Summary
Lipid droplets are the intracellular sites for neutral lipid storage. They are critical for lipid metabolism and energy homeostasis, and their dysfunction has been linked to many diseases. Accumulating evidence suggests that the roles lipid droplets play in biology are significantly broader than previously anticipated. Lipid droplets are the source of molecules important in the nucleus: they can sequester transcription factors and chromatin components and generate the lipid ligands for certain nuclear receptors. Lipid droplets have also emerged as important nodes for fatty acid trafficking, both inside the cell and between cells. In immunity, new roles for droplets, not directly linked to lipid metabolism, have been uncovered, as assembly platforms for specific viruses and as reservoirs for proteins that fight intracellular pathogens. Until recently, knowledge about droplets in the nervous system has been minimal, but now there are multiple links between lipid droplets and neurodegeneration: Many candidate genes for Hereditary Spastic Paraplegia also have central roles in lipid-droplet formation and maintenance, and mitochondrial dysfunction in neurons can lead to transient accumulating of lipid droplets in neighboring glial cells, an event that may, in turn, contribute to neuronal damage. As the cell biology and biochemistry of lipid droplets are increasingly well understood, the next few years should yield many new mechanistic insights into these novel functions of lipid droplets.
Introduction
Lipid droplets are the sites where cells store neutral lipids, such as triglycerides, steryl esters, and retinyl esters [1-3]. These stored lipids can then be used in times of need to generate energy, membrane components, and signaling lipids. Impairment of the machinery that makes or degrades lipid droplets has severe physiological consequences [1, 4-6], demonstrating that lipid droplets play central roles in cellular and organismal energy homeostasis, in particular, and overall lipid metabolism in general.
Lipid droplets also allow cells to safely sequester otherwise toxic lipids. For example, as amphipathic molecules, overabundant fatty acids can severely compromise membrane integrity. Once turned into triglycerides and incorporated into lipid droplets (Fig. 1A), they are relatively inert, stable, and harmless. This protective function is probably the reason for the abundant accumulation of lipid droplets in many disease states characterized by aberrant lipid supply and metabolism, such as obesity, atherosclerosis, and fatty liver disease [1, 6, 7].
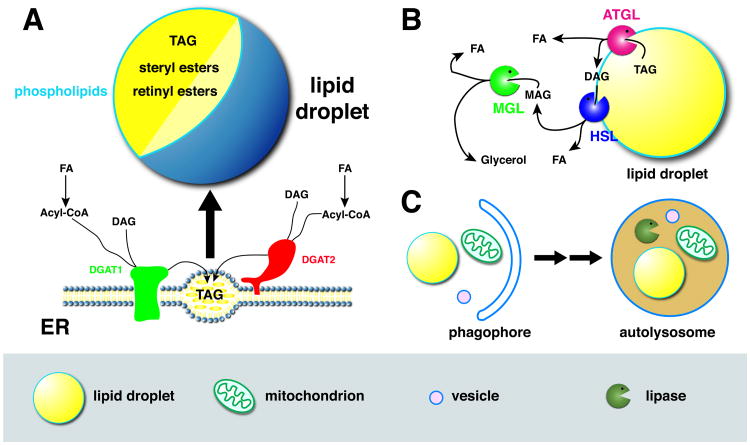
Figure 1. Lipid droplet basics.
A) Top: Lipid droplets have a central core of neutral lipids (triglycerides, steryl esters, retinyl esters) surrounded by a single layer of amphipathic lipids (mostly phospholipids) and proteins. Bottom: Lipid droplets arise from the ER, where two enzymes (DGAT1, DGAT2) synthesize triglycerides (triacylglycerol, TAG) from diacylglycerol (DAG) and acyl-CoA (ultimately derived from fatty acids (FAs)). Triglycerides initially accumulate between the two leaflets of the ER; these nascent droplets eventually give rise to mature lipid droplets, that may become detached from the ER or remain connected by narrow bridges. Accumulation of steryl and retinyl esters also promotes lipid-droplet formation. Like triglycerides, these molecules are produced by ER resident transferases, but for simplicity these enzymes are not included in the cartoon. B) Triglyceride breakdown via cytoplasmic lipases. The triglyceride lipase ATGL is droplet bound and, when activated, hydrolyzes TAG to DAG and FA. DAG is further broken down, via HSL (hormone-sensitive lipase) and MGL (monoglyceride lipase) to MAG (monoacylglycerol), glycerol, and FAs. C) Triglyceride breakdown via autophagy. A double membrane (phagophore) grows around various organelles (left), including lipid droplets, mitochondria, and vesicles, and traps them inside an autophagosome. After fusion of the autophagosome with lysosomes to form autolysosomes, the organelles are broken down by acid hydrolases, including lysosomal acid lipase (LAL).
Lipid droplets are particularly important in tissues specialized for energy storage or lipid turnover, such as adipose tissue, the liver, and the intestine [2, 3, 8]. Yet, they also accumulate in skeletal muscle, the adrenal cortex, macrophages, and mammary glands [1]. They control lipid signaling in immune cells and are the targets of attack by pathogens [9]. Finally, they have been observed in most cell types and occur throughout the animal kingdom, in plants, and unicellular organisms.
Recently, it has become apparent that lipid droplets play even broader cellular roles than previously appreciated. For example, they modulate the availability of proteins and signaling lipids in the nucleus, act as hubs for fatty acid trafficking, are used by viruses as assembly platforms, and their dysfunction in neurons and glia may lead to neurodegeneration. This review summarizes key recent findings into these emerging roles of lipid droplets, to share these exciting developments with researchers beyond the lipid-droplet field. Lipid droplets are still relatively understudied organelles, and given the versatile functions already revealed, it seems likely further roles in new areas of biology will be discovered.
Some basics concepts in lipid-droplet biology
In the last few years, there has been an explosive growth in our understanding of lipid-droplet structure, biogenesis, and turnover, extensively covered in many excellent recent reviews [1, 5, 10-15]. Among cellular organelles, lipid droplets have a unique structure (Fig. 1A): a central core of hydrophobic (neutral) lipids is surrounded by a single layer of amphipathic lipids and proteins (reminiscent of half a membrane). The triglycerides in the hydrophobic core are generated by an elaborate biosynthetic pathway (for a summary, see [10]), whose final step is catalyzed by the acyl-CoA:diacylglycerol acyltransferases DGAT1 and DGAT2 (Fig. 1A), converting DAG (diacylyglycerol) and fatty acids, first activated to acylCoA, into triglycerides. Both enzymes are located in the ER, where triglycerides accumulate at privileged sites that represent nascent lipid droplets [16]; mature lipid droplets are generated by continuous growth of these structures and finally become distinct from the ER, likely a process resembling budding [10, 13]. DGAT2 is only inserted in one leaflet of the ER membrane and can therefore diffuse onto the surface of lipid droplets, promoting triglyceride synthesis and continued droplet growth locally [17]. The hydrophobic core can also contain steryl esters, whose synthesis is catalyzed by acyl-CoA:cholesterol acyltransferases. Depending on cell type and conditions, steryl esters or triglycerides may predominate.
Breakdown of the droplet triglycerides can occur by two distinct pathways. Cytoplasmic triglyceride lipases bound to the surface of lipid droplets hydrolyze triglycerides to DAG and fatty acids. DAG can be further broken down, in two steps, into fatty acids and glycerol (Fig. 1C). In adipose tissue and many other cells, the bulk of triglyceride hydrolysis is catalyzed by a single lipase, adipose triglyceride lipase (ATGL) [5]. Lipid droplets can also be turned over by autophagy (Fig. 1C): like other cellular organelles, lipid droplets are taken up by autophagosomes, which fuse with lysosomes to form autolysosomes. The hydrolytic enzymes delivered from lysosomes then break down the autophagosome content; triglycerides, in particular, are predominantly hydrolyzed by lysosomal acid lipase (LAL) [5]. Discovered in hepatocytes [18], autophagy of lipid droplets (“lipophagy”) appears to make varied contributions to triglyceride breakdown, depending on cell type and physiological conditions [5].
Lipid droplets as modulators of nuclear functions
Lipid droplets arise from the ER and typically reside in the cytoplasm, often at considerable distance from the nucleus. Nevertheless, recent studies have uncovered intimate connections between lipid droplets and nuclear events. There is emerging evidence for a nuclear population of lipid droplets, which have been proposed to directly modulate lipid metabolism in the nucleus. In addition, lipid droplets in the cytoplasm can sequester transcription factors, enzymes, and chromatin components – and possibly many other proteins – and thus control their availability in the nucleus.
Nuclear lipid droplets
Two different groups have reported the presence of lipid droplets inside nuclei [19, 20]. Using neutral-lipid specific dyes, small, spherical structures were identified in the nuclei of cultured cells as well as in biochemically isolated nuclei [20]. Electron microscopy of serial sections revealed that at least some of these structures truly reside inside the nuclear compartment [19]. Biochemical fractionation suggests that these structures differ in their lipid composition from the lipid droplets in the cytoplasm [20]; however, they resemble those lipid droplets in their morphology and in the presence of neutral lipids and were thus named “nuclear lipid droplets”. It is not yet known how these lipid droplets arise, what proteins they associate with, or what their functional significance is. However, it is an intriguing possibility that they contribute to nuclear lipid homeostasis and locally modulate the availability of signaling lipids.
Exchange of proteins between lipid droplets and nuclei
Cytoplasmic lipid droplets can also profoundly affect nuclear events. For example, lipid droplets have been implicated in suppressing the activity of a transcription factor by keeping it out of the nucleus [21]. The lipid droplet protein Fsp27, also known as CIDEC, is expressed in adipocytes and promotes fusion between droplets, causing the formation of a single droplet per cell [22, 23]. A yeast two-hybrid screen revealed the transcription factor NFAT5 (Nuclear factor of activated T cells) as a potential Fsp27 interaction partner. NFAT5 is cytoplasmic under hypotonic conditions and translocates to the nucleus upon hypertonic stress to activate osmoprotective genes [24]. The physical interaction between Fsp27 and NFAT5 was confirmed in vivo, and Fsp27 knockdown in adipocytes lead to expression of NFAT5 target genes even in the absence of hypertonic stress [21]. To examine the underlying mechanism, Fsp27 was ectopically expressed in the heterologous HEK293 cells; under these conditions, Fsp27 was observed broadly throughout the cytoplasm. Fsp27 overexpression reduced the amount of nuclear NFAT5, as determined both by imaging and biochemistry, and blunted the expression of NFAT5 target genes when cells were exposed to hypertonic stress [21]. These results suggest that Fsp27 is able to sequester NFAT5 in the cytoplasm and interferes with its nuclear trafficking; since in adipocytes endogenous Fsp27 is associated with lipid droplets, this interaction would retain NFAT5 at the droplet surface (Fig. 2A), something that remains to be demonstrated directly. It will be interesting to determine if the interaction between Fsp27 and NFAT5 is regulated, e.g., by signaling pathways controlling lipolysis.
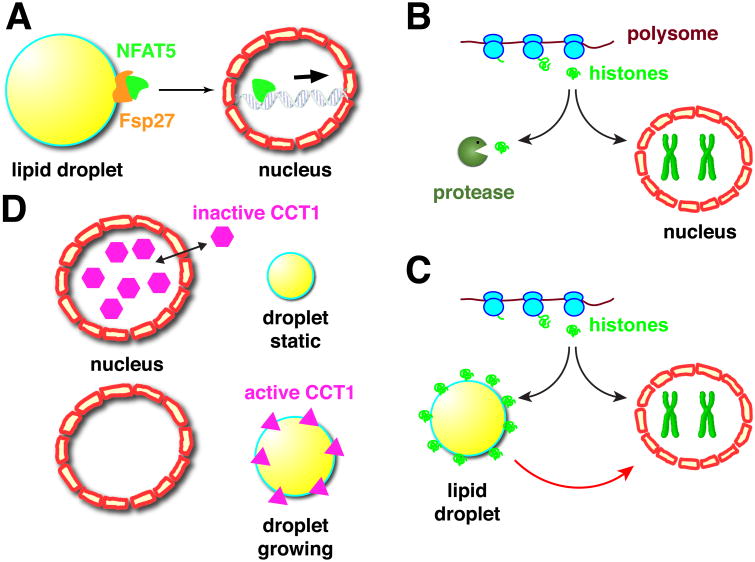
Figure 2. Protein exchange between lipid droplets and the nucleus.
A) The lipid-droplet protein Fsp27 can physically interact with the transcription factor NFAT5. This interaction is proposed to keep NFAT5 out of the nucleus, thus dampening transcriptional activation of NFAT5 target genes. B, C) Modulation of histone metabolism by lipid droplets. Typically, newly translated histones are either incorporated into chromatin in the nucleus or degraded (B). In Drosophila embryos, newly synthesized histones can be sequestered on lipid droplets before important into nuclei, shielding them from degradation (C). This sequestration allows long-term storage of histones in the cytoplasm and also ensures a consistent histone supply despite short-term fluctuations in histone production. D) The phospholipid synthesis enzyme CCT1 is usually present in both the nucleus and in the cytoplasm. These two pools exchange rapidly, with the majority of CCT1 present in the nucleus at any one point (top). When lipid droplets are growing, CCT1 is stably recruited to the droplet surface (bottom). Droplet binding results in a conformational change that activates the enzyme, boosting phospholipid synthesis, and thus allows the droplet surface layer to expand in concert with the growth of the core.
In Drosophila embryos, lipid droplets are associated with large amounts of specific histones [25], via the histone anchor Jabba [26]. This association is first detected during oogenesis and makes it possible for females to build up massive histone stores in the developing eggs (Fig. 2B,C): wild-type embryos contain enough excess histones for thousands of diploid nuclei; mutants lacking Jabba have drastically reduced histone stores [26]; indirect evidence suggests that extranuclear histones not bound to lipid droplets are degraded. Transplantation experiments revealed that in the embryo droplet-bound histones can transfer to nuclei [25] and presumably support chromatin assembly. Surprisingly, embryos lacking this droplet-bound histone supply develop largely normally [26, 27]. This is possible because of the intricate regulation of histone metabolism in early embryos (reviewed in [28]) which also contain abundant levels of histone messages deposited during oogenesis. When new synthesis of histones in the embryo is even mildly impaired, Jabba mutants cannot sustain development and die very early [26]. Thus, in this case, lipid droplets sequester a nuclear protein for long-term storage. This sequestration allows the organism to build up histones stores during oogenesis and keep them available when needed later for chromatin assembly (Fig. 2 C).
Lipid droplets of early Drosophila embryos also appear to affect histone metabolism in the short term, by buffering the histone supply [27]. When droplets are transplanted between embryos, the donor droplets can bind histones from the recipient embryo, suggesting that histones can be loaded onto droplets even in embryos. In Jabba mutants, the synthesis of histone H2A and its variant H2Av are imbalanced, and H2Av overaccumulates in the nuclei, an event linked to DNA damage [27]. This nuclear overaccumulation does not occur in wild-type embryos, presumably because here lipid droplets can trap histones produced in excess and prevent their unregulated entry into nuclei. Whether other species use similar droplet-based histone buffering remains to be determined, though histones have been detected on lipid droplets in housefly embryos, rat sebocytes, and mouse oocytes [25, 29, 30]
The enzyme CCT1 also displays dramatic exchange between lipid droplets and nuclei. CCT1 is one of two isoforms of CTP:phosphocholine cytidylyltransferase, an enzyme that catalyzes the rate-limiting step in the synthesis of the phospholipid phosphatidylcholine. In cultured fly cells, CCT1 is usually present in the nucleus, but under conditions in which cells synthesize new triglycerides and expand the hydrophobic core of droplets, CCT1 accumulates at the droplet surface [31, 32] (Fig. 2D). The presence at the droplet surface is critical to expand the droplet surface in concert with growth of the core: droplet binding activates the enzyme and thus leads to an increase in the cellular phosphatidylcholine supply. Whether CCT1's presence in the nucleus in the basal state is functionally important remains unclear. Nuclear accumulation is apparently not a mechanism to prevent access to the droplet surface: FRAP experiments revealed, CCT1 is not immobilized inside nuclei, but rapidly exchanges with a cytoplasmic pool. And overexpression of CCT2, an isoform exchanging between the cytoplasm and droplets, can fully rescue the effect of CCT1 depletion on droplet growth [31]. High nuclear accumulation and consequent low cytoplasmic pools of CCT1 might possibly modulate the kinetics of relocalization to droplets.
Prp19 is a subunit of the NineTeen Complex involved in a number of nuclear events, including spliceosome activation and transcription elongation [33]. In mouse adipocytes, it was also found associated with lipid droplets, and Prp19 knockdown resulted in reduced triglyceride accumulation [34]. It is not clear, if this dual localization to lipid droplets and to the nucleus represents unrelated functions of Prp19, or if the two populations are connected. Initial experiments with inhibitors of nuclear export revealed no changes in overall intracellular Prp19 distribution [34].
Modulation of lipid signaling
The PPARs (peroxisome proliferator-activated receptors) are transcription factors bound and activated by lipid ligands, including fatty acids and their derivatives. In oxidative tissues, such as the mammalian heart and liver, PPAR-α promotes the expression of proteins involved in lipid homeostasis [35]. In principle, fatty acids from exogenous sources or synthesized de novo might activate PPAR-α directly. However, free fatty acids are typically channeled, via activation to acyl-CoA, into specific metabolic pathways [36] and thus are not readily available for signaling. Studies in mice uncovered that PPAR-α signaling is severely compromised in the hearts of animals lacking ATGL [37]. As lack of ATGL function in the heart causes many profound changes (e.g., massive lipid accumulation and mitochondrial dysfunction), the effect on PPAR-α signaling might conceivably be indirect. Yet pharmacological stimulation of the PPAR-α pathway is sufficient to reverse these phenotypes, establishing signaling as a primary defect in these mutant hearts and suggesting a direct link between lipid droplets and PPAR-α activation [37]. It was proposed that ATGL-mediated triglyceride hydrolysis generates the ligands for PPAR-α [37] (Fig. 3A). This pathway may be tissue-specific as liver-specific knockdown of ATGL impaired the expression of PPAR-α target genes in this tissue, but PPAR-α agonists failed to reverse this effect [38].
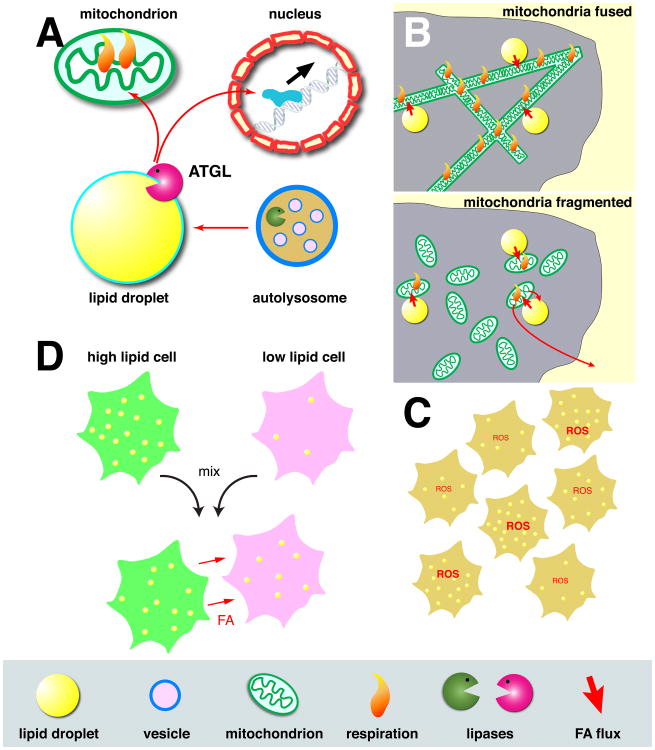
Figure 3.
Lipid droplets as hubs for fatty acid trafficking.
A) During starvation, fatty acids from phospholipid breakdown in autolysosomes are routed to lipid droplets, building up triglyceride stores there. This build-up balances the loss of fatty acids, generated by ATGL, to mitochondria, where they are burned as fuel. Fatty acids generated at lipid droplets by ATGL can also (perhaps directly, perhaps after derivatization) activate the PPAR-α transcription factor in the nucleus. B) Mitochondrial fusion state controls usage of lipid-droplet derived fatty acids. In starved cells, mitochondria are usually highly fused (top). The mitochondrial network thus created allows fatty acids from lipid droplets to be evenly distributed throughout mitochondria, promoting equal mitochondrial respiration. When fusion is prevented (bottom), mitochondria are fragmented, and efficient uptake of fatty acids and their metabolic breakdown occurs only in the mitochondria directly associated with lipid droplets. Unmetabolized fatty acids are re-exported, either into lipid droplets or into the extracellular space. C) Even in clonal populations of cells, there is great heterogeneity in the numbers of lipid droplets per cell. Cells with high lipid storage also have higher levels of reactive oxygen species (ROS), presumably the result of more active fatty acid metabolism. D). When cells with high and low lipid-droplet content are cultured together, fatty acids can be transferred from the high- to the low-lipid cells.
Lipid droplets as hubs for fatty acid trafficking
Lipid droplets act as a sink for overabundant fatty acids, and they can release lipids when needed for energy production, synthesis of membrane components, or signaling. It is becoming increasingly apparent that lipid trafficking to and from droplets is highly regulated in space (Fig. 3A). Fatty acids from triglyceride hydrolysis signal to nuclear receptors (above); fatty acids released during autophagy are shuttled through lipid droplets, as a way station before import into mitochondria for ATP production; production of steroid hormones in flies requires lipid exchange between the ER, lipid droplets, and mitochondria; and within a population of cells, high accumulation of droplets in a subset of cells has been proposed to protect the rest of the cells from fatty acid overload.
Lipid trafficking between lipid droplets and mitochondria
In starved mammalian cells, fatty acids fuel ATP production, via β oxidation in mitochondria. These fatty acids could derive from triglycerides (Fig. 1B,C) or from various membranous organelles. To follow the flux of fatty acids through various compartments, mouse embryonic fibroblasts (MEFs) were allowed to incorporate fluorescently labeled lipids into lipid droplets and into membranes, respectively, and their fate during starvation was monitored by imaging and biochemistry [39]. Fatty acids present as triglycerides in lipid droplets moved to mitochondria fairly quickly and were readily broken down. When ATGL (Fig. 1B) was knocked down, transfer of fatty acids was dramatically reduced and mitochondrial oxygen consumption rates dropped. Under the starvation conditions employed, lipophagy (Fig. 1C) was not induced and autophagy made no detectable contribution to transfer of fatty acids from droplets to mitochondria or to mitochondrial oxygen consumption rates.
The rapid relocalization of fatty acids to mitochondria is presumably accomplished by direct transfer. Lipid droplets and mitochondria indeed display close physical associations [40-42], and direct channeling of fatty acids from their site of release (droplets) to the site of consumption (mitochondria) would minimize the risk of toxic effects elsewhere, such as disruption of cellular membranes or inappropriate nuclear signaling.
Curiously, during starvation, the number and size of lipid droplets increased and total cellular triglyceride levels went up [39]. Using fluorescently labeled phospholipids and inhibition of autophagy pathways, this effect was traced to autophagic breakdown of membranous organelles. Presumably, fatty acids from phospholipid breakdown in autolysosomes are employed to replenish triglyceride stores in droplets upon starvation (Fig. 3A).
Mitochondria can also be remodeled by fusion and fission [43], allowing them to form highly interconnected networks or individual fragments. In starved cells, mitochondria were highly fused, a state that is apparently critical for efficient fatty acid import: usually labeled fatty acids from lipid droplets are homogenously distributed throughout the mitochondria, but when mitochondria were fragmented, the label was distributed unevenly. As a result, fatty acid could not be metabolized as efficiently; although cells with either fused or fragmented mitochondria upregulate β oxidation upon starvation, only those with fused mitochondria were able to maintain these levels. For cells with fragmented mitochondria, levels of β oxidation and, as a result, total mitochondrial respiration, dropped off with time, presumably because not all mitochondria had sufficient supply of fatty acids (Fig. 3B). The likely reason is that there are much fewer lipid droplets than mitochondrial fragments, and that only the mitochondria in direct physical contact with lipid droplets can take up fatty acids efficiently. In fused mitochondria, those fatty acids can diffuse through the entire network. In support of this interpretation, when glutamine was used as an alternative fuel, the fusion state of the mitochondria did not matter; as glutamine diffuses through the cytoplasm, its import into mitochondria is not restricted to a limited number of sites, unlike the supply of fatty acids from lipid droplets. Presumably since oversupply of unmetabolized fatty acids is dangerous, fatty acids were re-exported from the mitochondria, and either accumulated back in lipid droplets or were released from the cells into the extracellular space [39].
Efficiency of lipid exchange between mitochondria, ER, and lipid droplets may also underlie a recent observation that a promoter of mitochondrial fusion is important for lipid-droplet formation and steroid signaling in Drosophila. Marf (Mitochondrial associated regulatory factor, the fly ortholog of mammalian mitofusins) is a small GTPase that promotes fusion of the outer mitochondrial membrane; thus, loss of Marf leads to small, round mitochondria [44]. There is a particular dramatic phenotype in the ring gland, an endocrine tissue responsible for hormone secretion [45]: Mitochondrial morphology is altered, the ER is fragmented, and lipid-droplet number is dramatically reduced [44]. Ring gland lipid droplets receive sterols from the ER and store them as steryl esters; these, in turn, are the precursors for the production of the steroid hormone ecdysone, in the mitochondrial matrix. Efficient storage and turnover of steryl esters therefore presumably requires intimate contacts between the three organelles, and in Marf mutants the contacts between all three organelles were severely reduced [44]. Lack of Marf in the ring gland also greatly impairs ecdysone production, with dramatic organism-wide consequences [44].
Mitochondria may not be the only instance where close contacts with lipid droplets promote efficient transfer of fatty acids. Breakdown of fatty acids is not restricted to mitochondria, but can also occur in peroxisomes. In the yeast Saccharomyces cerevisiae, β oxidation is even entirely restricted to peroxisomes. Here, lipid droplets and peroxisomes display intimate physical connections, which have been proposed to promote efficient coupling of triglyceride breakdown with peroxisomal fatty oxidation [46].
Lipid droplet specialization across a cell population
The role of lipid droplets as buffers for fatty acid availability may even extend to lipid exchange between cells in the same tissue. A recent study identified a surprising heterogeneity in lipid-droplet content in hepatocytes [47]: in mouse liver, some cells have substantially larger numbers of lipid droplets than neighboring cells (Fig. 3C); this variability is especially apparent under conditions of high overall lipid storage in the liver. Similar variability was observed in primary hepatocytes in culture and with a cultured cell line of liver origin (AML12), suggesting that it is due to cell intrinsic properties, rather than a reflection of overall tissue structure.
Such heterogeneity might arise because some cells have acquired mutations in lipid metabolism. However, a cell sorting strategy demonstrated that heterogeneity is reversible and appears to be a population property. After growth on fatty acid rich media, cells were separated by flow cytometry into a low-lipid and a high-lipid subpopulation. After culture in standard media to promote breakdown of the stored lipids, the two populations were again grown under fatty-acid–rich conditions. Remarkably, both cultures showed the same broad distribution in lipid content. Inhibitor studies indicate that heterogeneity arises from fluctuations in biochemical networks controlling lipolysis, fatty acid oxidation, and protein synthesis.
At the level of a whole organism, heterogeneity of lipid droplet content is very common. Many animals have adipose tissues specialized for storing lipids. It was proposed that heterogeneity within a single cell population similarly sets aside a subpopulation of cells that collects lipids particularly well, stores them, and releases them to their neighbors when needed [47]. To test this idea, high-lipid cells were isolated in which lipid droplets had accumulated fluorescently labeled fatty acids. After co-culture with low lipid cells (marked with a different fluorescent dye to distinguish the two original populations), the high-lipid group had lost – and the low-lipid group had gained - some of the labeled fatty acids. Thus, the high-lipid cells can indeed supply lipids to their neighbors (Fig. 3D).
But what is the point of setting aside a subpopulation of cells with especially high lipid stores, if – in the long run – the lipids are presumably needed equally across cells? One possibility has to do with the fact that overaccumulation of free fatty acids is dangerous, both because of disruption of membranes and because of toxic metabolites generated by fatty acid breakdown. The high-lipid subpopulation indeed showed higher levels of oxidative damage, as seen by levels of reactive oxygen species (ROS) (Fig. 3C). Importantly, when fluorescently marked low-lipid cells were co-cultured with either low-lipid or high-lipid cells (unmarked) and challenged with fatty-acid–rich media, the marked cells displayed lower ROS levels in the presence of high-lipid cells. Thus, the presence of high-lipid cells protected their low-lipid neighbors. High-lipid cells may remove fatty acids more efficiently from the media, and thus the flux of free fatty acids into the low-lipid cells is reduced. Remarkably, in the co-culture experiment with high-lipid cells, ROS levels were not only reduced for the marked low-lipid cells, but also for the population as a whole.
Although the detailed mechanisms underlying these protective effects remain to be worked out, the reported experiments nicely demonstrate a novel strategy, namely heterogeneity in lipid droplet accumulation, to alleviate risks from overabundance of lipids. By accumulating more lipid droplets and more ROS, the high-lipid subpopulation reduces the overall risk of lipotoxicity. It is not yet clear how the high-lipid population is able to handle its increased risk: these cells may induce specific protective mechanisms, or they might repair their damage during the time when they find themselves in the low-lipid state (which will occur sooner or later, due to the stochastic nature of the heterogeneity). Since heterogeneity has been observed in cultured cells of various origins [47], this protective strategy may be employed not just by hepatocytes, but generally.
Lipid droplets and the fight against pathogens
It has been long known that lipid droplets play important roles in the immune system. They are sites of synthesis of eicosanoids, signaling lipids important for inflammation, host defense against pathogens, and cancer [48]. Various pathogens have, in turn, evolved strategies to tap into the lipid droplets of the host to ensure a sufficient lipid supply [49, 50]. Recent years have uncovered how certain viruses appropriate lipid droplets as assembly platforms and how cells use lipid droplets in novel ways to fight back.
Lipid droplets and viral assembly
Infection with Hepatitis C virus (HCV) is a global public health threat and can lead to liver cirrhosis and liver cancer [51]. For part of its life cycle, HCV crucially depends on lipid droplets (see [52, 53] for recent reviews): After infection, two newly expressed viral proteins transiently accumulate on lipid droplets: core, the major structural protein of the virus, and NS5A, a regulator of viral replication. Droplet-bound viral proteins then interact with the sites of viral RNA replication, a process facilitated by the droplet-localized Rab 18 [54] and by dynein-mediated intracellular relocalization of lipid droplets [55]. For the final maturation, the virus hijacks the pathway responsible for secretion of the VLDL lipoprotein particles, and virions are released into the extracellular space as low-density lipoviroparticles [56], whose lipids may ultimately derive from lipid droplets. HCV is not unique in its use of lipid droplets; several other viruses assemble with the help of lipid droplets [57, 58].
Droplet accumulation is a necessary step for virus maturation; if the interaction between core and lipid droplets is disrupted, either with mutations in core or pharmacologically, core protein stability is greatly reduced and virion assembly is impaired [59, 60]. Thus, preventing the recruitment of viral proteins to droplets is an attractive target for disrupting the viral life cycle. Droplet targeting requires cis-acting sequence motifs in both core and NS5A [61, 62], but also trans-acting host factors. In particular, DGAT1, one of the two enzymes mediating triglyceride synthesis (Fig. 1A), plays a crucial role: DGAT1 binds to both NS5A and core, and this interaction mediates recruitment to droplets and is required for efficient virion assembly [63, 64]. Interestingly, inhibiting the enzymatic activity of DGAT1 is sufficient to prevent droplet targeting of these proteins. Since DGAT1 generates only a subset of lipid droplets (the others depend on DGAT2; Fig. 1A), it was proposed that DGAT1 concentrates the viral proteins at the sites where it promotes lipid-droplet formation and thus guides them onto just these lipid droplets [64] (Fig. 4A).
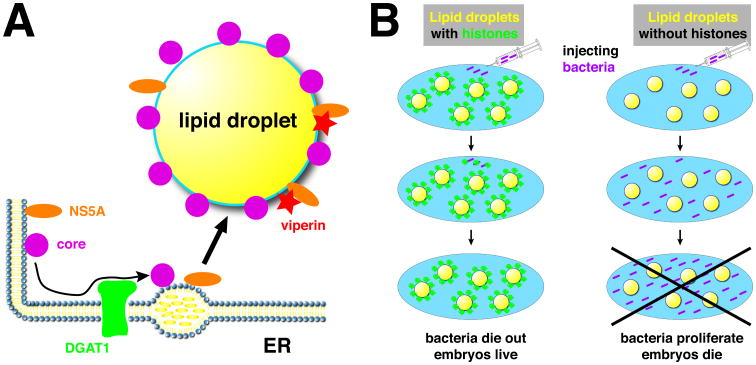
Figure 4.
Novel interactions between lipid droplets and pathogens.
A) During Hepatitis C virus replication, newly synthesized core and NS5A proteins are (after processing) attached to the ER. Via physical interactions with DGAT1, they are guided to nascent lipid droplets and thus accumulate on the lipid-droplet surface. This accumulation is a necessary step for later virion assembly. The anti-viral protein viperin also accumulates on lipid droplets, where it physically interacts with NS5A. This interaction suppresses NS5A's function in viral replication. B). When bacteria are injected into the cytoplasm of wild-type Drosophila embryos (left), they are killed via lipid-droplet associated histones, allowing embryo survival. After injection into mutants lacking droplet-bound histones (right), the bacteria proliferate and kill the host.
Lipid droplets as stores for antiviral and antibacterial proteins
Viperin (Virus inhibitory protein, ER associated, interferon inducible) is an interferon-induced protein with broad anti-viral activity [65, 66]. Viperin is targeted to the cytoplasmic face of the ER and is also enriched around lipid droplets [62]; targeting to both locations is mediated by an N-terminal amphipathic alpha helix [62]. Intriguingly, two of the viruses combatted by viperin, HCV and Dengue virus, employ droplets for their assembly. Using confocal microscopy and fluorescence resonance energy transfer (FRET), viperin was shown to interact with the HCV nonstructural protein NS5A at the droplet surface, via its C-terminal region [67]. This interaction as well as the N-terminal droplet-targeting helix are required for viperin's antiviral activity against HCV [67]. For Dengue virus, in contrast, while a physical interaction with the viral protein NS3 was important, droplet binding was dispensable for the anti-viral effect [68]. The N-terminal alpha helix in viperin was also important to restrict the replication of chikungunya virus, though presumably through localization at the ER, rather than lipid droplets [69]. Thus, at least in some cases, viperin apparently targets a droplet-dependent step of viral replication and its enrichment on the droplet surface is necessary for its activity (Fig. 4A).
As discussed earlier, lipid droplets can be associated with histones. This observation potentially has implications for immunity since histones are increasingly recognized as antibacterial agents [70, 71]: In vitro, histones have broad anti-bacterial activity [72], and histones present in extracellular secretions have been reported to contribute to protection against bacterial pathogens [73, 74]. Analysis in Drosophila suggests that histones bound to lipid droplets can similarly provide a defense against intracellular bacterial invaders [75]. Droplets biochemically purified from Drosophila embryos are associated with high levels of certain histones [25] and are highly bactericidal in vitro [75]. A number of independent approaches, including using histone antibodies and mutations in the histone anchor, Jabba, [26], showed that killing activity was due to histones.
To test if droplet-bound histones are protective in vivo, wild-type and Jabba mutant embryos were injected with GFP-labeled Escherichia coli; while bacterial numbers diminished in the wild type, they dramatically increased in the mutants [75] (Fig. 4B). In the same injection assay, wild-type embryos also showed significantly higher levels of survival when challenged with a number of gram-positive and gram-negative bacteria. This new immune mechanism may also operate at other developmental stages: when adult flies were infected with the intracellular pathogen Listeria monocytogenes, Jabba mutants were impaired in restricting bacterial titers and were killed much more readily [75]. Loading up lipid droplets with histones to kill bacterial invaders may be a conserved innate immunity mechanism since when mice were challenged with lipopolysaccharide – to mimic bacterial infections – the levels of droplet-bound histone H1 increased in the liver [75].
Lipid droplets and the nervous system
Lipid metabolism plays crucial roles in the nervous system, for many membrane functions and signaling events [76-78]. Yet until recently, there has been only sparse and unconnected information on the role of lipid droplets in neurons and other cells of the nervous system. For example, lipid droplets have been detected in the axons of Aplysia neurons cultured in vitro [79] and in cultured neurons and brain sections of Huntington Disease models [80]. There are also links between α synuclein, a protein whose dysfunction or overexpression can cause Parkinson's disease, and lipid droplets: α synuclein has been reported to bind to lipid droplets in vitro [81] and in cultured cells [82], and overexpression in yeast promotes droplet accumulation [83]; the relevance of these observations for α synuclein's in vivo function and for neurodegeneration are not yet explored. However, recent papers have identified the presence of lipid droplets in neurons and in glia under certain disease conditions and suggest that disrupted lipid-droplet function can contribute to neurodegeneration.
Hereditary spastic paraplegias and lipid droplets
Hereditary spastic paraplegias (HSPs) are inherited disorders characterized by motor-sensory axon degeneration, weakness in lower extremities, and spasticity [84]. Mutations in over 50 loci can cause HSP, and the cellular functions of the encoded proteins show a surprising heterogeneity. Recently, a number of HSP candidate genes have been shown to have crucial roles in lipid-droplet biology: atlastin, REEP1, seipin, spartin, and kinesin-1. Atlastin mediates fusion of ER tubules and also controls the size of lipid droplets [85]. REEP1 maintains the high curvature of ER tubules, and when overexpressed together with Atlastin increases lipid-droplet size [85, 86]. Seipin, an integral membrane protein at the ER-droplet junction, is important for lipid droplet formation and maintenance [87, 88]. Spartin localizes to lipid droplets, interacts with E3 ubiquitin ligases, and modulates the turnover of lipid-droplet proteins [89-91]; spartin knockout mice have increased lipid-droplet numbers in their adipose tissue [92]. KIF5A encodes the microtubule motor kinesin-1; the same motor powers the motion of lipid droplets in Drosophila [93]. Finally, several of the HSP candidate genes encode enzymes implicated in phospholipid or fatty acid metabolism [94, 95]; their dysfunction might therefore alter the supply or composition of the lipids stored in lipid droplets. These observations raise the intriguing possibility that aberrant lipid-droplet biogenesis or function might contribute to axonal degeneration. However, since all of these proteins also have functions unrelated to lipid droplets (e.g., controlling ER structure or promoting vesicle trafficking), the link between lipid droplets and HSPs remains tentative.
A much more direct connection to lipid droplets has recently emerged from the analysis of the HSP gene DDHD2 [96]. Patients with mutations in DDHD2 exhibit very early onset of the disease (<2 years) and are often intellectually disabled [97]. The DDHD2 gene is highly expressed in the brain and encodes a serine hydrolase that in vitro displays phospholipase activity. To determine its function in vivo, DDHD2 activity was abrogated genetically, using knockout mice, as well as pharmacologically, with selective inhibitors [96]. In both cases, adults accumulated large amounts of triglycerides in the brain and the spinal cord, but there was little to no effect in other tissues; brain phospholipid content was unchanged. These observations suggest that DDHD2 has a specific function in triglyceride metabolism of the central nervous system. It likely acts as a triglyceride lipase since recombinant DDHD2 expressed in cultured cells displays triglyceride hydrolase activity and, compared to the wild type, total triglyceride hydrolase activity is significantly reduced in brain lysates of mutant mice [96]. Finally, the main triglyceride hydrolase in the fat body of the moth Manduca sexta shares extensive sequence homology with DDHD2 [98].
The brains of knockout mice displayed abundant lipid droplets, while lipid droplets were only rarely detected in wild-type brains [96]. They accumulated predominantly in neurons and were present in cytoplasm, dendrites and axons. The DDHD2 knockout mice also exhibited deficits in motor coordination and cognition [96], reminiscent of the defects in the human patients [97]. Intriguingly, in the patients, cerebral magnetic resonance spectroscopy revealed an abnormal spectrum, with a peak characteristic of lipid accumulation [97], though it is not yet known whether this peak represents triglycerides. While the mechanisms that link droplet accumulation and neuronal impairment remain obscure, one intriguing observation is that in the DDHD2 knockout mice some of the large droplets observed were associated with noticeable swellings of the neuronal processes and thus might present obstacles to intracellular trafficking in the relatively thin axons and dendrites.
Glial lipid droplets
Glial cells are non-neuronal cells that surround neurons and play important supportive roles in the central and peripheral nervous system. Lipid droplets have been observed in culture in primary glia as well in glia-derived cell lines [99, 100]. When CPT2, a mitochondrial enzyme necessary for β oxidation of long-chain fatty acids, is abolished in flies, massive amounts of triglycerides accumulate specifically in the brain of adults; glial cells, but not neurons, accumulated abundant lipid droplets [101]. This seems to be a cell-autonomous effect because CPT2 is expressed predominantly in glia and CPT2 expression solely in glia is sufficient to reverse triglyceride accumulation in the brain. Flies lacking CPT2 have a dramatically reduced lifespan, and glial specific CPT2 expression was able to partially rescue this defect, indicating that triglyceride metabolism in glia may make an important contribution to overall organismal energy metabolism.
Lipid droplets can also accumulate in glia non-cell-autonomously, in response to mitochondrial dysfunction in neighboring neurons [102]. For a subset of Drosophila mutants known to cause neurodegeneration in adult photoreceptors [103], abundant lipid droplets transiently accumulate in the glial cells next to photoreceptors, prior to or concomitant with the onset of neurodegeneration. No droplets were observed in the wild type or in the neurons of mutant animals [102] (Fig. 5A, B). The mutants that exhibited this phenotype all affect mitochondrial function and in particular cause increased levels of reactive oxygen species (ROS). Elevated ROS is indeed critical for droplet formation in glia as pharmacological or genetic reduction of ROS prevented droplet accumulation. Glial lipid droplets were also detected in a mouse model of neurodegeneration due to mitochondrial dysfunction, suggesting an evolutionarily conserved pathway.
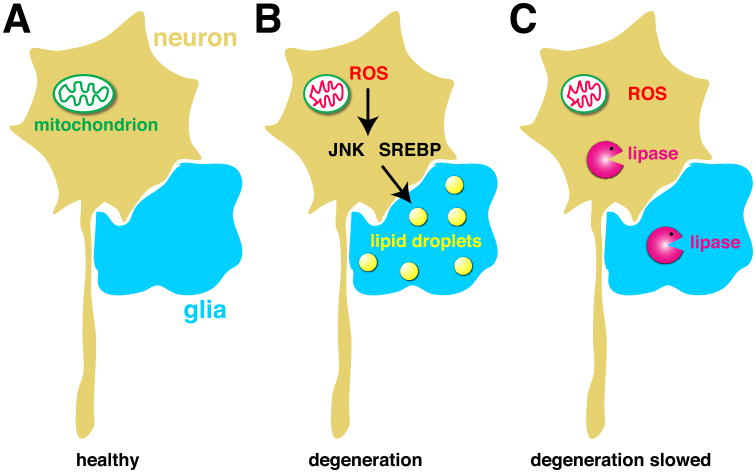
Figure 5. A link between lipid droplets and neurodegeneration.
A) In healthy neurons and glia, few, if any, lipid droplets are present. B) High levels of ROS in neurons, due to mitochondrial dysfunction, leads to neurodegeneration and to accumulation of lipid droplets in glia. Droplet accumulation requires the JNK and SREBP pathways in neurons. C) Overexpression of triglyceride lipases in neurons or glia or both abolishes glial lipid droplets and delays neurodegeneration, indicating a mechanistic role for lipid metabolism and glial lipid droplets.
How do ROS promote glial lipid droplets? The full pathway has yet to be worked out, but activation of c-Jun-N-terminal Kinase (JNK) and Sterol Regulatory Element Binging Protein (SREBP) pathways are critical; JNK mediates stress responses [104] and SREBP controls transcription of many metabolic genes and, in particular, promotes lipogenesis [105, 106]. Although droplets accumulate in glia, the trigger originates in neurons: when the mitochondrial genes identified were knocked down in glia, there was no effect; knockdown only in neurons was sufficient to promote glial lipid droplets. In addition, expression of an anti-oxidant enzyme or knockdown of JNK solely in neurons was able to reduce glial droplet accumulation. Thus, mitochondrial dysfunction and elevated ROS in photoreceptors causes accumulation of lipid droplets in glia in a non cell-autonomous manner.
Thus, damage to neurons due to mitochondrial dysfunction leads both to transient formation of lipid droplets in glia and to neurodegeneration. Are these lipid droplets an ultimately futile protective response, do they promote neurodegeneration, or are they innocent bystanders? Activation of JNK or SREBP in neurons in the absence of ROS still leads to glial lipid droplets, but not neurodegeneration. Thus, glial lipid droplets per se are not detrimental for neurons. The culprit might be lipids damaged by ROS as the mutants leading to neurodegeneration displayed dramatically elevated levels of peroxidated lipids. Furthermore, expression of two different lipases, the ATGL homolog Brummer or the LAL homolog Lip4 (Fig. 1 B, C), dramatically reduced both lipid droplet accumulation and the levels of peroxidated lipids and also delayed neurodegeneration (Fig. 5C). These observations strongly suggest that neurodegeneration is driven by altered lipid metabolism, though the exact role of lipid droplets remains to be elucidated.
The fatal neurodegenerative disease Amyotrophic Lateral Sclerosis (ALS) has recently also been linked to lipid droplets. A particular subtype of ALS is caused by mutations in the human VAMP-associated protein B. When equivalent mutations are introduced into the fly ortholog DVAP, expression of the mutant protein causes degeneration of fly photoreceptors. In genetic screens for enhancers and suppressors of this phenotype, one of the most represented functional categories was proteins linked to lipid droplets, including proteins involved in droplet biogenesis and droplet motility [107]. The proteins such identified will provide a rich source for follow-up studies to dissect how lipid droplets might impact neurodegeneration.
Perspective
The crucial roles of lipid droplets in energy homeostasis and lipid metabolism have focused a lot of recent attention on these still relatively understudied organelles. Yet the examples discussed above show that lipid droplets play even broader roles and touch biological processes only loosely connected to their traditionally studied functions.
In particular, lipid droplets contribute to protein trafficking and protein maturation in the cell. They exchange proteins with the nucleus, modulate protein stability, and allow concentrated accumulation of anti-viral and anti-bacterial proteins. We do not know enough to judge if these processes are independently evolved and all just happen to take advantage of lipid droplets or whether they are indicative of a general cellular pathway of protein trafficking. Lipid droplets have been proposed to act as general protein sequestration sites [107]; such sequestration might modulate the ability of these proteins to interact with binding partners, promote assembly of protein complexes, store damaged proteins safely before degradation, or allow moving droplets to deliver proteins [107, 108]. As many published lipid-droplet proteomes contain proteins from other compartments, there are ample candidates for testing how widespread protein sequestration on droplets is. For the verified examples, much work needs to be done to understand how the sequestered proteins are targeted to lipid droplets, whether they are bound stably or dynamically, and how release from droplets is controlled. And why are these proteins sequestered on lipid droplets and not elsewhere in the cell? Is droplet localization, say, of histones, just an accident of evolution, or do lipid droplets provide a unique cellular niche?
The emerging roles of lipid droplets as hubs for fatty acid trafficking (Fig. 3) suggest that the pathways fatty acids take from and to lipid droplets are highly regulated. But apart from some insights into the importance of direct contacts between lipid droplets and mitochondria [42], little is known about the molecular mechanisms controlling this trafficking. For fatty acid trafficking modulated by droplet heterogeneity between cells (Fig. 3C,D), there are intriguing hints that heterogeneity is a regulatable property since the extent of heterogeneity is different between cells of different origin [47], but the control pathways remain to be worked out.
For lipid droplets in the nervous system, it is now established that both neurons and glia can accumulate lipid droplets under certain disease conditions. But what role they play under these conditions and whether droplets are normally present in the nervous system is far from clear. For example, in the fly models of neurodegeneration (Fig. 5), it was proposed that accumulation of lipid droplets in glia promotes neurodegeneration, as long as high ROS levels provide a second insult [102]. However, lipase overexpression in glia only mildly delayed neurodegeneration, while lipase overexpression in neurons, where no droplets were detected, had a much stronger protective effect. It will be very interesting, in these examples and in the mouse models of HSP, to examine if ablating droplet biogenesis in specific cell types modulates the disease phenotypes, for better or for worse, and how these effects compare to disruption or upregulation of turnover pathways (Fig. 1B, C). Real-time imaging of the trafficking of labeled fatty acids (like in [39]) and characterization of the lipidomes and proteomes of these droplets will provide complementary information to characterize exactly how lipid metabolism is derailed in the disease conditions.
Given the diverse novel roles proposed for lipid droplets, droplets should be on the radar screen of many a biologist trying to uncover the mechanistic basis of an ill-characterized process. With the recent insights into biogenesis and turnover of lipid droplets [14], one can now systematically determine how a process if affected if droplets are entirely absent, are structurally abnormal, or cannot be degraded. Because lipid droplets are ubiquitous organelles, but have been carefully studied in only a few cell types, it seems likely that, as our understanding of these unique and dynamic organelles deepens, their cellular and physiological roles will keep expanding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross DA, Silver DL. Cytosolic lipid droplets: from mechanisms of fat storage to disease. Crit Rev Biochem Mol Biol. 2014;49:304–326. doi: 10.3109/10409238.2014.931337. [DOI] [PubMed] [Google Scholar]
- 9.Saka HA, Valdivia R. Emerging roles for lipid droplets in immunity and host-pathogen interactions. Annu Rev Cell Dev Biol. 2012;28:411–437. doi: 10.1146/annurev-cellbio-092910-153958. [DOI] [PubMed] [Google Scholar]
- 10.Pol A, Gross SP, Parton RG. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J Cell Biol. 2014;204:635–646. doi: 10.1083/jcb.201311051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Cordes KR, Farese RV, Jr, Walther TC. Lipid droplets at a glance. J Cell Sci. 2009;122:749–752. doi: 10.1242/jcs.037630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiam AR, Farese RV, Jr, Walther TC. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol. 2013;14:775–786. doi: 10.1038/nrm3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilfling F, Haas JT, Walther TC, Farese RV., Jr Lipid droplet biogenesis. Curr Opin Cell Biol. 2014;29:39–45. doi: 10.1016/j.ceb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashemi HF, Goodman JM. The life cycle of lipid droplets. Curr Opin Cell Biol. 2015;33C:119–124. doi: 10.1016/j.ceb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassan A, Herms A, Fernandez-Vidal A, Bosch M, Schieber NL, Reddy BJ, Fajardo A, Gelabert-Baldrich M, Tebar F, Enrich C, et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J Cell Biol. 2013;203:985–1001. doi: 10.1083/jcb.201305142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 2013;24:384–399. doi: 10.1016/j.devcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uzbekov R, Roingeard P. Nuclear lipid droplets identified by electron microscopy of serial sections. BMC Res Notes. 2013;6:386. doi: 10.1186/1756-0500-6-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Layerenza JP, Gonzalez P, Garcia de Bravo MM, Polo MP, Sisti MS, Ves-Losada A. Nuclear lipid droplets: a novel nuclear domain. Biochim Biophys Acta. 2013;1831:327–340. doi: 10.1016/j.bbalip.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Ueno M, Shen WJ, Patel S, Greenberg AS, Azhar S, Kraemer FB. Fat-specific protein 27 modulates nuclear factor of activated T cells 5 and the cellular response to stress. J Lipid Res. 2013;54:734–743. doi: 10.1194/jlr.M033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol. 2011;195:953–963. doi: 10.1083/jcb.201104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jambunathan S, Yin J, Khan W, Tamori Y, Puri V. FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One. 2011;6:e28614. doi: 10.1371/journal.pone.0028614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aramburu J, Drews-Elger K, Estrada-Gelonch A, Minguillon J, Morancho B, Santiago V, Lopez-Rodriguez C. Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem Pharmacol. 2006;72:1597–1604. doi: 10.1016/j.bcp.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Cermelli S, Guo Y, Gross SP, Welte MA. The lipid droplet proteome reveals that droplets are a protein storage depot. Curr Biol. 2006;16 doi: 10.1016/j.cub.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Thiel K, Thul PJ, Beller M, Kühnlein RP, Welte MA. Lipid droplets control the maternal histone supply of Drosophila embryos. Curr Biol. 2012;22:2104–2113. doi: 10.1016/j.cub.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Johnson MR, Ke Z, Chen L, Welte MA. Drosophila lipid droplets buffer the H2Av supply to protect early embryonic development. Curr Biol. 2014;24:1485–1491. doi: 10.1016/j.cub.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horard B, Loppin B. Histone storage and deposition in the early Drosophila embryo. Chromosoma. 2015 doi: 10.1007/s00412-014-0504-7. Epublished ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Nagai A, Sato T, Akimoto N, Ito A, Sumida M. Isolation and identification of histone H3 protein enriched in microvesicles secreted from cultured sebocytes. Endocrinology. 2005;146:2593–2601. doi: 10.1210/en.2004-1478. [DOI] [PubMed] [Google Scholar]
- 30.Kan R, Jin M, Subramanian V, Causey CP, Thompson PR, Coonrod SA. Potential role for PADI-mediated histone citrullination in preimplantation development. BMC Dev Biol. 2012;12:19. doi: 10.1186/1471-213X-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krahmer N, Guo Y, Wilfling F, Hilger M, Lingrell S, Heger K, Newman HW, Schmidt-Supprian M, Vance DE, Mann M, et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011;14:504–515. doi: 10.1016/j.cmet.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chanarat S, Strasser K. Splicing and beyond: the many faces of the Prp19 complex. Biochim Biophys Acta. 2013;1833:2126–2134. doi: 10.1016/j.bbamcr.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Cho SY, Shin ES, Park PJ, Shin DW, Chang HK, Kim D, Lee HH, Lee JH, Kim SH, Song MJ, et al. Identification of mouse Prp19p as a lipid droplet-associated protein and its possible involvement in the biogenesis of lipid droplets. J Biol Chem. 2007;282:2456–2465. doi: 10.1074/jbc.M608042200. [DOI] [PubMed] [Google Scholar]
- 35.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mashek DG, Li LO, Coleman RA. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2007;2:465–476. doi: 10.2217/17460875.2.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rambold AS, Cohen S, Lippincott-Schwartz J. Fatty Acid Trafficking in Starved Cells: Regulation by Lipid Droplet Lipolysis, Autophagy, and Mitochondrial Fusion Dynamics. Dev Cell. 2015;32:678–692. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw CS, Jones DA, Wagenmakers AJ. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol. 2008;129:65–72. doi: 10.1007/s00418-007-0349-8. [DOI] [PubMed] [Google Scholar]
- 41.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1271–1278. doi: 10.1152/ajpregu.00472.2006. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Sreenivasan U, Hu H, Saladino A, Polster BM, Lund LM, Gong DW, Stanley WC, Sztalryd C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res. 2011;52:2159–2168. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoppins S. The regulation of mitochondrial dynamics. Curr Opin Cell Biol. 2014;29:46–52. doi: 10.1016/j.ceb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Sandoval H, Yao CK, Chen K, Jaiswal M, Donti T, Lin YQ, Bayat V, Xiong B, Zhang K, David G, et al. Mitochondrial fusion but not fission regulates larval growth and synaptic development through steroid hormone production. Elife. 2014;3:e03558. doi: 10.7554/eLife.03558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang X, Warren JT, Gilbert LI. New players in the regulation of ecdysone biosynthesis. J Genet Genomics. 2008;35:1–10. doi: 10.1016/S1673-8527(08)60001-6. [DOI] [PubMed] [Google Scholar]
- 46.Binns D, Januszewski T, Chen Y, Hill J, Markin VS, Zhao Y, Gilpin C, Chapman KD, Anderson RG, Goodman JM. An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol. 2006;173:719–731. doi: 10.1083/jcb.200511125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herms A, Bosch M, Ariotti N, Reddy BJ, Fajardo A, Fernandez-Vidal A, Alvarez-Guaita A, Fernandez-Rojo MA, Rentero C, Tebar F, et al. Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Curr Biol. 2013;23:1489–1496. doi: 10.1016/j.cub.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bozza PT, Bakker-Abreu I, Navarro-Xavier RA, Bandeira-Melo C. Lipid body function in eicosanoid synthesis: an update. Prostaglandins Leukot Essent Fatty Acids. 2011;85:205–213. doi: 10.1016/j.plefa.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A. 2008;105:9379–9384. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elamin AA, Stehr M, Singh M. Lipid Droplets and Mycobacterium leprae Infection. J Pathog. 2012;2012:361374. doi: 10.1155/2012/361374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herker E, Ott M. Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol Metab. 2011;22:241–248. doi: 10.1016/j.tem.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filipe A, McLauchlan J. Hepatitis C virus and lipid droplets: finding a niche. Trends Mol Med. 2015;21:34–42. doi: 10.1016/j.molmed.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Salloum S, Wang H, Ferguson C, Parton RG, Tai AW. Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets. PLoS Pathog. 2013;9:e1003513. doi: 10.1371/journal.ppat.1003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, McLauchlan J. Hepatitis C Virus Core Protein Induces LIpid Droplet Redistribution in a Microtubule- and Dynein-Dependent Manner. Traffic. 2008;9:1268–1282. doi: 10.1111/j.1600-0854.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 56.Andre P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Brechot C, Paranhos-Baccala G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheung W, Gill M, Esposito A, Kaminski CF, Courousse N, Chwetzoff S, Trugnan G, Keshavan N, Lever A, Desselberger U. Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication. J Virol. 2010;84:6782–6798. doi: 10.1128/JVI.01757-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boulant S, Targett-Adams P, McLauchlan J. Disrupting the association of hepatitis C virus core protein with lipid droplets correlates with a loss in production of infectious virus. J Gen Virol. 2007;88:2204–2213. doi: 10.1099/vir.0.82898-0. [DOI] [PubMed] [Google Scholar]
- 60.Liefhebber JM, Hague CV, Zhang Q, Wakelam MJ, McLauchlan J. Modulation of triglyceride and cholesterol ester synthesis impairs assembly of infectious hepatitis C virus. J Biol Chem. 2014;289:21276–21288. doi: 10.1074/jbc.M114.582999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boulant S, Montserret R, Hope RG, Ratinier M, Targett-Adams P, Lavergne JP, Penin F, McLauchlan J. Structural determinants that target the hepatitis C virus core protein to lipid droplets. J Biol Chem. 2006;281:22236–22247. doi: 10.1074/jbc.M601031200. [DOI] [PubMed] [Google Scholar]
- 62.Hinson ER, Cresswell P. The antiviral protein, viperin, localizes to lipid droplets via its N-terminal amphipathic alpha-helix. Proc Natl Acad Sci U S A. 2009;106:20452–20457. doi: 10.1073/pnas.0911679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herker E, Harris C, Hernandez C, Carpentier A, Kaehlcke K, Rosenberg AR, Farese RV, Jr, Ott M. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat Med. 2010;16:1295–1298. doi: 10.1038/nm.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camus G, Herker E, Modi AA, Haas JT, Ramage HR, Farese RV, Jr, Ott M. Diacylglycerol acyltransferase-1 localizes hepatitis C virus NS5A protein to lipid droplets and enhances NS5A interaction with the viral capsid core. J Biol Chem. 2013;288:9915–9923. doi: 10.1074/jbc.M112.434910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helbig KJ, Beard MR. The role of viperin in the innate antiviral response. J Mol Biol. 2014;426:1210–1219. doi: 10.1016/j.jmb.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Seo JY, Yaneva R, Cresswell P. Viperin: a multifunctional, interferon-inducible protein that regulates virus replication. Cell Host Microbe. 2011;10:534–539. doi: 10.1016/j.chom.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Helbig KJ, Eyre NS, Yip E, Narayana S, Li K, Fiches G, McCartney EM, Jangra RK, Lemon SM, Beard MR. The antiviral protein viperin inhibits hepatitis C virus replication via interaction with nonstructural protein 5A. Hepatology. 2011;54:1506–1517. doi: 10.1002/hep.24542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helbig KJ, Carr JM, Calvert JK, Wati S, Clarke JN, Eyre NS, Narayana SK, Fiches GN, McCartney EM, Beard MR. Viperin is induced following dengue virus type-2 (DENV-2) infection and has anti-viral actions requiring the C-terminal end of viperin. PLoS Negl Trop Dis. 2013;7:e2178. doi: 10.1371/journal.pntd.0002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teng TS, Foo SS, Simamarta D, Lum FM, Teo TH, Lulla A, Yeo NK, Koh EG, Chow A, Leo YS, et al. Viperin restricts chikungunya virus replication and pathology. J Clin Invest. 2012;122:4447–4460. doi: 10.1172/JCI63120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nikapitiya C, Dorrington T, Gomez-Chiarri M. The role of histones in the immune responses of aquatic invertebrates. ISJ. 2013;10:94–101. [Google Scholar]
- 71.Kawasaki H, Iwamuro S. Potential roles of histones in host defense as antimicrobial agents. Infect Disord Drug Targets. 2008;8:195–205. doi: 10.2174/1871526510808030195. [DOI] [PubMed] [Google Scholar]
- 72.Hirsch JG. Bactericidal action of histone. J Exp Med. 1958;108:925–944. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee DY, Huang CM, Nakatsuji T, Thiboutot D, Kang SA, Monestier M, Gallo RL. Histone H4 is a major component of the antimicrobial action of human sebocytes. J Invest Dermatol. 2009;129:2489–2496. doi: 10.1038/jid.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho JH, Park IY, Kim HS, Lee WT, Kim MS, Kim SC. Cathepsin D produces antimicrobial peptide parasin I from histone H2A in the skin mucosa of fish. FASEB J. 2002;16:429–431. doi: 10.1096/fj.01-0736fje. [DOI] [PubMed] [Google Scholar]
- 75.Anand P, Cermelli S, Li Z, Kassan A, Bosch M, Sigua R, Huang L, Ouellette AJ, Pol A, Welte MA, et al. A novel role for lipid droplets in the organismal antibacterial response. Elife. 2012;1:e00003. doi: 10.7554/eLife.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 77.Davletov B, Montecucco C. Lipid function at synapses. Curr Opin Neurobiol. 2010;20:543–549. doi: 10.1016/j.conb.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 79.Savage MJ, Goldberg DJ, Schacher S. Absolute specificity for retrograde fast axonal transport displayed by lipid droplets originating in the axon of an identified Aplysia neuron in vitro. Brain Res. 1987;406:215–223. doi: 10.1016/0006-8993(87)90785-2. [DOI] [PubMed] [Google Scholar]
- 80.Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, de Vries R, Arias E, Harris S, Sulzer D, et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington's disease. Nat Neurosci. 2010;13:567–576. doi: 10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thiam AR, Antonny B, Wang J, Delacotte J, Wilfling F, Walther TC, Beck R, Rothman JE, Pincet F. COPI buds 60-nm lipid droplets from reconstituted water-phospholipid-triacylglyceride interfaces, suggesting a tension clamp function. Proc Natl Acad Sci U S A. 2013;110:13244–13249. doi: 10.1073/pnas.1307685110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 83.Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fink JK. Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms. Acta Neuropathol. 2013;126:307–328. doi: 10.1007/s00401-013-1115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klemm RW, Norton JP, Cole RA, Li CS, Park SH, Crane MM, Li L, Jin D, Boye-Doe A, Liu TY, et al. A conserved role for atlastin GTPases in regulating lipid droplet size. Cell Rep. 2013;3:1465–1475. doi: 10.1016/j.celrep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Falk J, Rohde M, Bekhite MM, Neugebauer S, Hemmerich P, Kiehntopf M, Deufel T, Hubner CA, Beetz C. Functional mutation analysis provides evidence for a role of REEP1 in lipid droplet biology. Hum Mutat. 2014;35:497–504. doi: 10.1002/humu.22521. [DOI] [PubMed] [Google Scholar]
- 87.Cartwright BR, Goodman JM. Seipin: from human disease to molecular mechanism. J Lipid Res. 2012;53:1042–1055. doi: 10.1194/jlr.R023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fei W, Du X, Yang H. Seipin, adipogenesis and lipid droplets. Trends Endocrinol Metab. 2011;22:204–210. doi: 10.1016/j.tem.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Eastman SW, Yassaee M, Bieniasz PD. A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover. J Cell Biol. 2009;184:881–894. doi: 10.1083/jcb.200808041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hooper C, Puttamadappa SS, Loring Z, Shekhtman A, Bakowska JC. Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets. BMC Biol. 2010;8:72. doi: 10.1186/1741-7007-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Edwards TL, Clowes VE, Tsang HT, Connell JW, Sanderson CM, Luzio JP, Reid E. Endogenous spartin (SPG20) is recruited to endosomes and lipid droplets and interacts with the ubiquitin E3 ligases AIP4 and AIP5. Biochem J. 2009;423:31–39. doi: 10.1042/BJ20082398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Renvoise B, Stadler J, Singh R, Bakowska JC, Blackstone C. Spg20-/- mice reveal multimodal functions for Troyer syndrome protein spartin in lipid droplet maintenance, cytokinesis and BMP signaling. Hum Mol Genet. 2012;21:3604–3618. doi: 10.1093/hmg/dds191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, Gross SP. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–1107. doi: 10.1016/j.cell.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tesson C, Nawara M, Salih MA, Rossignol R, Zaki MS, Al Balwi M, Schule R, Mignot C, Obre E, Bouhouche A, et al. Alteration of fatty-acid-metabolizing enzymes affects mitochondrial form and function in hereditary spastic paraplegia. Am J Hum Genet. 2012;91:1051–1064. doi: 10.1016/j.ajhg.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rainier S, Bui M, Mark E, Thomas D, Tokarz D, Ming L, Delaney C, Richardson RJ, Albers JW, Matsunami N, et al. Neuropathy target esterase gene mutations cause motor neuron disease. Am J Hum Genet. 2008;82:780–785. doi: 10.1016/j.ajhg.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inloes JM, Hsu KL, Dix MM, Viader A, Masuda K, Takei T, Wood MR, Cravatt BF. The hereditary spastic paraplegia-related enzyme DDHD2 is a principal brain triglyceride lipase. Proc Natl Acad Sci U S A. 2014;111:14924–14929. doi: 10.1073/pnas.1413706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schuurs-Hoeijmakers JH, Geraghty MT, Kamsteeg EJ, Ben-Salem S, de Bot ST, Nijhof B, van de V, II, van der Graaf M, Nobau AC, Otte-Holler I, et al. Mutations in DDHD2, encoding an intracellular phospholipase A(1), cause a recessive form of complex hereditary spastic paraplegia. Am J Hum Genet. 2012;91:1073–1081. doi: 10.1016/j.ajhg.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arrese EL, Patel RT, Soulages JL. The main triglyceridelipase from the insect fat body is an active phospholipase A(1): identification and characterization. J Lipid Res. 2006;47:2656–2667. doi: 10.1194/jlr.M600161-JLR200. [DOI] [PubMed] [Google Scholar]
- 99.Gavgiotaki E, Filippidis G, Kalognomou M, Tsouko AA, Skordos I, Fotakis C, Athanassakis I. Third Harmonic Generation microscopy as a reliable diagnostic tool for evaluating lipid body modification during cell activation: The example of BV-2 microglia cells. J Struct Biol. 2015;189:105–113. doi: 10.1016/j.jsb.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 100.Lucken-Ardjomande Häsler S, Vallis Y, Jolin HE, McKenzie AN, McMahon HT. GRAF1a is a brain-specific protein that promotes lipid droplet clustering and growth, and is enriched at lipid droplet junctions. J Cell Sci. 2014;127:4602–4619. doi: 10.1242/jcs.147694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulz J, Jasikova L, Skriba A, Roithova J. Role of gold(I) alpha-oxo carbenes in the oxidation reactions of alkynes catalyzed by gold(I) complexes. J Am Chem Soc. 2014;136:11513–11523. doi: 10.1021/ja505945d. [DOI] [PubMed] [Google Scholar]
- 102.Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, et al. Glial Lipid Droplets and ROS Induced by Mitochondrial Defects Promote Neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamamoto S, Jaiswal M, Charng WL, Gambin T, Karaca E, Mirzaa G, Wiszniewski W, Sandoval H, Haelterman NA, Xiong B, et al. A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell. 2014;159:200–214. doi: 10.1016/j.cell.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 105.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rawson RB. The SREBP pathway--insights from Insigs and insects. Nat Rev Mol Cell Biol. 2003;4:631–640. doi: 10.1038/nrm1174. [DOI] [PubMed] [Google Scholar]
- 107.Welte MA. Proteins under new management: lipid droplets deliver. Trends Cell Biol. 2007;17:363–369. doi: 10.1016/j.tcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 108.Hodges BD, Wu CC. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res. 2011;51:262–273. doi: 10.1194/jlr.R003582. [DOI] [PMC free article] [PubMed] [Google Scholar]