Abstract
Background
Bu-zhong-yi-qi-tang (BZYQT), an herbal formula of traditional Chinese medicine, has been an effective regimen of allergic diseases for nearly 800 years. Our previous report has demonstrated its anti-inflammatory effects in patients with perennial allergic rhinitis, and the aim of this study is to investigate the anti-asthmatic effect of BZYQT.
Methods
Female BALB/cByJNarl mice were sensitized with normal saline (control group) or OVA. Mice sensitized by OVA were fed with distilled water (OVA group), oral 0.5 g/Kg (low-dose group) or 1 g/Kg (high-dose group) of BZYQT solution once daily on days 36-40 besides their routine diet. Airway hyper-responsiveness (AHR), eosinophil infiltration, levels of cytokines and total immunoglobulin E (IgE) in broncho-alveolar lavage fluid (BALF) were determined. The lungs and tracheas were removed, and histopathologic examination was subsequently performed.
Results
AHR was significantly reduced in both low- and high-dose BZYQT groups compared with the OVA group after inhalation of the highest dose of methacholine (50 mg/ml). The levels of eotaxin, Th2-related cytokines (IL-4, IL-5, IL-13), IgE, and eosinophil infiltration in BALF were significantly decreased in both BZYQT groups compared with the OVA group. Histopathologic examination revealed that eosinophil infiltration of the lung and trachea tissues was remarkably attenuated in both BZYQT groups.
Conclusions
Oral administration of BZYQT solution may exert anti-asthmatic effect by relieving AHR in OVA-sensitized mice, which is compatible with our clinical experience. Although detailed mechanism is to be determined, we surmise that it may be correlated with the immune-modulatory effects of inhibiting Th2 responses on the basis of our limited results.
Introduction
Asthma has become an important public health problem worldwide, and patients with bronchial asthma are usually characterized with symptoms of wheezing, cough, and shortness of breath [1]. Chronic inflammation of airways has been identified to play a key role in the pathogenesis of bronchial asthma, which used to be taken as a disease of bronchial smooth muscles [2, 3]. Airway inflammation in asthma is characterized by acute onset airway hyper-responsiveness (AHR) and infiltration of inflammatory cells, especially eosinophils [4–6]. Furthermore, eosinophils have been related to the severity of asthma, and several mediators resulting in eosinophil activation may also lead to contraction of airway smooth muscles [7, 8]. The onset of asthma is generally attributed to genetic and environmental factors; however, predominant Th2 cell activity has been identified as part of the core pathogenesis of asthma [9]. Activated Th2 cells will secrete cytokines, such as IL-4, IL-5, and IL-13 [9, 10]. IL-4 has been proved to promote IgE production and T cell differentiation into Th2 cells [11, 12]. IL-5, however, induces the differentiation, maturation, and migration of eosinophils to the local tissue of inflammation [13, 14]. Overproduction of IL-13 enhances airway hyper-responsiveness, and it is also implicated in the pathogenesis of airway eosinophilia [15]. Therefore, suppression of Th2 cytokines may have the potential to alleviate chronic airway inflammation and, subsequently, the symptoms of asthma [10].
Bu-Zhong-Yi-Qi-Tang (BZYQT), composed of ten medical herbs (Table 1), is a well-known formula of traditional Chinese medicine and has been widely used for treatment of allergic diseases [16]. In the past decades, it has been frequently reported that BZYQT possesses a variety of immune-modulatory effects, such as stimulation of peripheral blood mononuclear cells to produce G-CSF and TNF-alpha in Hepatocellular carcinoma (HCC) patients [17], suppression of the proliferation of human hepatoma cell lines by inhibition of DNA synthesis followed by apoptosis [18], suppression of contact hypersensitivity during chronic stage [19], and reduction of IgE levels in the atopic dermatitis animal model [20]. However, none of these previous reports has investigated its effect on relieving clinical airway symptoms, such as AHR, and the potential correlation with Th2 activity and eosinophil infiltration. In view of these facts, this study is designed to determine whether BZYQT alleviates hyper-responsiveness and chronic inflammation of the airways in the asthmatic murine model sensitized by ovalbumin.
Table 1. Composition of Bu-Zhong-Yi-Qi-Tang (every 7.56g of water extract are derived from 27g of raw herb).
| Plant Name | Plant Part | Ratio |
|---|---|---|
| Astragalus mongholicus Bunge. | Root | 6.0 |
| Panax ginseng C.A.Mey. | Root | 4.0 |
| Glycyrrhiza uralensis Fisch. | Root and rhizome | 4.0 |
| Zingiber officinale Rosc. | Rhizome | 3.0 |
| Ziziphus jujuba Mill. var. inermis Rehd. | Fruit | 2.0 |
| Angelica dah-rica Fisch. ex Hoffm. | Root | 2.0 |
| Citrus reticulata Blanco. | Pericarp | 2.0 |
| Atractylodes macrocephala Koidz. | Rhizome | 2.0 |
| Bupleurum chinense DC. | Root | 1.0 |
| Cimicifuga foetida L. | Rhizome | 1.0 |
| Total | 27.0 |
Materials and Methods
Animals and Ethics Statement
Inbred female BALB/cByJNarl mice aged 6–8 weeks and weighed around 15–20 g were purchased from the National Laboratory Animal Center (Taipei, Taiwan) [21]. All mice were housed and maintained at the animal center of Chang Gung University before the experiment. The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital (Approval ID: AN-94082).
Preparation of bu-zhong-yi-qi-tang (BZYQT)
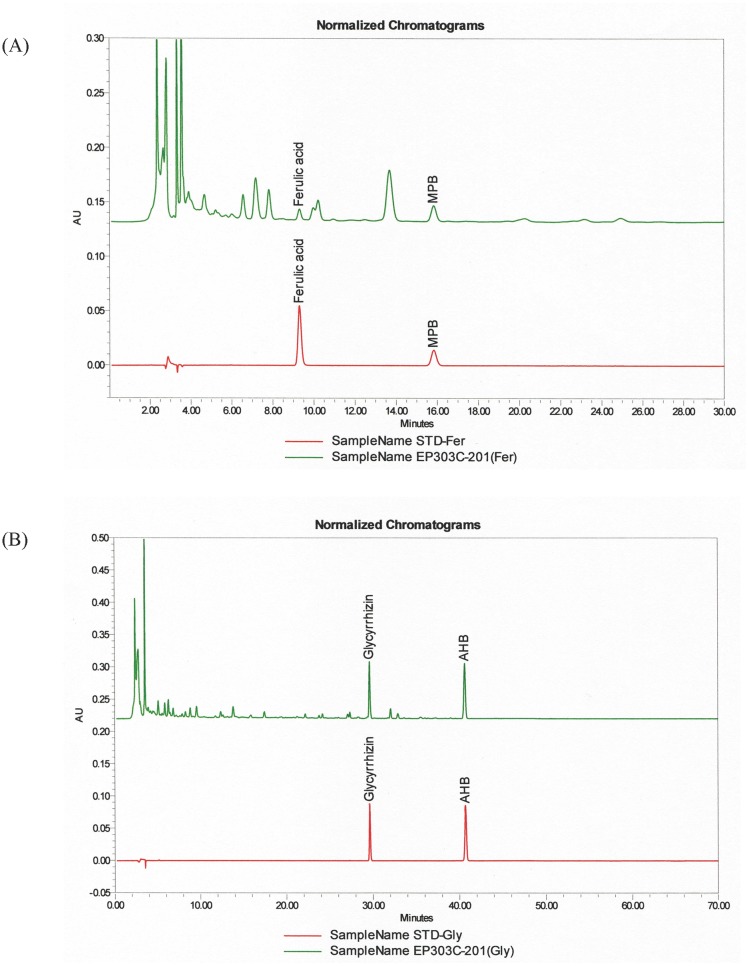
BZYQT was purchased from the GMP pharmaceutical factory of Chuang Song Zong Pharmaceutical Co. (Kaohsiung, Taiwan), and the authenticity was proved by HPLC (Fig 1). The original preparation is concentrated powder of the aqueous-extract mixture of 10 common Chinese herbal plants, which are listed in Table 1[16]. The concentrated powder of BZYQT was dissolved (1 g/ml) in distilled water at 26°C, and was ready for administration to mice via gastric tube.
Fig 1. HPLC data of Bu-Zhong-Yi-Qi-Tang (BZYQT) (Upper: BZYQT; Lower: standard sample).
The peaks indicate the existence of glycyrrhizin, AHB, Ferulic acid, and MPB in both panels, which proves the authenticity of BZYQT.
Mice sensitization, challenge, and drug administration
A total of 30 female BALB/c(ByJNarl) mice were randomly divided into 4 groups:
Control group (n = 10): sensitized and challenged with normal saline, and treated with distilled water
OVA group (n = 4): sensitized and challenged with OVA, and treated with distilled water
Low-dose BZYQT (LD) group (n = 8): sensitized and challenged with OVA, and treated with 0.5 g/Kg of BZYQT solution
High-dose BZYQT (HD) group (n = 8): sensitized and challenged with OVA, and treated with 1 g/Kg of BZYQT solution
Mice in OVA and BZYQT groups were sensitized by three intra-peritoneal (I.P.) injections of 25 μg OVA (grade V; Sigma, St. Louis, USA), which had been emulsified in 4 mg aluminum hydroxide in 100 μl normal saline on days 0, 14, 28 [22]. Mice in the control group were injected with 100 μl normal saline. Mice were challenged with 100 μg OVA dissolved in 50μl normal saline (OVA group and BZYQT groups), or with 50 μl normal saline alone (control group), intra-nasally (I.N.) on days 42–44. Mice in each group were fed once daily with distilled water (control group and OVA group), or with solution of BZYQT (low-/ high-dose BZYQT groups) via gastric tube on days 36–40 besides their routine diet.
Measurement of airway hyper-responsiveness (AHR)
AHR was measured on day 45, 24 hours after last OVA challenge, with stimulation of methacholine to conscious, unrestrained mice in plethysmograph (Buxco Electronics, Troy, NY, USA). Mice in each group were exposed to a series of incremental dosages of aerosolized methacholine (6.25, 12.5, 25, 50 mg/ml) for one minute, and AHR was recorded as enhanced pause (Penh), which was calculated with the program provided by Buxco Electronics [22]. Enhanced pause (Penh) represents airway hyper-responsiveness and is defined as pause × (PEP/PIP) (pause, time of expiration; PEP, peak expiratory pressure; and PIP, peak inspiratory pressure) [23].
Collection of broncho-alveolar lavage fluid (BALF)
Mice were sacrificed with the cervical dislocation method on the day following AHR measurement (Day 46). To collect BALF, lungs of each mouse were lavaged with 1 ml of normal saline for three times. The different cell types and numbers were determined after cytospin preparations and Liu stain of BALF. The percentage of eosinophil was determined on the basis of eosinophil cell counts in 500 BALF cells according to morphology and staining characteristics. After centrifugation, the supernatant of BALF was collected, frozen and stored at -80°C until further cytokine analysis [22].
Determination of cytokines, IgE, and chemokine levels in BALF samples
BALF samples were diluted to either 1:2(for cytokine analysis), or 1:4 (for eotaxin analysis). The concentration of IL-4, IL-5, IL-13, and eotaxin in BALF samples were measured with enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocols. Total immunoglobulin E (IgE) levels in BALF samples were also measured by ELISA, directly using OD 450 nm readings.[22, 24]
Histopathologic examination of lungs
After BALF collection, the lungs and tracheas were removed from mice, and were fixed with 10% of buffered formalin for one week. After they were fixed, the specimens were embedded in OCT (Tissue-Tek, Sakura Finetek, Torrance, CA, USA)[24]. Each tissue specimen was sliced into 6-μm sections, and was stained with H&E method for light microscopic inspection.
Statistical analysis
The results are presented in mean ± SD (standard deviation). The statistical significance was determined by one-way analysis of variance (ANOVA) with a Bonferroni post-hoc test, and a difference with P-value < 0.05 was considered significant.
Results
Oral administration of BZYQT attenuated OVA-induced AHR
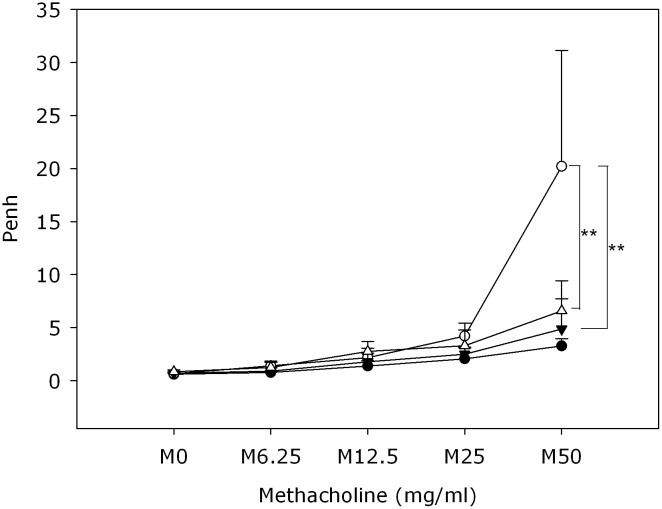
The mice in low-/high-dose BZYQT groups were administered with solution of BZYQT once daily on days 36–40 in order to determine the effect of BZYQT on OVA-induced airway inflammation. AHR was determined in all mice after inhalation of a series of dosages of methacholine ranging from 6.25 to 50 mg/ml. There were no significant differences in AHR observed between the OVA group and the BZYQT groups with the inhalation of 0-25mg/ml methacholine. The highest dose of methacholine (50 mg/ml) inhalation, however, induced significantly higher AHR in the OVA group than that in the control group, low-dose BZYQT group, and high-dose BZYQT group (Fig 2). Moreover, there was no significant difference between Penh values of both low- and high-dose BZYQT groups after the highest level (50mg/ml) of methacholine stimulation. These findings indicated that both low- and high-dose BZYQT treatment might significantly suppress OVA-induced AHR with similar potency.
Fig 2. Effect of oral administration of BZYQT on airway hyperresponsiveness (AHR).
After last OVA challenge, mice were exposed to a series of incremental dosages of methacholine (6.25, 12.5, 25, 50 mg/ml). The Penh values were then recorded, indicating AHR of mice under each dosage of methacholine stimulation. Control group (●, n = 10), OVA group (○, n = 4), Low dose (0.5 g/Kg) BZYQT group (△, n = 8), High dose (1 g/Kg) BZYQT group (▼, n = 8). Data were presented in mean ± standard deviation. ** p< 0.01 compared with the OVA group.
Effect of BZYQT on Th2-related cytokines, eotaxin and IgE levels in BALF
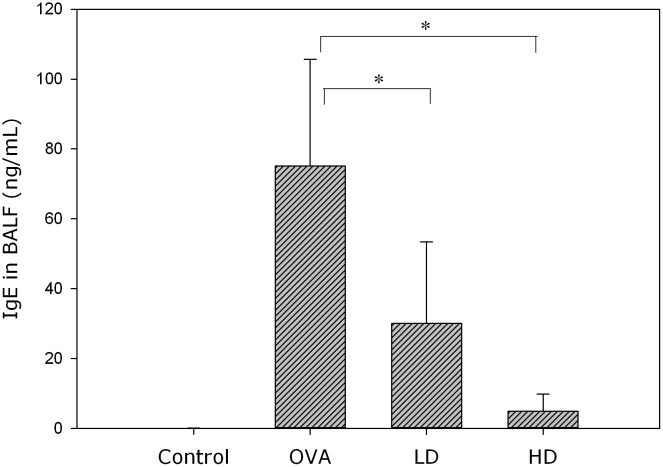
All the mice were sacrificed on day 46, and BALF was collected from their lungs. After centrifugation, the supernatant of BALF was analyzed for levels of Th2-related cytokines (IL-4, IL-5, IL-13), eotaxin and IgE with ELISA. The two BZYQT groups were found to have significantly lower levels of IL-4, IL-5, IL-13 (Fig 3), IgE (Fig 4), and eotaxin (Fig 5B) than the OVA group.
Fig 3. BZYQT regulated Th2 cytokines in BALF of asthmatic murine model sensitized by OVA.
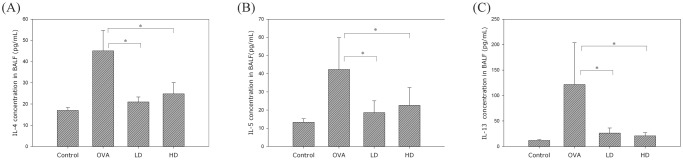
The concentrations of IL-4 (A), IL-5 (B), and IL-13 (C) were determined by ELISA. Data were presented in mean ± standard deviation. * p < 0.05 compared with the OVA group.
Fig 4. BZYQT significantly attenuated IgE levels in BALF of OVA-sensitized mice.
These data were measured by ELISA, and presented in mean ± standard deviation. ** p < 0.01 compared with the OVA group.
Fig 5. The concentration of eosinophil chemoattractant eotaxin (B) and the percentage of eosinophil cell count (A) in BALF were significantly down-regulated in the BZYQT groups.
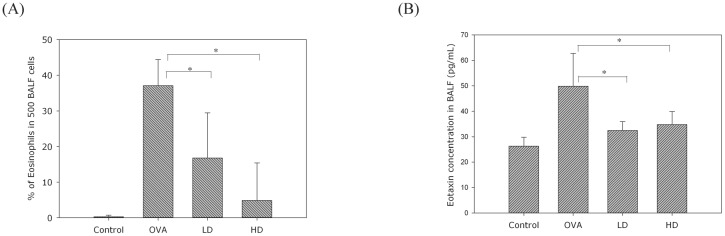
Data were presented in mean ± standard deviation. * p < 0.05 compared with the OVA group.
BZYQT attenuated OVA-induced eosinophil infiltration in BALF
To evaluate the effect of BZYQT on eosinophil infiltration, BALF samples after cytospin preparations were stained with Liu stain, and the percentage of eosinophil was determined on the basis of eosinophil cell counts in 500 BALF cells according to morphology and staining characteristics. The percentage of eosinophil was significantly higher in the OVA group than the control group, and it was significantly attenuated in both BZYQT groups (Fig 5A).
Histopathologic examination of effect of BZYQT on eosinophil infiltration
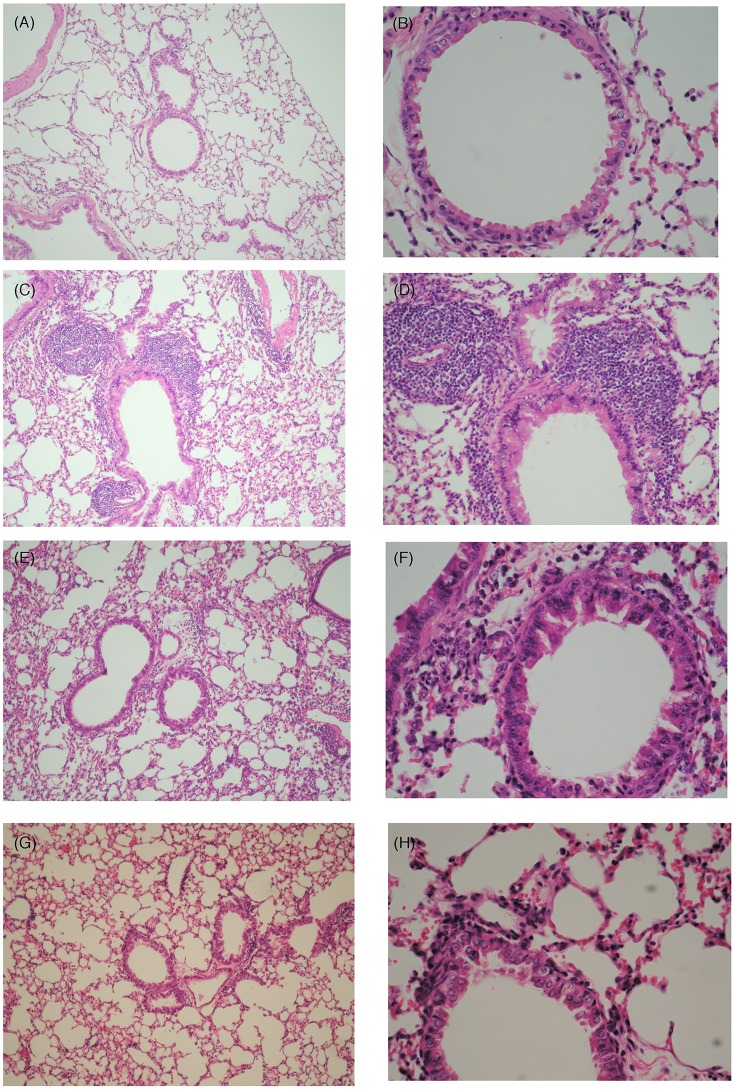
The lungs and tracheas were removed, fixed with formalin, sliced into sections, and stained with H&E method. For each animal, the extent of the lung infiltrate was determined by establishing the percentage of compromised bronchioli within 30 of such structures, randomly selected at low-power fields (with a X4 objective). In addition, with the X40 objective, 8random digital images per group were taken within areas of overt peribronchiolar inflammation. Total eosinophil cell counts were determined from these images and expressed as number of cells per square millimeter. Such quantification was focused on peribronchiolar areas that were the main sites of inflammatory reaction.
Histopathologic examination of the specimens revealed severe infiltration of eosinophils over the perivascular and peribronchiolar areas of the lung (45,2±5.4/HPF) and trachea tissues of the OVA group (Fig 6C and 6D). Oral administration of low-(23.8±4.2/HPF) and high-dose (5.1±3.9/HPF) BZYQT solutions, however, significantly reduced eosinophil infiltration into lung tissues (Fig 6).
Fig 6. Histopathologic examination of sections of lung and trachea tissues with H&E stain.
Specimens were derived from the control (low power view, A; high power view, B), OVA (low power view, C; high power view, D), LD (low power view, E; high power view, F), and HD (low power view, G; high power view, H) groups, respectively. Notably, eosinophil infiltration over the perivascular and peribronchiolar areas were enhanced in OVA group, and then attenuated in both BZYQT groups significantly.
Discussion
The pathophysiology of asthma is extremely complicated, involving the network of Th2 cells and cytokines, and airway inflammation has been proved to be a major feature of the pathogenesis of chronic asthma. After exposure to allergens, Th2 cells secrete cytokines, such as IL-4, IL-5, and IL-13 [25], which promote airway hyper-responsiveness, IgE production, and infiltration of inflammatory cells, such as eosinophil, into the airways [26]. These pathophysiological changes were confirmed in the OVA-sensitized mice in our study. On the other hand, research on cytokine-related gene knockout mice has demonstrated that the symptoms of asthma may be aggravated by Th2 cytokines [27, 28].
Our study evaluated the beneficial effects of BZYQT on airway hyper-responsiveness and Th2 overexpression in OVA-sensitized murine model of asthma. The levels of inflammatory cell (eosinophil) infiltration, Th2 cytokines (IL-4, IL-5, IL-13), eosinophil-related chemokine (eotaxin), IgE and AHR were all significantly enhanced in the OVA-sensitized group. However, all the above levels were remarkably reduced in both low- and high-dose BZYQT groups.
IL-5 plays a key role in the terminal differentiation, maturation, and survival of eosinophils, and together with the chemokine eotaxin, promotes eosinophil recruitment to the site of inflammation [12, 14]. It has been reported that murine model which is in lack of IL-5 expression would not develop AHR or eosinophilia though under allergen challenge[29, 30]. Anti-IL-5 regimens may ameliorate airway allergic inflammation and reduce eosinophils in the blood and sputum; however, AHR and late allergic responses were not significantly improved[29–31]. Recent studies has revealed that anti-IL-5 regimens may reduce frequency of exacerbation in severe asthma more significantly in subgroups of patients, in which eosinophils play a pathogenic role[32–34]. In our study, BZYQT has been demonstrated to decrease the levels of IL-5 and the number of eosinophil in BALF and in the lung tissues surrounding the airways as well. Although further studies are needed to clarify the detailed mechanism, we suspect that BZYQT exerted anti-asthmatic effect through down-regulating Th2 response, especially IL-5 expression, and eosinophilic inflammation was ameliorated subsequently.
IL-4 facilitates the differentiation and proliferation of Th2 cells, the switching of B cells to secrete IgE, and the development of AHR as well [12, 34]. On the other hand, inhibition of IL-4 by monoclonal antibodies attenuates airway eosinophilia and IgE level in allergic mice [35]; therefore, the production of IgE is closely related to the concentration of IL-4. Furthermore, it has been reported that IL-4 deficient mice demonstrated to be spared from asthmatic attack and airway inflammation[36]. Th2 cytokines, especially IL-4 and IL-13, are crucial mediators of asthma, and both are proved to play an important role in up-regulating the eosinophil chemo-attractants of eotaxin [37]. There is evidence showing that eosinophilia may be correlated with the induction of AHR and the severity of asthma [8, 38]. BZYQT has been used in traditional Chinese medicine for allergic asthma treatment and prevention. Besides relieving AHR, which we have already known clinically, we demonstrated its efficacy in reducing IL-4 and IgE levels in BALF in murine model of allergic asthma. Moreover, histopathologic analysis also confirmed that administration of BZYQT may significantly reduce eosinophil infiltration into the perivascular and peribronchiolar areas of the lung and trachea tissues. Though further evidence may be need, it is indicated in our study that BZYQT reduced the levels of IgE of OVA-sensitized mice by down-regulating IL-4, and its suppression of IL-4 and IL-13 may also contribute to relieve lung eosinophila and AHR.
Bu-Zhong-Yi-Qi-Tang (BZYQT), which comprises crude ingredients extracted from ten herbs, has been a well-known traditional Chinese medicine formula used in preventive therapy for allergic diseases during non-acute stages for 800 years. BZYQT has been proved to have immune-modulatory effects on allergic diseases. A previous clinical study had revealed that long-term therapy with BZYQT suppresses total serum levels of IgE, PGE2, LTC4, and COX-2 mRNA expression in IL-4-stimulated PMN in patients with perennial allergic rhinitis, indicating that BZYQT is beneficial to the non-acute stage of allergic rhinitis via anti-inflammatory mechanisms [16]. Moreover, BZYQT can also decrease the IgE level in serum of mice by inhibiting the development of IL-4-producing CD4+ T cells [39]. Another study had revealed that oral administration of BZYQT suppresses IgE antibody production and histamine release in type I allergic reaction in mice immunized with ovalbumin, revealing the potential efficacy of BZYQT in treating type I allergic diseases, such as asthma [40]. Ishimitsu had been reported a similar study revealed that BZYQT suppresses IgE and Th2-type cytokine production [41]. Consistent with previous findings, our results demonstrated that BZYQT alleviated airway eosinophilia and hyper-responsiveness, which may be accomplished by decreasing the concentration of Th2 cytokines, eotaxin, and IgE in OVA-sensitized mice. However, due to the complexity of the component of Chinese medical formula, it remains unclear whether a specific compound inside acts alone or multiple compounds may interact to achieve this efficacy we demonstrated in this study. It is anticipated that future studies may reveal the molecular mechanism of the immune-modulatory effects of BZYQT.
Conclusions
Oral administration of BZYQT demonstrated immune-modulatory effects by ameliorating AHR and airway eosinophilia in the murine model of allergic asthma induced by ovalbumin, and those effects may be accomplished by down-regulating Th2 responses. However, further studies are needed to clarify its underlying mechanism.
Acknowledgments
This study was supported by a grant from the New Century Health Care Promotion Foundation.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by a grant from the New Century Health Care Promotion Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 2004;22:789–815. [DOI] [PubMed] [Google Scholar]
- 2. Santing RE, Olymulder CG, Zaagsma J, Meurs H. Relationships among allergen-induced early and late phase airway obstructions, bronchial hyperreactivity, and inflammation in conscious, unrestrained guinea pigs. The J Allergy Clin Immunol 1994;93:1021–1030. [DOI] [PubMed] [Google Scholar]
- 3. Kay AB. Asthma and inflammation. J Allergy Clin Immunol 1991;87:893–910. [DOI] [PubMed] [Google Scholar]
- 4. Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745. [DOI] [PubMed] [Google Scholar]
- 5. Berend N, Salome CM, King GG. Mechanisms of airway hyperresponsiveness in asthma. Respirology 2008;13:624–631. [DOI] [PubMed] [Google Scholar]
- 6. Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis 1990;142:1407–1413. [DOI] [PubMed] [Google Scholar]
- 7. Chang HC, Gong CC, Chan CL, Mak OT. A nebulized complex traditional Chinese medicine inhibits Histamine and IL-4 production by ovalbumin in guinea pigs and can stabilize mast cells in vitro. BMC Complement Altern Med 2013;13:174 10.1186/1472-6882-13-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. N Engl J Med 1990;323:1033–1039. [DOI] [PubMed] [Google Scholar]
- 9. Georas SN, Guo J, De Fanis U, Casolaro V. T-helper cell type-2 regulation in allergic disease. Eur Respir J 2005;26:1119–1137. [DOI] [PubMed] [Google Scholar]
- 10. Huang WC, Kuo ML, Li ML, Yang RC, Liou CJ, Shen JJ. Gynostemma pentaphyllum decreases allergic reactions in a murine asthmatic model. Am J Chin Med 2008;36:579–592. [DOI] [PubMed] [Google Scholar]
- 11. Barrios RJ, Kheradmand F, Batts L, Corry DB. Asthma: pathology and pathophysiology. Arch Pathol Lab Med 2006;130:447–451. [DOI] [PubMed] [Google Scholar]
- 12. Lee MY, Shin IS, Lim HS, Shin HK. A water extract of Samchulkunbi-tang attenuates airway inflammation by inhibiting inos and MMP-9 activities in an ovalbumin-induced murine asthma model. BMC Complement Altern Med 2012;12:257 10.1186/1472-6882-12-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothenberg ME, Hogan SP. The eosinophil. Annu. Rev. Immunol 2006;24:147–174. [DOI] [PubMed] [Google Scholar]
- 14. Kariyawasam HH, Robinson DS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol 2007;19:681–686. [DOI] [PubMed] [Google Scholar]
- 15. Scichilone N, Messina M, Battaglia S, Catalano F, Bellia V. Airway hyperresponsiveness in the elderly: prevalence and clinical implications. Eur Respir J 2005;25:364–375. [DOI] [PubMed] [Google Scholar]
- 16. Yang SH, Yu CL. Antiinflammatory effects of Bu-zhong-yi-qi-tang in patients with perennial allergic rhinitis. J Ethnopharmacol 2008;115:104–109. [DOI] [PubMed] [Google Scholar]
- 17. Kao ST, Yang SL, Hsieh CC, Yang MD, Wang TF, Lin JG. Immunomodulation of Bu-Zhong-Yi-Qi-Tang on in vitro granulocyte colony-stimulating-factor and tumor necrosis factor-alpha production by peripheral blood mononuclear cells. Immunopharmacol Immunotoxicol 2000;22:711–720. [DOI] [PubMed] [Google Scholar]
- 18. Kao ST, Yeh CC, Hsieh CC, Yang MD, Lee MR, Liu HS, et al. The Chinese medicine Bu-Zhong-Yi-Qi-Tang inhibited proliferation of hepatoma cell lines by inducing apoptosis via G0/G1 arrest. Life Sci 2001;69:1485–1496. [DOI] [PubMed] [Google Scholar]
- 19. Nakada T, Watanabe K, Matsumoto T, Santa K, Triizuka K, Hanawa T. Effect of orally administered Hochu-ekki-to, a Japanese herbal medicine, on contact hypersensitivity caused by repeated application of antigen. Int Immunopharmacol 2002;2:901–911. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi H, Mizuno N, Kutsuna H, Teramae H, Ueoku S, Onoyama J, et al. Hochu-ekki-to suppresses development of dermatitis and elevation of serum IgE level in NC/Nga mice. Drugs Exp Clin Res 2003;29:81–84. [PubMed] [Google Scholar]
- 21. Sy LB, Wu YL, Chiang BL, Wang YH, Wu WM. Propolis extracts exhibit an immunoregulatory activity in an OVA-sensitized airway inflammatory animal model. Int Immunopharmacol 2006;6:1053–1060. [DOI] [PubMed] [Google Scholar]
- 22. Liou CJ, Huang WC, Kuo ML, Yang RC, Shen JJ. Long-term oral administration of Gynostemma pentaphyllum extract attenuates airway inflammation and Th2 cell activities in ovalbumin-sensitized mice. Food Chem Toxicol 2010;48:2592–2598. 10.1016/j.fct.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 23. Park SJ, Shin WH, Seo JW, Kim EJ. Anthocyanins inhibit airway inflammation and hyperresponsiveness in a murine asthma model. Food Chem Toxicol 2007;45:1459–1467. [DOI] [PubMed] [Google Scholar]
- 24. Wu CJ, Chen LC, Kuo ML. Attenuated Salmonella typhimurium reduces ovalbumin-induced airway inflammation and T-helper type 2 responses in mice. Clin Exp Immunol 2006;145:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dai HY, Cao KJ, Zhao AB, Chen XY. [Study on the effects of hematopoiesis of anemic mice after marrow-suppressed treated by tonifying kidney, invigorating spleen, and removing blood stasis]. Zhong Yao Cai 2011;34:250–253. [PubMed] [Google Scholar]
- 26. Shin IS, Jeon WY, Shin HK, Lee MY. Effects of montelukast on subepithelial/peribronchial fibrosis in a murine model of ovalbumin induced chronic asthma. Int Immunopharmacol 2013;17:867–873. 10.1016/j.intimp.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 27. Venkayya R, Lam M, Willkom M, Grunig G, Corry DB, Erle DJ. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol 2002;26:202–208. [DOI] [PubMed] [Google Scholar]
- 28. Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, et al. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest 2004;113:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med 1996;183:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka H, Kawada N, Yamada T, Kawada K, Takatsu K, Nagai H. Allergen-induced airway inflammation and bronchial responsiveness in interleukin-5 receptor alpha chain-deficient mice. Clin Exp Allergy 2000;30:874–881. [DOI] [PubMed] [Google Scholar]
- 31. Deckers J, Branco Madeira F, Hammad H. Innate immune cells in asthma. Trends Immunol 2013;34:540–547. 10.1016/j.it.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 32. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009;360:973–984. 10.1056/NEJMoa0808991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 2009;360:985–993. 10.1056/NEJMoa0805435 [DOI] [PubMed] [Google Scholar]
- 34. Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol 2013;4:263 10.3389/fmicb.2013.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gluck J, Rymarczyk B, Rogala B. Serum IL-33 but not ST2 level is elevated in intermittent allergic rhinitis and is a marker of the disease severity. Agents Actions 2012;61:547–550. 10.1007/s00011-012-0443-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, et al. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy: journal of the British Society for Allergy and Clinical Immunology 1994;24:73–80. [DOI] [PubMed] [Google Scholar]
- 37. Beal DR, Stepien DM, Natarajan S, Kim J, Remick DG. Reduction of eotaxin production and eosinophil recruitment by pulmonary autologous macrophage transfer in a cockroach-allergen induced asthma model. Am J Physiol Lung Cell Mol Physiol 2013;305:L866–877. 10.1152/ajplung.00120.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gleich GJ, Frigas E, Loegering DA, Wassom DL, Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. Open J Immunol 1979;123:2925–2927. [PubMed] [Google Scholar]
- 39. Kaneko M, Kishihara K, Kawakita T, Nakamura T, Takimoto H, Nomoto K. Suppression of IgE production in mice treated with a traditional Chinese medicine, bu-zhong-yi-qi-tang (Japanese name: hochu-ekki-to). Immunopharmacology 1997;36:79–85. [DOI] [PubMed] [Google Scholar]
- 40. Suzuki T, Takano I, Nagai F, Fujitani T, Ushiyama K, Okubo T, et al. Suppressive effects of Hochu-ekki-to, a traditional Chinese medicine, on IgE production and histamine release in mice immunized with ovalbumin. Biol Pharm Bull 1999;22:1180–1184. [DOI] [PubMed] [Google Scholar]
- 41. Ishimitsu R, Nishimura H, Kawauchi H, Kawakita T, Yoshikai Y. Dichotomous effect of a traditional Japanese medicine, Bu-zhong-yi-qi-tang on allergic asthma in mice. Int Immunopharmacol 2001;1:857–865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.