Abstract
Forgetfulness is common symptom with age. Especially for midlife women, hormonal cessation by menopausal change is one of the causes in cognitive disorders. And neuropathological changes in brain can lead to mild cognitive impairment (MCI) and eventually dementia. Prevention of MCI is important for decreasing progression to dementia. This article presents therapeutic approaches based on pathophysiologic changes in brain for preventing cognitive decline.
Keywords: Hormone replacement therapy, Mild cognitive impairment, Postmenopause
Introduction
Cognitive disorders are classified as cognitive aging, mild cognitive impairment (MCI), and dementia.1 Cognitive aging is physiologic forgetfulness and represents an erosion of existing abilities, beginning almost imperceptibly in middle age and accelerating during old age.1 MCI is episodic memory loss without dementia. It is demonstrable memory impairment, but other cognitive abilities are not impaired and daily activities are largely intact.1 Neurophysiological changes in the brain begin and clinical manifestations like cognitive decline are present with age, that`s defined as MCI. When these symptoms are persistent with cognitive impairment, it eventually will progress to dementia. Dementia is major cognitive decline that has a substantial impact on occupational activities and other aspects of usual daily living.1 Dementia is caused by various disease or conditions. The most common cause of dementia is Alzheimer's disease (AD).
Ward et al.2 reported that the prevalence of MCI is 31.9% in Korean people older than 60 years. People with MCI are considered to be at risk for developing dementia, since research has shown that over half of those with MCI will develop dementia within 5 years, especially AD.3 But it also means that some people with MCI seem to remain stable or return to normal over time. Thus MCI can be regarded as preclinical dementia, and the prevention of it is very important clinically.
Neuropathology
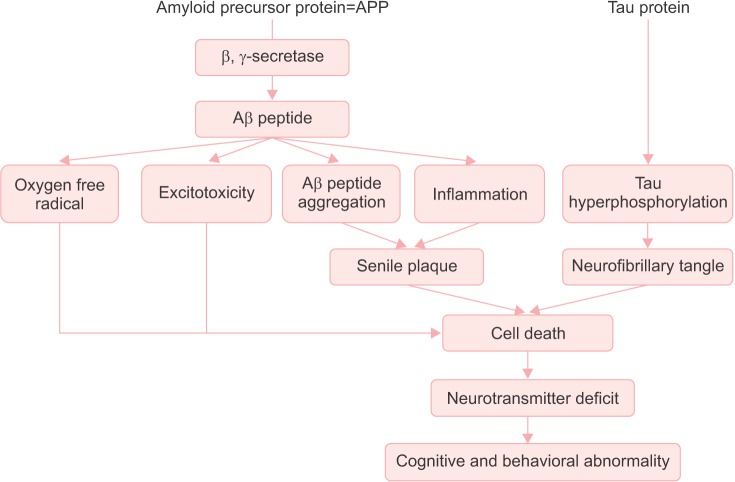
MCI is associated with the early stages of the neurobiological and neuropathological changes of AD; including the accumulation of senile plaques, neurofibrillary tangles, synaptic and neurotransmitter associated deficits, and significant neuronal cell death.4 These changes are thought to be related to the cause, development, and course of the disease.
Senile plaques, which are composed of β-amyloid polypeptides, seem to form as a result of disorders in processing β-amyloid and its precursor protein.5 And the inflammation around plaques seem to destroy neighboring neurons.5 Neuronal death in the brain plays a key role in AD progression and is directly linked to neuroinflammation. Thus, the regulation of neuroinflammatory processes might be a practical strategy for the treatment of AD.6
Neurofibrillary tangles are made up of a protein called tau, and eventually lead to destruction of the neuron.5
Cholinergic deficits in AD have been well documented.7 Insufficient levels of acetylcholine (Ach) at synapse will impair the neuromodulatory function of this neurotransmitter.7 The reduction of Ach is correlated with the neuropathology and cognitive impairment.
Therapeutic Approaches Based on Pathologic Mechanism in Brain (Fig. 1)
Fig. 1. Therapeutic approaches based on pathologic mechanism in brain.
1. Antioxidants
Vitamin E is known to be potent antioxidant properties and free radicals may contribute to the pathological processes of cognitive impairment. Sano et al.8 had reported antioxidants that protect against oxidative damage may reduce the neuronal damage and slow the progression of AD. Antioxidants are considered to be a promising approach to neuroprotection and constitute a major part of the panel of clinical and experimental drugs that are currently considered for AD prevention and therapy.9 But, recent reports by Cochrane Database conclude that no convincing evidence that vitamin E is of benefit in the treatment of AD or MCI.10
2. Anti-inflammatory drugs
Inflammation surrounding β-amyloid plaques with resultant destruction of neurons is thought to be a key factor in the pathogenesis of AD.5 Observational studies have suggested that non-steroidal anti-inflammatory drugs (NSAIDs) protect against AD,11,12 but results have been inconsistent.13,14 Systemic review and meta-analysis of observational studies conclude NSAIDs seems to lower the risk of developing AD in adults aged over 55 years.15 And benefits may be greater the longer NSAIDs are used.15 But the evidence of potential preventive use of aspirin is not robust.15
3. Menopausal hormone therapy (HT)
During development and adulthood, the human brain is a target for estrogens and other gonadal steroid hormones. Estrogen influences neural function and neurological disease directly, through effects on neurons and glia, and indirectly, through effects on oxidative stress, inflammation, the cerebral vasculature and the immune system.16 The Women's Health Initiative Memory Study (WHIMS) reported that effect of estrogen plus progestin on global cognitive function in postmenopausal women aged 65 years or older did not improve cognitive function when compared with placebo.17 While most women receiving estrogen plus progestin did not experience clinically relevant adverse effects on cognition compared with placebo, a small increased risk of clinically meaningful cognitive decline occurred in the estrogen plus progestin group.17 Next year the effect of estrogen-alone therapy, also evaluated in WHIMS. They concluded that for women aged 65 years or older, estrogen therapy had an adverse effect on cognition, which was greater among women with lower cognitive function at initiation of treatment.18
WHIMS of Younger Women (WHIMSY) later reported that conjugated equine estrogens (CEEs)-based therapies produced no overall sustained benefit or risk to cognitive function when administered to postmenopausal women aged 50 to 55 years.19 And they are not able to address whether initiating HT during menopause and maintaining therapy until any symptoms are passed affects cognitive function, either in the short- or long term.19
A recent analysis of the population-based Cache County Study followed 1,768 women who had provided a detailed history on age at menopause and use of HT examined whether the association of HT with AD.20 They reported that women who used HT within 5 years of menopause had a 30% reduced risk for developing AD in later life, especially if the duration of use was 10 years or longer. Moreover, HT may be beneficial if taken near menopause.20
An ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS), called the KEEPS Cognitive and Affective Study (KEEPS Cog) reported that HT initiated during the perimenopausal period could delay or preferably prevent future development of neurodegenerative diseases like MCI and AD.21 Furthermore, compared to placebo, women assigned to the oral CEE group improved significantly on measures of depression-dejection and anxiety-tension. The oral CEE group also showed a trend in improvement on measures of anger-hostility and memory recall of printed material.21
Ultimately, the recent results of the KEEPS and the KEEPS Cog study are that if there proves to be a window of time in the early post menopause when initiation of long-term HT has a net beneficial effect, then women can feel reassured that treatment of their menopausal symptoms when they are likely to be the most severe, can concurrently protect their vascular system, reduce bone reabsorption, or even elicit neuroprotection from dementia in late life.21,22
4. Cholinesterase inhibitor (CI)
CIs are the only agents approved by the U.S. Food and Drug Administration for the treatment of AD. They inhibit the degradation of Ach within the neuronal synapse. Instruments that measure cognition, behavior, and functional ability have shown that CIs are beneficial in patients with AD.5
CIs could theoretically delay or prevent progression to AD from the MCI state, but have failed to reach sufficient strength of evidence to be recommended.23 From the current evidence, if there is a benefit of CIs in slowing progression to AD, it appears to be transient as well as limited.24
5. Others
Based on the observation of decreased cholinergic neurotransmission in brain disorders characterized by cognitive impairment, cholinergic precursor loading therapy could relieve the cognitive symptoms.25 Several studies have shown that acetyl-L-carnitine and choline alphoscerate are able to increase the synthesis and the release of Ach in patients with MCI, Alzheimer-related dementia, cerebrovascular damage and aging.26,27,28,29
In Europe, Ginkgo biloba is commonly prescribed for all memory impaired patients, with the idea that it improves blood and oxygen flow to the brain and supports memory function, mental sharpness and circulation.30
Lifestyle Change
"Maintain a healthy lifestyle" with adequate exercise, avoidance of obesity, mental and physical stimulation, control of stress, treatment of medical illnesses and depression, and control of vascular risk factors such as diabetes, hypertension, and hyperlipidemia is suggested to prevent cognitive decline.31,32
A healthy diet helps prevent hypertension (via reduced saturated fats and sodium), prediabetes (reduce sweets and caloric intake and consume more fibre), and stroke (dietary change to reduce cholesterol).30
There is evidence that individuals whose diets are high in omega-3 fatty acids, especially docosahexaenoic acid (DHA), have a 50% reduction in their risk of developing dementia.33 About 180 mg of DHA daily intake is suggested and this amount can be achieved by eating the fish about three times per week.30
And the potential for physical activity and exercise training to improve cognitive function is that women with higher levels of baseline physical activity were less likely to develop cognitive decline.34,35
About the mental activity, keeping the brain active by continuing to learn new skills is helpful to build cognitive reserve.36 This staves off dementia.
Free radical damages can accelerate the brain aging, so by consuming antioxidants such as vitamin E will help the body quench free radical associated changes.30
Conclusion
The prevention and treatment of cognitive impairment has considered important within aging population. Interventions from lifestyle measures to pharmacological treatments may be beneficial for cognitive function. And recent studies suggest that physical exercise, mental activity, and nutritional supplements may reduce the risk of cognitive disorders and AD.
Acknowledgement
This study was supported by research funds from Chosun University Hospital, 2012.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Henderson VW. Cognitive symptoms and disorders in the midlife woman. Female Patient. 2011;36:1–5. [Google Scholar]
- 2.Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14–21. doi: 10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 4.Haroutunian V, Hoffman LB, Beeri MS. Is there a neuropathology difference between mild cognitive impairment and dementia? Dialogues Clin Neurosci. 2009;11:171–179. doi: 10.31887/DCNS.2009.11.2/vharoutunian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLaGarza VW. Pharmacologic treatment of Alzheimer's disease: an update. Am Fam Physician. 2003;68:1365–1372. [PubMed] [Google Scholar]
- 6.Zhou W, Hu W. Anti-neuroinflammatory agents for the treatment of Alzheimer's disease. Future Med Chem. 2013;5:1559–1571. doi: 10.4155/fmc.13.125. [DOI] [PubMed] [Google Scholar]
- 7.Giacobini E. Invited review: Cholinesterase inhibitors for Alzheimer's disease therapy: from tacrine to future applications. Neurochem Int. 1998;32:413–419. doi: 10.1016/s0197-0186(97)00124-1. [DOI] [PubMed] [Google Scholar]
- 8.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimers Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 9.Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer's disease as preventive and therapeutic approach. Free Radic Biol Med. 2002;33:182–191. doi: 10.1016/s0891-5849(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 10.Farina N, Isaac MG, Clark AR, Rusted J, Tabet N. Vitamin E for Alzheimer's dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2012;11:CD002854. doi: 10.1002/14651858.CD002854.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 12.in t' Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimers disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 13.Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology. 1999;53:197–201. doi: 10.1212/wnl.53.1.197. [DOI] [PubMed] [Google Scholar]
- 14.Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, et al. A randomized controlled trial of prednisone in Alzheimer's disease. Alzheimer's Disease Cooperative Study. Neurology. 2000;54:588–593. doi: 10.1212/wnl.54.3.588. [DOI] [PubMed] [Google Scholar]
- 15.Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer's disease: systematic review and meta-analysis of observational studies. BMJ. 2003;327:128. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Villiers TJ, Pines A, Panay N, Gambacciani M, Archer DF, Baber RJ, et al. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric. 2013;16:316–337. doi: 10.3109/13697137.2013.795683. [DOI] [PubMed] [Google Scholar]
- 17.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 18.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 19.Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, LeBlanc ES, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429–1436. doi: 10.1001/jamainternmed.2013.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao H, Breitner JC, Whitmer RA, Wang J, Hayden K, Wengreen H, et al. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79:1846–1852. doi: 10.1212/WNL.0b013e318271f823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wharton W, Gleason CE, Miller VM, Asthana S. Rationale and design of the Kronos Early Estrogen Prevention Study (KEEPS) and the KEEPS Cognitive and Affective sub study (KEEPS Cog) Brain Res. 2013;1514:12–17. doi: 10.1016/j.brainres.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wharton W, Gleason CE, Dowling NM, Carlsson CM, Brinton EA, Santoro MN, et al. The KEEPS-Cognitive and Affective Study: baseline associations between vascular risk factors and cognition. J Alzheimers Dis. 2014;40:331–341. doi: 10.3233/JAD-130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 24.Blacker D. Mild cognitive impairment-no benefit from vitamin E, little from donepezil. N Engl J Med. 2005;352:2439–2441. doi: 10.1056/NEJMe058086. [DOI] [PubMed] [Google Scholar]
- 25.Tayebati SK, Amenta F. Choline-containing phospholipids: relevance to brain functional pathways. Clin Chem Lab Med. 2013;51:513–521. doi: 10.1515/cclm-2012-0559. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery SA, Thal LJ, Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer's disease. Int Clin Psychopharmacol. 2003;18:61–71. doi: 10.1097/00004850-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 27.De Jesus Moreno Moreno M. Cognitive improvement in mild to moderate Alzheimer's dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial. Clin Ther. 2003;25:178–193. doi: 10.1016/s0149-2918(03)90023-3. [DOI] [PubMed] [Google Scholar]
- 28.Di Perri R, Coppola G, Ambrosio LA, Grasso A, Puca FM, Rizzo M. A multicentre trial to evaluate the efficacy and tolerability of alpha-glycerylphosphorylcholine versus cytosine diphosphocholine in patients with vascular dementia. J Int Med Res. 1991;19:330–341. doi: 10.1177/030006059101900406. [DOI] [PubMed] [Google Scholar]
- 29.Amenta F, Di Tullio MA, Tomassoni D. The cholinergic approach for the treatment of vascular dementia: evidence from pre-clinical and clinical studies. Clin Exp Hypertens. 2002;24:697–713. doi: 10.1081/ceh-120015346. [DOI] [PubMed] [Google Scholar]
- 30.Chertkow H. Treating mild cognitive impairment. Can Rev Alzheimers Dis Other Dement. 2006;8:12–22. [Google Scholar]
- 31.Shifren JL, Gass ML. The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21:1038–1062. doi: 10.1097/GME.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 32.Burke D, Hickie I, Breakspear M, Götz J. Possibilities for the prevention and treatment of cognitive impairment and dementia. Br J Psychiatry. 2007;190:371–372. doi: 10.1192/bjp.bp.106.033407. [DOI] [PubMed] [Google Scholar]
- 33.Engelhart MJ, Geerlings MI, Ruitenberg A, Van Swieten JC, Hofman A, Witteman JC, et al. Diet and risk of dementia: Does fat matter?: The Rotterdam Study. Neurology. 2002;59:1915–1921. doi: 10.1212/01.wnl.0000038345.77753.46. [DOI] [PubMed] [Google Scholar]
- 34.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 35.Andrade C, Radhakrishnan R. The prevention and treatment of cognitive decline and dementia: An overview of recent research on experimental treatments. Indian J Psychiatry. 2009;51:12–25. doi: 10.4103/0019-5545.44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory SM, Parker B, Thompson PD. Physical activity, cognitive function, and brain health: what is the role of exercise training in the prevention of dementia? Brain Sci. 2012;2:684–708. doi: 10.3390/brainsci2040684. [DOI] [PMC free article] [PubMed] [Google Scholar]