely potent chemoattractant that promotes the adhesion of neutrophils, macrophages and other inflammatory cells to the vascular endothelium, thereby increasing vascular permeability. CysLTs can enhance the permeability and contractility of postcapillary venules [6]. LTB4-mediated effects are believed to occur through two G-protein coupled receptors (GPCRs): LTB4 receptor 1, or BLT1 (high affinity), and LTB4 receptor 2, BLT2 (low affinity) [7]. Increased expression of 5-LO in pulmonary artery endothelial cells (PAECs) has been reported in disease states such as primary pulmonary hypertension [8], chronic hypoxia [9] and antigen challenge [10]. Although the mechanism remains unclear, the induction of 5-LO expression may reflect endothelial dysfunction in the pulmonary vasculature, which has been found to be associated with the above diseases.
A third eicosanoid enzymatic pathway is the cytochrome P-450 epoxygenase (EPOX) pathway, which catalyzes two distinct enzymatic activities. EPOX hydroxylase enzymes generate HETEs that have cardiovascular and pro-inflammatory activities. Epoxyeicosatrienoic acids (EETs) that are derived from EPOX have multiple biological activities, including cardioprotection and anti-inflammatory properties [11–13]. The bioconversion of arachidonic acid (AA) into four EET regioisomers, 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET, occurs via EPOX [14,15]. Rat CYP2C11 generates relatively equal proportions of 14,15-EET and 11,12-EET: 39% and 41%, respectively [16]. In human endothelial cells, 11,12-EET was found to significantly inhibit the expression of VCAM-1 in response to TNF-α, IL-1α, and LPS. By contrast, 14,15-EET had negligible effects, whereas 5,6-EET, 8,9-EET, and 11,12-DHET all led varying degrees of inhibition, but to a lesser extent than 11,12-EET. 11,12-EET also inhibited TNF-α-induced E-selectin and ICAM-1 expression [17]. Our previous studies have also shown that 11,12-EET and 14,15-EET can inhibit the oxidized low-density lipoprotein (ox-LDL)-induced expression of ICAM-1, MCP-1/CCL2 and E-selectin in rat pulmonary arterial endothelial cells (RPAECs) [18]. However, the exact mechanism of the suppressive effect of EETs on inflammation remains unclear.
Ox-LDL is associated with atherosclerotic events that involve the modulation of AA metabolism and the activation of inflammatory signaling. Lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1) receptor acts as a cell surface receptor for ox-LDL on endothelial cells, and its expression is enhanced in proatherogenic settings [19, 20]. The LOX-1 receptor is upregulated by several stimuli, including ox-LDL, proinflammatory cytokines, endothelin-1, protein kinase-C, and angiotensin II [21]. We have previously demonstrated that EETs can induce protection against ox-LDL-induced endothelial dysfunction by blocking the binding of ox-LDL to the LOX-1 receptor, which subsequently decreases the expression of proinflammatory molecules [18].
In the present study, we found for the first time that ox-LDL can induce LTB4 production and activation in RPAECs. These increases in LTB4 production and activation can further induce the expression and release of ICAM-1 and MCP-1/CCL2 in RPAECs. Based on two lines of evidence, we speculated that LTB4 might mediate the atherosclerotic inflammatory response following ox-LDL treatment. These included the findings that LTB4 induces endothelium-dependent vascular responses [22] and that LTB4 may be an early mediator of atherosclerosis in patients presenting with obstructive sleep apnea [23, 24]. Indeed, LTB4 exerts a potent pro-inflammatory function via its interactions with cysLTs and BLT receptor subtypes, which are expressed in the inflammatory and structural cells that form the vascular wall. There has currently been a renewed interest in using LT antagonists for the treatment of atherosclerosis and its associated ischemic complications [25].
We have previously demonstrated that ox-LDL can mediate the release of the inflammatory factors ICAM-1 and MCP-1/CCL2 in RPAECs through activation of the MAPK/NF-κB pathway [18]. In this study, we found that the ox-LDL-induced LTB4 synthesis was also regulated by the same pathway. LTB4 synthesis regulated the expression and release of the pro-inflammatory factors ICAM-1 and MCP-1/CCL2 following ox-LDL stimulation. Therefore, we suggest that ox-LDL promotes LTB4 production by regulating the 5-LO pathway in endothelial cells. Following the above, we investigated the mechanisms behind the association of EETs and ox-LDL-induced LTB4 production.
Materials and Methods
2.1 Ethics statement
All of the animal procedures and experiments conducted in this study were approved by the Ethics Committee of the Zhejiang University, School of Medicine. All animals received humane care, in accordance with the guide prepared by the Committee of Care and Use of Laboratory Animals of Zhejiang University (Permit No.ZJU201403-1-02-045). Male Sprague–Dawley rats (200–350 g, purchased from the Laboratory Animal Center, Zhejiang University; Ltd Certificate No. SCXK 2012–0002) were housed in groups of five and kept at 20–23°C under a 12/12 h light/dark cycle in 45–65% humidity. Standard laboratory food and water were provided ad libitum.
2.2. Reagents and antibodies
11,12-EET and 14,15-EET were purchased from Sigma-Aldrich (St. Louis, MO). Ox-LDL was purchased from Yiyuan Biotechnologies (Guangzhou, China) and is considered to be stable for six weeks after receipt when handled aseptically and stored at 2–8°C. Ox-LDL is a large protein (MW 3500 kDa) and has a diameter of 25.8 nm. It is composed of approximately 20–25% protein and 75–80% lipid. The lipid portion can be further described as being composed of 9% free cholesterol, 42% cholesteryl ester, 20–24% phospholipid, and 5% triglyceride. The ox-LDL that is produced by Yiyuan biotechnology is isolated from blood-bank-produced human plasma via ultra-centrifugation (1.019–1.063 g/cc). Following this, human LDL is oxidized using Cu2SO4 (oxidant) in PBS. Oxidation is terminated by adding an excess of EDTA-Na2. Each lot is analyzed via agarose gel electrophoresis for migration versus LDL. The lot of ox-LDL used in this study migrated 2.0-fold further than native LDL. TRIzol Reagent was purchased from Takara (Dalian, China). Primary antibodies against β-actin (Cell Signaling Technology, Danvers, MA), 5-LO and BLT1 (Santa Cruz Biotechnology, Santa Cruz, CA) were used in immunoblotting analysis. Rat LTB4, ICAM-1 and MCP-1/CCL2 enzyme-linked immunoassay (ELISA) kits were purchased from Boster (Wuhan, China). All other reagents and preparations were obtained as indicated.
2.3. Isolation and culture of RPAECs
Rat pulmonary artery endothelial cells (RPAECs) were isolated from pulmonary arteries as previously described with modification [26]. Briefly, male Sprague-Dawley rats (200–350 g) were sacrificed with 20% urethane. Following this, their hearts and lungs were excised. The mainstem pulmonary artery and two vessel generations were isolated from each heart, dissected, split, and fixed onto a 35 mm petri dish. The artery was inverted and the intimal lining was stacked on the dish. One week later, RPAECs were dissociated from the arteries. The cells were maintained in RPMI 1640 (HyClone, Logan, UT) that was supplemented with 10% fetal bovine serum (FBS) (HyClone) at 37°C in the presence of 5% CO2. Primary cells were allowed to grow and were passaged at confluency by trypsin digestion into culture flasks. RPAECs were characterized based on morphological criteria and by indirect immunofluorescence using an antibody specific to rat Factor VIII antigen; they were used up to passage 6.
2.4. RNA isolation and quantitative real-time PCR (qRT-PCR)
After the experimental treatment, total RNA was extracted from RPAECs using TRIzol Reagent (Takara, TaKaRa Biotechnology, Dalian, China) according to the manufacturer’s instructions. First-strand cDNA was generated from 4 μg of total RNA using oligo-dT to prime reverse transcription according to the manufacturer’s protocol. PCR primers were purchased from Shanghai Bioengineering Ltd (Shanghai, China). After an initial denaturation step of 10 min at 95°C, a two-step cycle procedure was used (denaturation at 95°C for 15 s, annealing and extension at 60°C for 1 min) for 40 cycles. Real-time PCR reactions were each performed in a total volume of 20 μL reaction mixture, containing 2 μL cDNA, 10.4 μL 2× SYBR Green 1 Master Mix (Takara), and 0.4 μL of each primer, using a Real-Time PCR System 7500 (Applied Biosystems). GAPDH was used as an internal control to normalize the samples. The transcript number was calculated using a 2-ΔΔCt method (relative) [27]. The primers that were used in this experiment are indicated in Table 1.
Table 1. Primer sequences used in the present study.
| Genes | Primer Sequences(5’-3’) |
|---|---|
| Rat ICAM-1 | Sense: AGATCATACGGGTTTGGGCTTC |
| Antisense: TATGACTCGTGAAAGAAATCAGCTC | |
| Rat MCP-1/CCL2 | Sense: ATGCAGGTCTCTGTCACGCT |
| Antisense: GGTGCTGAAGTCCTTAGGGT | |
| Rat 5-LO | Sense: CAAACCCCTGGAGAGAAGAAC |
| Antisense: GCAATACCGAACACCTCAGAC | |
| Rat BLT1 | Sense: GCACCTGGAGTTTTGAAGTGA |
| Antisense: TACGAACCTTTTGGGACACA | |
| Rat GAPDH | Sense: CATGTTCGTCATGGGTGTGAACCA |
| Antisense: ATGGCATGGACTGTGGTCATGAGT |
2.5. Western blot analysis
After treatment, the cells were washed three times with ice-cold PBS and lysed in 100 μL radioimmunoprecipitation assay (RIPA) lysis buffer (Biyuntian Biotechnology, Haimen, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) (Haoxin Biotechnology, Hangzhou, China). Protein concentration was measured using a BCA Protein Assay Kit (cwbiotech, Beijing, China). Cell lysates (30 μg) were separated by SDS-polyacrylamide gel electrophoresis and probed with antibodies specific for 5-LO, BLT1 (1:200, Santa Cruz, CA, USA) and β-actin (1:1000, Cell Signaling Technology, Beverly, MA, USA) overnight at 4°C; following this, they were probed with goat anti-rabbit 800 antibodies (1:5000) for 2 h at room temperature. Immunoreactive bands were visualized using a two-color infrared imaging system (Odyssey, LI-COR, USA).
2.6. Enzyme-linked immunosorbent assay (ELISA)
After treatment, supernatant was centrifuged, collected, and stored at -80°C prior to use. The presence of MCP-1/CCL2, ICAM-1 and LTB4 in the culture media of RPAECs was detected using ELISA kits (Boster, Wuhan, China); paired matched antibodies were used according to the manufacturer's instructions. Color absorbance at 450 nm was determined using a Bio-Rad microplate reader.
2.7. Statistical analysis
Data were presented as the mean ± S.E.M. GraphPad Prism V5.0 software was used for statistical analysis. Statistical tests were performed using SPSS software (version 16.0; SPSS, Chicago, IL). Differences between mean values of multiple groups were analyzed by either one-way analysis of variance (ANOVA) or Student's t test. Statistical significance was accepted at P < 0.05.
Results
3.1. Ox-LDL triggers inflammatory responses and upregulates the expression and activity of the 5-LO pathway in rat pulmonary arterial endothelial cells
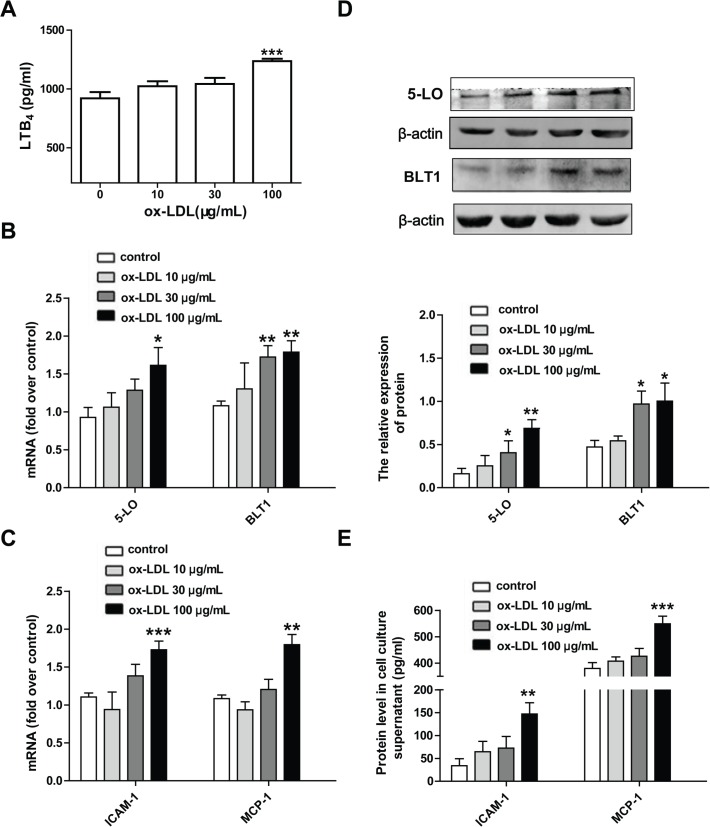
To determine whether the 5-LO pathway is involved in ox-LDL-induced endothelial inflammation, cultured RPAECs were treated with ox-LDL (10–100 μg/mL). Following this, the expression and release of the pro-inflammatory factors ICAM-1 and MCP-1/CCL2 were measured. We found that ox-LDL induced a marked elevation of ICAM-1 and MCP-1/CCL2 expression and release in a concentration-dependent manner in RPAECs (Fig 1C and 1E). Furthermore, 24 hours of stimulation with ox-LDL increased the levels of 5-LO and BLT1 mRNAs and proteins in RPAECs in a concentration-dependent manner (Fig 1B and 1D). Ox-LDL (100 μg/mL) also significantly increased the levels of the 5-LO metabolite LTB4 level in culture supernatants compared to non-stimulated control (P<0.001) (Fig 1A).
Fig 1. Ox-LDL induces the expression and release of ICAM-1 and MCP-1/CCL2 and up-regulates the 5-LO pathway.
(A) RPAECs were exposed to different concentration of ox-LDL (10–100 μg/mL) for 24 h. LTB4 protein levels in cell culture supernatant was quantified by ELISA. (B, D) 5-LO and BLT1 mRNA and protein levels were respectively measured by qRT-PCR and western blot. (C, E) ICAM-1, MCP-1/CCL2 mRNA and protein levels in cell culture supernatant were quantified by qRT-PCR and ELISA. The data shown are means ± S.E.M. of results from at least four independent experiments. *P<0.05, **P<0.01, ***P<0.001 compared with the control group.
3.2. EETs inhibit ox-LDL-induced LTB4, ICAM-1 and MCP-1/CCL2 expression and secretion by suppressing the 5-LO pathway
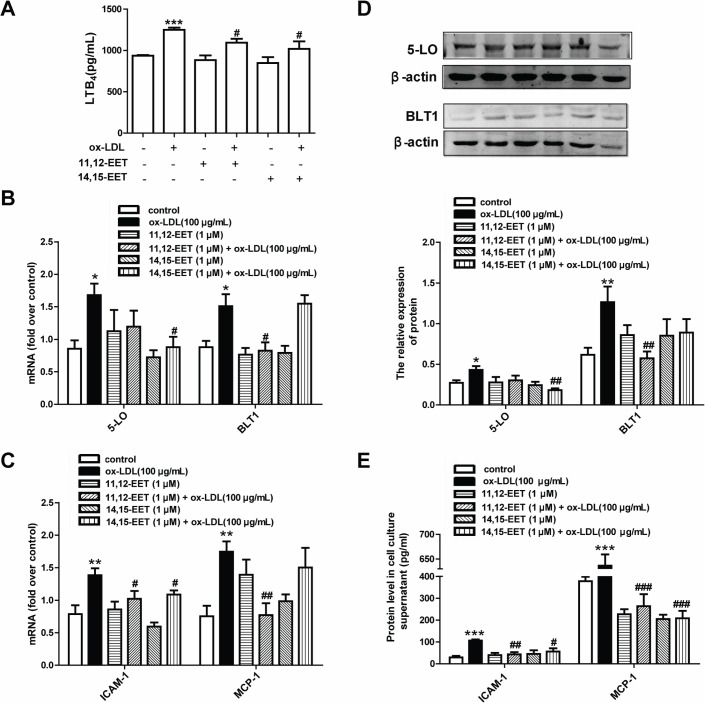
To confirm whether EETs affect the expression and activity of the 5-LO pathway, RPAECs were treated with 11,12-EET and 14,15-EET for 30 min and then stimulated with ox-LDL (100 μg/mL) for 24 h. ELISA was performed to assess the levels of the pro-inflammatory factors LTB4, ICAM-1 and MCP-1/CCL2. The levels of 5-LO and BLT1 proteins and mRNAs were examined by western blot analysis and real-time PCR. Pre-incubation with 11,12-EET or 14,15-EET at 1 μM significantly suppressed the ox-LDL stimulation induced expression of LTB4, ICAM-1 and MCP-1/CCL2 mRNAs and proteins in RPAECs (Fig 2A, 2C and 2E). We next examined the influence of EETs on the ox-LDL-induced expression of 5-LO and the BLT1 LTB4 receptor. As shown in Fig 2B and 2D, the presence of 14,15-EET dramatically decreased 5-LO mRNA and protein expression, whereas 11,12-EET decreased BLT1 expression.
Fig 2. EETs inhibit ox-LDL-induced expression and production of ICAM-1 and MCP-1/CCL2 by down-regulating the 5-LO pathway in RPAECs.
(A) RPAECs were incubated with the indicated concentrations of 11,12-EET or 14,15-EET (1 μM) for 30 min and then stimulated with ox-LDL (100 μg/mL) for 24 h. LTB4 protein levels in cell culture supernatant were quantified by ELISA. (B, D) 5-LO and BLT1 mRNA and protein levels were respectively determined by qRT-PCR and western blot. (C, E) ICAM-1 and MCP-1/CCL2 mRNA expression and protein levels in cell culture supernatant were quantified by qRT-PCR and ELISA. The data represent means ± S.E.M. from six independent experiments. *P<0.05, **P<0.01, ***P<0.001 compared with the control group. # P<0.05 compared with the ox-LDL (100 μg/mL)-treated group.
3.3. LTB4 increases the expression and release of the pro-inflammatory factors ICAM-1 and MCP-1/CCL2
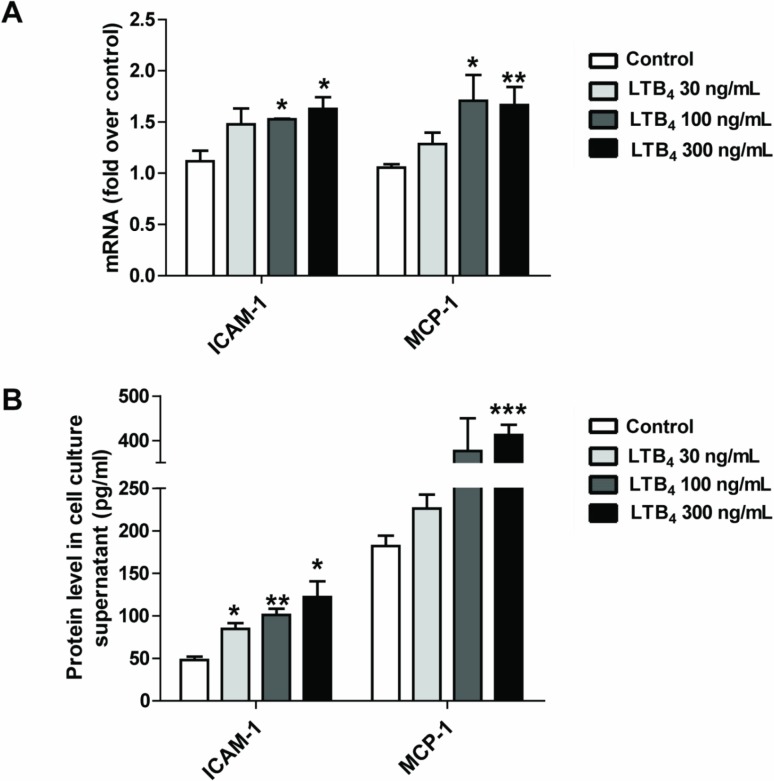
LTB4 plays an important role in inflammatory responses during endothelial dysfunction. A previous report indicated that LTB4 might be an early mediator of atherosclerosis [23]. To determine whether LTB4 can regulate the expression and production of pro-inflammatory factors, primary RPAECs were treated with LTB4 (30–300 ng/mL), and levels of MCP-1/CCL2, a chemokine, and ICAM-1, an adhesion molecule, were measured. We found that treatment with 100–300 ng/mL of LTB4 robustly increased the expression of ICAM-1 and MCP-1/CCL2 mRNAs in RPAECs in a concentration-dependent manner (Fig 3A). We further confirmed these results by measuring ICAM-1 and MCP-1 /CCL2 release, and we found that 24 hours of LTB4 treatment augmented the secretion of these molecules into the culture media of RPAECs in a concentration-dependent manner (Fig 3B).
Fig 3. LTB4 induces ICAM-1 and MCP-1/CCL2 expression and release in rat pulmonary arterial endothelial cells.
RPAECs were exposed to different concentrations of LTB4 (30–300 ng/mL) for 24 h. ICAM-1 and MCP-1/CCL2 mRNA and protein levels were respectively measured by qRT-PCR (A) and ELISA (B). The data represent means ± S.E.M. from six independent experiments. * P <0.05, ** P <0.01, *** P <0.001 compared with the control group.
3.4. Role of LTB4 in ox-LDL-induced ICAM-1 and MCP-1/CCL2 expression
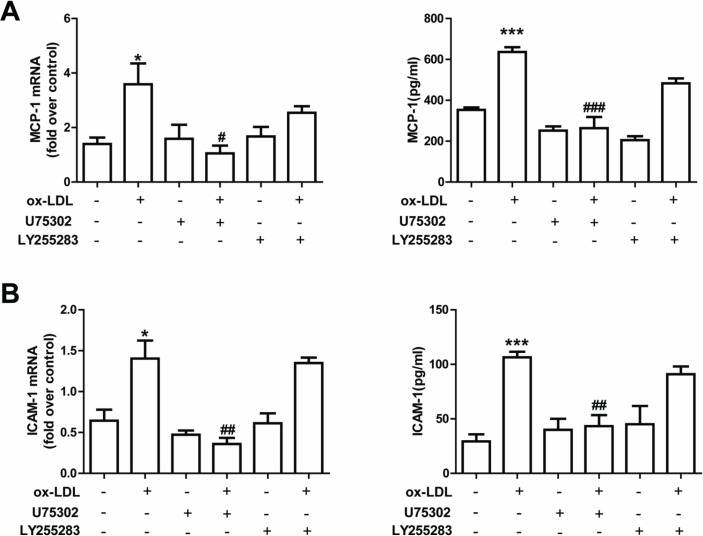
LTB4 exerts biological function through two receptors: BLT1 and BLT2 [4]. To determine whether ox-LDL-induced ICAM-1 and MCP-1/CCL2 expression is mediated by LTB4 receptors, primary RPAECs were treated with LTB4 receptor antagonists in the presence or absence of ox-LDL. ICAM-1 and MCP-1/CCL2 levels in supernatant were determined by ELISA.
At 24 hours after treatment with LTB4, we found a concentration-dependent increase in ICAM-1 and MCP-1/CCL2 expression and release (Fig 3A and 3B). Furthermore, pretreatment using a BLT1 receptor antagonist, U75302, inhibited ox-LDL-induced ICAM-1 and MCP-1/CCL2 production (Fig 4A and 4B). However, the pretreatment using a BLT2 receptor antagonist, LY255283, did not produce the same effects (Fig 4A and 4B). These results indicate that one way in which ox-LDL induces inflammatory responses in RPAECs is by activating the LTB4/BLT1 receptor.
Fig 4. Role of LTB4 receptor antagonists on ICAM-1 and MCP-1/CCL2 expression in RPAECs.
Cells were pretreated with a BLT1 receptor antagonist (U75302) (10 μM) and a BLT2 receptor antagonist (LY255283) (10 μM) and then stimulated with ox-LDL (100 μg/mL) for 24 h. Expression levels of MCP-1/CCL2 (A) and ICAM-1 (B) mRNAs and proteins were determined by qRT-PCR and ELISA, respectively. The data represent means ± S.E.M. from five independent experiments. * P <0.05, ** P <0.01, *** P <0.001 compared with the control group. # P <0.05 and ## P <0.01 compared with the ox-LDL (100 μg/mL)-stimulated group.
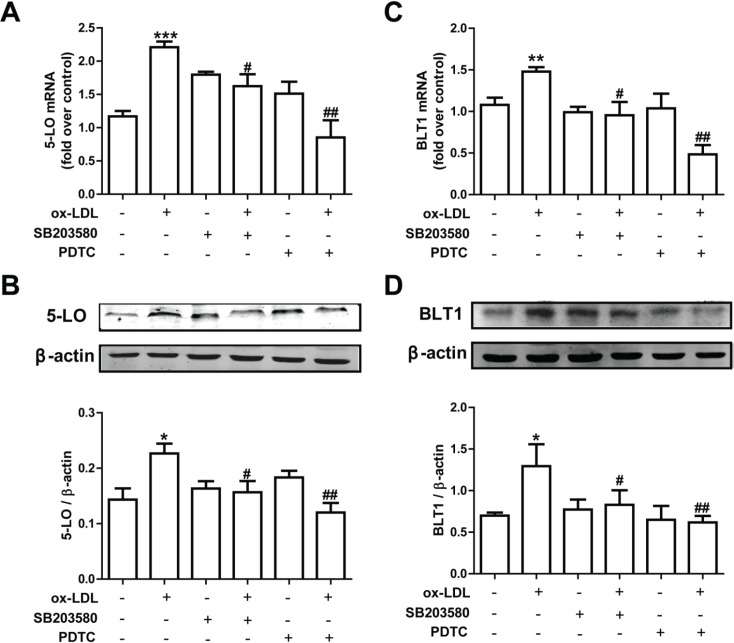
3.5. Pharmacological suppression of the p38 MAPK and NF-κB pathways inhibits 5-LO and BLT1 expression in RPAECs
To assess the potential molecular mechanisms behind ox-LDL-mediated 5-LO pathway activation, we examined how using inhibitors of the 5-LO pathway affected the expression of 5-LO and BLT1 mRNAs and proteins in RPAECs. After 30 minutes of exposure to a p38 inhibitor (SB203580) and an NF-κB inhibitor (PDTC), we found marked reductions in 5-LO and BLT1 mRNA and protein levels, which had been previously upregulated following treatment with ox-LDL. Thus, these data suggest that ox-LDL stimulation upregulates 5-LO and BLT1 expression by activating p38 MAPK and NF-κB signaling pathways.
Discussion
The current study is the first to describe the cellular mechanisms that are responsible for the inhibitory effects of epoxygenase-derived eicosanoids, including 11,12-EET and 14,15-EET, on ox-LDL-mediated pro-inflammatory mediators (ICAM-1 and MCP-1/CCL2) in RPAECs. Leukotrienes have long been described as lipid mediators and were initially shown to have significant effects on the pathogenesis of allergic rhinitis and bronchial asthma. Recent research has revealed a new role for leukotrienes in the vascular inflammation that is associated with atherosclerotic vascular disease [28, 29]. As noted, LTB4, a 5-LO-derived eicosanoid, is a prominent pro-inflammatory and chemotactic mediator and can regulate the production of many additional inflammatory mediators by activating nuclear signals [30]. In an earlier exploratory study, we tested the effects of low-density lipoprotein (LDL) on LTB4 production, ICAM-1 and MCP-1/CCL2 expression in RPAECs. We found ox-LDL is more potent, and LDL does not induce significant inflammatory responses in RPAECs (S2 Fig). Therefore, we selected ox-LDL stimulation as the model in our current study. In this study, we showed that ox-LDL promoted LTB4 production in RPAECs (Fig 1A). We used hydrogen peroxide (H2O2) as a control to determine whether our results were due to an oxidant effect or an ox-LDL-specific effect. We examined the expression and release of ICAM-1 and MCP-1/CCL2 and the production of LTB4 following H2O2 stimulaiton in rat pulmonary artery endothelial cells. H2O2 (125–500 μM)-induced inflammatory responses were not found to be statistically significant (S3 Fig). Therefore, it is a specific effect induced by ox-LDL. We then demonstrated that 1 μM concentrations of 11,12-EET and 14,15-EET (Fig 2A) decreased ox-LDL-induced LTB4 production in RPAECs, which represents the first description that EETs can affect the ox-LDL-stimulated production of LTB4 in pulmonary arterial endothelial cells.
Although the exact mechanism behind the suppressive effects of EETs on LTB4 production remains unclear, the induction of LTB4 production in RPAECs revealed that ox-LDL has a potential role in initiating inflammation through the 5-LO pathway. Additional observations suggested that EETs might directly inhibit 5-LO activity. Liu et al. [31] demonstrated that soluble epoxide hydrolase (sEH) inhibition stabilizes CYP450 production of EETs and that inhibition of sEH enhances the anti-inflammatory effects that are produced by a protein inhibitor of 5-lipoxygenase activation in a murine model. Revermann et al. [32] reported that a pirinixic acid derivative, LP105, is a potent inhibitor of monocyte 5-LO and can reduce AngII-induced vascular remodeling in mice. Our results regarding the anti-inflammatory effects of 11,12-EET and 14,15-EET in RPAECs are consistent with these findings. Pretreatment with 11,12-EET or 14,15-EET at 1 μM causes significant suppression of 5-LO and its metabolite LTB4, both of which are increased by ox-LDL stimulation (Fig 2A, 2B and 2D). These results suggest that a beneficial effect is imparted by the increased formation of protective arachidonic acid derivatives, particularly with respect to EETs.
It has been reported that LTB4-BLT signaling is involved in atherogenesis [33–35]. In early atherogenesis, the 5-LO metabolite LTB4 enhances inflammatory responses by several mechanisms, one of which is the induction of pro-inflammatory mediator release and foam cell formation that is orchestrated by BLT receptors [5]. It has been reported that symptomatic atherosclerotic plaques express elevated levels of both 5-LO and LTB4. This evidence indicates that LTB4 might be a key mediator of 5-LO-dependent plaque instability [36]. In line with these reports, we showed here that the inhibition of BLT1 attenuated the ox-LDL stimulation induced upregulation of ICAM-1 and MCP-1/CCL2 expression in RPAECs (Fig 4). Moreover, LTB4 activation is capable of stimulating the production of ICAM-1 and MCP-1/CCL2 in RPAECs (Fig 3A and 3B), suggesting that LTB4 is a major activator of inflammation. In a study [37] that compared healthy subjects with patients presenting with carotid atherosclerosis, a significant increase was found in the expression of all of the components of the 5-LO pathway, in addition to levels of BLT1 and BLT2 mRNA (measured by real-time PCR), in peripheral blood mononuclear cells; levels of LTB4 were also increased in plasma (measured by ELISA). However, whether ox-LDL promotes BLT1 and BLT2 expression has not yet been investigated. In our study, we found that expression of the BLT1 LTB4 receptor was decreased by treatment with 11,12-EET (Fig 2B, Fig 2D). These results suggest that the LTB4/BLT1 receptor pathway plays a role in generating the anti-inflammatory effects that are produced by EETs in ox-LDL-stimulated RPAECs. BLT1 expression was decreased by 11,12-EET, whereas 14,15-EET dramatically decreased 5- LO expression. The differential action of the two EETs in mediator production and 5-LO pathway expression was due to the differences in chemical structure, which can significantly impact their effects on vascular activity. These phenomena has been previously reported that 11,12-EET significantly attenuates the TNF-α-stimulated endothelial activation and leukocyte adhesion by inhibiting IκBα degradation and NF-κB activation, whereas 14,15-EET results in a lower activation of NF-κB [38]. Another study suggests that EETs may act in a cell type-specific manner [39]. These results may explain the differential actions of the two EETs in the present study.
As a principle enzyme of leukotriene production, 5-LO is regulated by Ca2+, ox-LDL, ROS, and HNE in the NF-κB/ERK and Sp1/p38 MAPK pathways [40, 41]. In this study, we found that ox-LDL promoted the expression of 5-LO and BLT1 mRNAs and proteins through the p38 MAPK/ NF-κB pathway in rat PAECs. Results from qRT-PCR and western blotting indicated that ox-LDL promotes the expression of 5-LO and BLT1 mRNAs and proteins in a concentration-dependent manner. The maximum effect produced by ox-LDL on 5-LO expression was at a concentration of 100 μg/mL: the same concentration at which the p38 MAPK and NF-κB pathways became the most activated in a previously reported study [18]. To determine whether the p38 MAPK and NF-κB pathways were involved in the ox-LDL-induced expression of 5-LO and BLT1, a p38 MAPK inhibitor, SB203580, and an NF-κB inhibitor, PDTC, were employed. Following treatment with these inhibitors, Ox-LDL-induced increases to the expression of 5-LO and BLT1 mRNAs and proteins were significantly inhibited (Fig 5). Furthermore, our earlier data also demonstrated that EETs suppress ox-LDL-induced p38 phosphorylation in RPAECs [18]. This phosphorylation of p38 MAPK might modulate the nuclear translocation of various transcription factors, including NF-κB [42]. These data support that the p38 MAPK and NF-κB signal transduction pathways play a role in ox-LDL-regulated 5-LO and BLT1 expression. Our results demonstrate that 5-LO and BLT1 expression is regulated by ox-LDL-induced NF-κB activation through the p38 MAPK pathway. Because 5-LO is a crucial factor in leukotriene production, inhibition of this pathway may represent a potential therapeutic target for treating vascular endothelium mediated inflammatory responses in patients with cardiovascular disease. With the rising number of indications for anti-LT therapy, drug development strategies centered on 5-LO inhibition are becoming increasingly popular. The most advanced drug that has been developed thus far is an N-hydroxyurea derivative known as Atreleuton. Atreleuton was recently examined in a phase II study conducted on atherosclerotic patients with established cardiovascular disease following acute coronary syndrome. The results of this trial demonstrated that Atreleuton potently reduces leukotriene production and may also affect the formation of atherosclerotic plaques [43]. Another study also revealed that the Cys-LT1 receptor antagonist Montelukast might be beneficial toward the secondary prevention of cardiovascular disease [44].
Fig 5. The p38 MAPK and NF-κB signaling pathways are involved in ox-LDL-mediated expression of 5-LO and BLT1.
RPAECs were pre-treated with a p38 inhibitor (10 μM SB203580) or an NF-κB inhibitor (20 μM PDTC) for 30 min and then incubated with ox-LDL (100 μg/mL) for another 24 h. The levels of 5-LO and BLT1 mRNAs and proteins were determined by qRT-PCR (A, C) and western blot (B, D), respectively. The data represent the means ± S.E.M. from four independent experiments. * P <0.05 and ** P <0.01 compared with the untreated group. # P <0.05 and ## P <0.01 compared with the ox-LDL (100 μg/mL)-stimulated group.
Conclusion
In conclusion, the results of the present study confirmed the following: (1) Ox-LDL promotes the production of LTB4, ICAM-1 and MCP-1/CCL2 in conditions of endothelial inflammation by up-regulating 5-LO expression through p38 MAPK/NF-κB activation; (2) EETs produce anti-inflammatory effects by inhibiting LTB4, ICAM-1 and MCP-1/CCL2 release in RPAECs through the 5-LO pathway; (3) the LTB4/BLT1 receptor pathway is involved in the production of EET-mediated anti-inflammatory effects. We proposed a potential mechanism to explain the protective effects of EETs on endothelial dysfunction in RPAECs.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
We thank Yicheng Xie from the University of British Columbia in Canada for the kind suggestions on manuscript preparation. We also thank the language service of American Journal Experts for revising the English writing of this paper.
Data Availability
All relevant data are within the paper and Supporting Information.
Funding Statement
This work was supported by grants from the Natural Science Foundation of Zhejiang Province of China (No. Y2100218, http://www.zjnsf.gov.cn/) and the National Science Foundation of China (No. 81270095, http://www.nsfc.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Di Gennaro A, Haeggstrom JZ. The leukotrienes: immune-modulating lipid mediators of disease. Adv Immunol. 2012; 116: 51–92. 10.1016/B978-0-12-394300-2.00002-8 [DOI] [PubMed] [Google Scholar]
- 2. Okunishi K, Peters-Golden M. Leukotrienes and airway inflammation. Biochim Biophys Acta. 2011; 1810: 1096–1102. 10.1016/j.bbagen.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Papierniak ES, Lowenthal DT, Harman E. Novel therapies in asthma: leukotriene antagonists, biologic agents, and beyond. Am J Ther. 2013; 20: 79–103. 10.1097/MJT.0b013e31826915c2 [DOI] [PubMed] [Google Scholar]
- 4. Back M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc Drugs Ther. 2009; 23: 41–48. 10.1007/s10557-008-6140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res. 2010; 86: 243–253. 10.1093/cvr/cvq016 [DOI] [PubMed] [Google Scholar]
- 6. Charo IF, Taub R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat Rev Drug Discov. 2011; 10: 365–376. 10.1038/nrd3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim N, Luster AD. Regulation of immune cells by eicosanoid receptors. Scientific World Journal. 2007; 7: 1307–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wright L, Tuder RM, Wang J, Cool CD, Lepley RA, Voelkel NF. 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 1998; 157: 219–229. [DOI] [PubMed] [Google Scholar]
- 9. Voelkel NF, Tuder RM, Wade K, Hoper M, Lepley RA, Goulet JL, et al. Inhibition of 5-lipoxygenase-activating protein (FLAP) reduces pulmonary vascular reactivity and pulmonary hypertension in hypoxic rats. J Clin Invest. 1996; 97: 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu SJ, Tang LO, Watney E, Chi EY, Henderson WR Jr. In situ amplification of 5-lipoxygenase and 5-lipoxygenase-activating protein in allergic airway inflammation and inhibition by leukotriene blockade. J Immunol. 2000; 165: 4640–4648. [DOI] [PubMed] [Google Scholar]
- 11. Fleming I. DiscrEET regulators of homeostasis: epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends Pharmacol Sci. 2007; 28: 448–452. [DOI] [PubMed] [Google Scholar]
- 12. Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010; 56: 336–344. 10.1097/FJC.0b013e3181f04b1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliv Rev. 2011; 63: 597–609. 10.1016/j.addr.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 14. Capdevila JH, Falck JR. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins Other Lipid Mediat. 2002; 68–69: 325–344. [DOI] [PubMed] [Google Scholar]
- 15. Pfister SL, Gauthier KM, Campbell WB. Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol.2010; 60: 27–59. 10.1016/B978-0-12-385061-4.00002-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karara A, Makita K, Jacobson HR, Falck JR, Guengerich FP, DuBois RN, et al. Molecular cloning, expression, and enzymatic characterization of the rat kidney cytochrome P-450 arachidonic acid epoxygenase. J Biol Chem. 1993; 268: 13565–13570. [PubMed] [Google Scholar]
- 17. Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999; 285: 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang JX, Zhang SJ, Liu YN, Lin XX, Sun YH, Shen HJ, et al. EETs alleviate ox-LDL-induced inflammation by inhibiting LOX-1 receptor expression in rat pulmonary arterial endothelial cells. Eur J Pharmacol. 2014; 727: 43–51. 10.1016/j.ejphar.2014.01.045 [DOI] [PubMed] [Google Scholar]
- 19. Wang GF, Shi CG, Sun MZ, Wang L, Wu SX, Wang HF, et al. Tetramethylpyrazine attenuates atherosclerosis development and protects endothelial cells from ox-LDL. Cardiovasc Drugs Ther. 2013; 27: 199–210. 10.1007/s10557-013-6440-6 [DOI] [PubMed] [Google Scholar]
- 20. Zhang H, Ma G, Yao Y, Qian H, Li W, Chen X, et al. Olmesartan attenuates the impairment of endothelial cells induced by oxidized low density lipoprotein through downregulating expression of LOX-1. Int J Mol Sci. 2012; 13: 1512–1523. 10.3390/ijms13021512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006; 69: 36–45. [DOI] [PubMed] [Google Scholar]
- 22. Bäck M, Sakata K, Qiu H, Haeggström JZ, Dahlén SE. Endothelium-dependent vascular responses induced by leukotriene B4. Prostaglandins Other Lipid Mediat. 2007; 83(3): 209–212. [DOI] [PubMed] [Google Scholar]
- 23. Lefebvre B, Pépin JL, Baguet JP, Tamisier R, Roustit M, Riedweg K, et al. Leukotriene B4: early mediator of atherosclerosis in obstructive sleep apnoea? Eur Respir J. 2008; 32(1): 113–120. 10.1183/09031936.00137107 [DOI] [PubMed] [Google Scholar]
- 24. Stanke-Labesque F, Pépin JL, de Jouvencel T, Arnaud C, Baguet JP, Petri MH, et al. Leukotriene B4 pathway activation and atherosclerosis in obstructive sleep apnea. J Lipid Res. 2012; 53(9): 1944–1951. 10.1194/jlr.P022814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riccioni G, Bäck M, Capra V. Leukotrienes and atherosclerosis. Curr Drug Targets. 2010; 11(7): 882–887. [DOI] [PubMed] [Google Scholar]
- 26. Adkison JB, Miller GT, Weber DS, Miyahara T, Ballard ST, Frost JR, et al. Differential responses of pulmonary endothelial phenotypes to cyclical stretch. Microvasc Res. 2006; 71: 175–184. [DOI] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 28. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 29. Riccioni G, Back M. Leukotrienes as modifiers of preclinical atherosclerosis? Scientific World Journal. 2012; 2012: 490968 10.1100/2012/490968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le Bel M, Brunet A, Gosselin J. Leukotriene B4, an endogenous stimulator of the innate immune response against pathogens. J Innate Immun. 2014; 6(2): 159–168. 10.1159/000353694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu JY, Yang J, Inceoglu B, Qiu H, Ulu A, Hwang SH, et al. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010; 79: 880–887. 10.1016/j.bcp.2009.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Revermann M, Mieth A, Popescu L, Paulke A, Wurglics M, Pellowska M, et al. A pirinixic acid derivative (LP105) inhibits murine 5-lipoxygenase activity and attenuates vascular remodelling in a murine model of aortic aneurysm. Br J Pharmacol. 2011; 163: 1721–1732. 10.1111/j.1476-5381.2011.01321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aiello RJ, Bourassa PA, Lindsey S, Weng W, Freeman A, Showell HJ. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler Thromb Vasc Biol. 2002; 22: 443–449. [DOI] [PubMed] [Google Scholar]
- 34. Back M, Bu DX, Branstrom R, Sheikine Y, Yan ZQ, Hansson GK. Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci U SA. 2005; 102: 17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heller EA, Liu E, Tager AM, Sinha S, Roberts JD, Koehn SL, et al. Inhibition of atherogenesis in BLT1-deficient mice reveals a role for LTB4 and BLT1 in smooth muscle cell recruitment. Circulation. 2005; 112: 578–586. [DOI] [PubMed] [Google Scholar]
- 36. Cipollone F, Mezzetti A, Fazia ML, Cuccurullo C, Iezzi A, Ucchino S, et al. Association between 5-lipoxygenase expression and plaque instability in humans. Arterioscler Thromb Vasc Biol. 2005; 25: 1665–1670. [DOI] [PubMed] [Google Scholar]
- 37. Sánchez-Galán E, Gómez-Hernández A, Vidal C, Martín-Ventura JL, Blanco-Colio LM, Muñoz-García B, et al. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009; 81(1): 216–225. 10.1093/cvr/cvn277 [DOI] [PubMed] [Google Scholar]
- 38. Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliv Rev. 2011; 63(8): 597–609. 10.1016/j.addr.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 39. Thomson SJ, Askari A, Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. Int J Vasc Med. 2012; 2012: 605101 10.1155/2012/605101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee SJ, Kim CE, Seo KW, Kim CD. HNE-induced 5-LO expression is regulated by NF-{kappa}B/ERK and Sp1/p38 MAPK pathways via EGF receptor in murine macrophages. Cardiovasc Res. 2010; 88: 352–359. 10.1093/cvr/cvq194 [DOI] [PubMed] [Google Scholar]
- 41. Coffey MJ, Serezani CH, Phare SM, Flamand N, Peters-Golden M. NADPH oxidase deficiency results in reduced alveolar macrophage 5-lipoxygenase expression and decreased leukotriene synthesis. J Leukoc Biol. 2007; 82: 1585–1591. [DOI] [PubMed] [Google Scholar]
- 42. Bao MH, Zhang YW, Zhou HH. Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38 MAPK/NF-kappaB pathway. J Ethnopharmacol. 2013; 146: 543–551. 10.1016/j.jep.2013.01.019 [DOI] [PubMed] [Google Scholar]
- 43. Steinhilber D, Hofmann B. Recent advances in the search for novel 5-lipoxygenase inhibitors. Basic Clin Pharmacol Toxicol. 2014; 114(1): 70–77. 10.1111/bcpt.12114 [DOI] [PubMed] [Google Scholar]
- 44. Hofmann B, Steinhilber D. 5-Lipoxygenase inhibitors: a review of recent patents (2010–2012). Expert Opin Ther Pat. 2013; 23(7): 895–909. 10.1517/13543776.2013.791678 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and Supporting Information.