Abstract
Background
Delirium is a common complication in elderly hospitalized patients. It prolongs the length of hospital stay, raises costs, increases the workload of the nursing staff, and may necessitate transfer of the patient to a nursing home. The risk of postoperative delirium is particularly high in elderly patients with pre-existing cognitive deficits.
Methods
In an open study, we systematically assessed the frequency of postoperative delirium in patients over age 70 on two surgical wards of a general hospital. In a six-month “prevalence phase,” from March to August 2011, we counted the number of patients with postoperative delirium, but did not initiate any intervention. Thereafter, in a ten-month “intervention phase” from September 2011 to June 2012, a nurse with special training in the management of delirium carried out an intervention involving component measures of the Hospital Elder Life Program (HELP) on one of the two wards, with the aim of preventing postoperative delirium. The patients on the other ward served as a control group.
Results
In the prevalence phase, 20.2% of all patients developed postoperative delirium (95% confidence interval [CI], 14.6–26.4). In the intervention phase, postoperative delirium arose in 20.8% (95% CI, 11.3–32.1) of the patients on the ward with no specific interventions, but in only 4.9% (95% CI, 0.0–11.5) of those on the ward where the intervention was carried out. The difference was presumably due to the measures initiated by the specially trained nurse, including validation, improvement of sleep, cognitive activation, early mobilization, improved sensory stimulation, and improved nutritional and fluid intake. Important predictors of postoperative delirium included a low score on the Mini–Mental State Examination, advanced age, and preoperative infection.
Conclusion
The frequency of postoperative delirium in elderly patients with cognitive deficits can be lowered with nursing measures carried out by a specially trained nurse, close postoperative supervision, and cognitive activation.
The term “delirium” is used to describe all acute mental disturbances that have an organic cause and are accompanied by disorientation and cognitive impairment (1, 2).
With an incidence of 14% to 56%, delirium is the commonest complication in over 70-year-olds receiving treatment as inpatients (3), and is associated with a mortality rate of 25% to 33% (4). Dementia increases the likelihood of delirium, and is far and away its most important risk factor (5). Especially in medical and surgical wards and in intensive care, 50% of all patients are disoriented postoperatively (6). Delirium increases the length of hospital stay, the costs, the amount of care required, and the risk that the patient will be institutionalized (7– 10).
In the past few years in Germany, some promising pilot projects have shown that the incidence of postoperative delirium on general hospital medical and surgical wards can be reduced by a variety of interventions (11– 14).
Existing models, such as the procedures used at the St Franziskus Hospital in Münster, show that the incidence of postoperative delirium can be reduced if the patient has a single defined contact person for the duration of their hospital stay and is screened on admission for cognitive deficits (15, 16).
Non-medical interventions are important supportive elements of treatment that are not adequately utilized in the everyday management of delirious patients (17, 18). They include aids to orientation: a clock, a calendar, or a familiar photo can all have a supportive effect in the patient’s treatment. Protecting patients from stimuli is an important aid in non-medical treatment of delirium. This should include not changing the patient’s room and having no staff changes, so far as possible. Sensory aids for the patient such as glasses or hearing aids should be used. Adequate lighting is important, so that a proper sleep-wake cycle is ensured. Personal attention and the involvement of friends or relatives have a positive effect (19– 21).
The aim of the present study was to answer the following questions:
What is the incidence of postoperative delirium in a general surgical ward in a general hospital?
What preoperative factors are predictive of delirium?
Can a specialist geriatric psychiatric nurse reduce the incidence of postoperative delirium by means of non-pharmacological interventions?
The present study is something of a new departure in that the focus was, first, on cognitively impaired patients undergoing general surgical treatment in the context of a real-life mixture of patients, and, second, on non-drug, nursing interventions. We aim to show that delirium prophylaxis is not primarily a task for surgeons or physicians, but for trained nursing staff.
Methods
The methods used, especially the statistical analysis, are described in detail in the supplementary eMethods section.
Study design
The study was carried out in two general surgical wards of a teaching hospital providing standard care; one was designated the intervention ward and the other the control ward. A variety of medical and ancillary teams were responsible for patients’ treatment. The patient groups had similar demographic characteristics. The treatment teams were made up of surgeons, specialized surgical nurses, physiotherapists, and social workers. The two wards were similar in terms of numbers of nursing and medical staff per number of beds. In neither of the wards was there any special training in geriatrics or geriatric psychiatry.
During the first phase of the study (prevalence phase; March to August 2011), on both wards the number of over 70-year-olds who already showed cognitive impairment and confusion preoperatively was recorded. Socioeconomic data, diagnoses, physical diseases, and preoperative medication were also recorded. In addition, the following investigations or measurements were carried out pre- and postoperatively:
Mini–Mental State Examination (MMSE) (22)
Activities of Daily Living (ADL) (23)
Confusion Assessment Method (CAM) (24)
Delirium Rating Scale (DRS) (25)
Nurses’ Observation Scale for Geriatric Patients (NOSGER) (26)
Barthel Index (27)
Montgomery–Åsberg Depression Rating Scale (MADRS) (28)
Body mass index
Hours of sleep (Table 1).
Table 1. Assessment instruments used.
| Instrument | Brief description | Scoring |
|---|---|---|
| Barthel Index | Assessment instrument for systematic assessment of level of independence | Scoring for some of the activities of daily living: 0 points = completely dependent 100 points = independent The present study dichotomized the score: ≥85 points (= “needs occasional assistance” to “does not need assistance” help) and <85 points (= “needs assistance” to “completely dependent”). |
| Confusion Assessment Method (CAM) | Diagnostic instrument to identify delirium in geriatric patients | Delirium vs. no delirium |
| Montgomery–Åsberg Depression Scale (MADRS) | Third-party questionnaire used for quantitative assessment of depression in patients | The scores for the ten items are added together: maximum 60 points >28 = severe depression |
| Mini–Mental State Examination (MMSE) | Suitable screening instrument to determine cognitive deficits in everyday clinical practice | Scale runs from 0 to 30 points: 30 points = no deficits <27 points = disease-related deficits 0 points = very severely compromised cognitive function |
| Nurses’ Observation Scale for Geriatric Patients (NOSGER) |
Standardized third-party assessment by nursing staff (by observation) to determine presence of dementia in geriatric patients | Overall mean values for patient groups living independently, in a residential home, or in a nursing home: higher overall scores are associated with more pronounced signs of dementia |
During the subsequent 10-month intervention phase of the study (September 2011 to June 2012), one of the two wards became the intervention ward, the other the control ward.
On the intervention ward, interventions were implemented in the following areas by the geriatric psychiatric nurse (delirium liaison nurse) pre- and postoperatively, according to the individual needs of the patients (Table 2):
Table 2. Interventions and frequencies of their use in the intervention group (n = 61).
| Intervention | N (%) | Specific measure (%) |
|---|---|---|
| Early mobilization | 51 (83.6) | Walking (83.6) Taking meals at the table (78.7) For self-care (78.7) To avoid decubitus ulcers (78.7) |
| Improved sensory stimulation | 41 (67.2) | Visual aids (glasses) (67.2) Hearing aid (18.0) Well-adjusted lighting (1.6) Personal items (23.3) Reduced/increased stimulation (3.0) |
| Improved fluid and nutritional intake | 31 (50.8) | Patient’s own choice of food (27.9) Nutritional advice (11.5) Eating as a social ritual (34.4) Dentures (18.0) Dysphagia screening (13.1) |
| Improved sleep | 57 (93.4) | Take patient’s sleeping habits into account (93.4) Change the structuring of the day (18) |
| Cognitive activation | 57 (93.4) | Reorientation aids (e.g., calendar, clock, daily newspaper) (62.3) Group interventions: group social meetings; topic-based discussions; reminiscence therapy (63.9) Individual interventions: memory training (55.7) Enjoyment therapy (49.2) Education of relatives (23.0) Relatives: help to support patient (23.0) |
| Validation (Feil method) | 61 (100) | Training program for nursing staff as a form of self-preservation therapy (100) Communication (100) Convey sense of safety (98.4) Avoid room changes (98.4) Enable distractions/entertainment (100) |
Study population
The study included all patients admitted to the two surgical wards between March 2011 and June 2012, so long as they fulfilled the following criteria:
Over 70 years of age
No clinically manifest signs of delirium
Capable of understanding the study and consenting to participation.
Patients with any of the following characteristics were excluded:
Advanced dementia, unable to give consent
Severe delirium preoperatively
End-stage disease
Refusal to participate in the study.
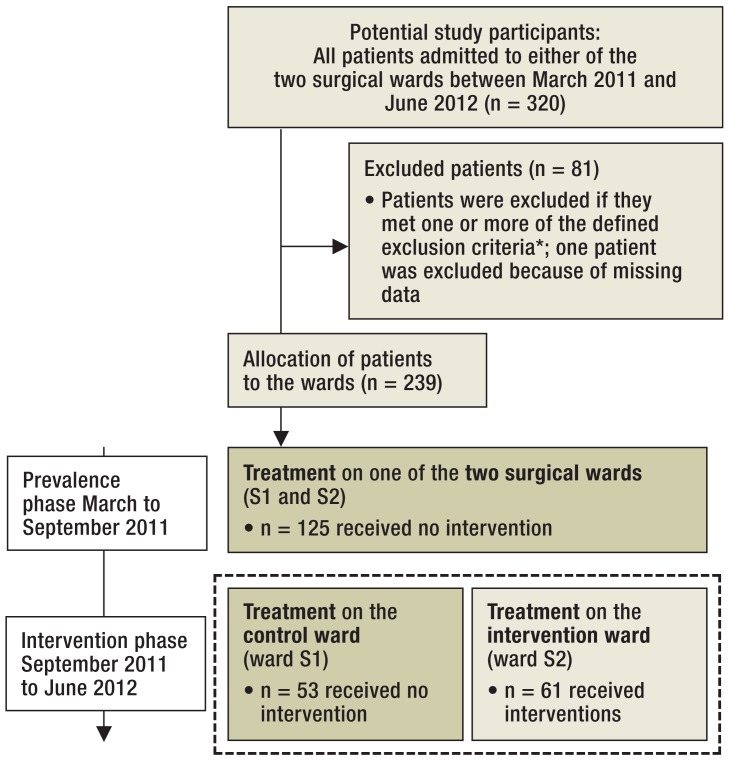
The study design was approved by the hospital ethics committee on 7 October 2010. The Figure shows the flow chart for included and excluded patients during both phases of the study.
Figure.
Patient grouping according to the analyses carried out: No patient received specific treatment for delirium prevention. For this reason, the data of those shown in the dark-olive boxes were used as a study sample (n = 178) to determine factors predictive of postoperative delirium. The patient groups in the dashed boxes (n = 114) were both treated during the same study period, so their data were used to determine the effect of intervention.
*Patients admitted to the wards were included in the study if they were over 70 years old, showed no clinical signs of delirium, and were able to understand the study and consent to participate. Patients were excluded if they had advanced dementia if they were unable to give consent due to advanced dementia, if they were already suffering preoperatively from severe delirium, refused on principle to participate in any study, or were in the terminal stage of their disease
The analysis performed to identify the prevalence and preoperative predictors of postoperative delirium included all patients in the study who were treated on either of the two surgical wards between March 2011 and June 2012 (n = 178). Mean patient age was 76.8 years, and 53.9% of patients (n = 96) were women. Patients’ preoperative characteristics are summarized in Table 3.
Table 3. Preoperative differences between patients with and those without postoperative delirium (PD).
| Total (N = 178) | With PD (n = 36) | Without PD (n = 142) | U or χ 2 | Z*1 | p*2 | OR (95% CI) or r | ||
|---|---|---|---|---|---|---|---|---|
| Sociodemographic characteristics | ||||||||
| Age | M (SD) | 76.8 (5.8) | 80.0 (6.5) | 76.0 (5.4) | 1580.00 | −3.54 | <0.001*3, 4 | −0.27 |
| Sex (female) | n (%) | 96 (53.9) | 20 (55.6) | 76 (53.5) | 0.05 | – | 0.827 | 1.09 (0.52–2.27) |
| Education <8 years | n (%) | 95 (53.4) | 27 (75.0) | 68 (47.9) | 8.48 | – | 0.004*4 | 3.27 (1.43–7.44) |
| Relatives (vs. no relatives) | n (%) | 165 (92.7) | 34 (94.4) | 131 (92.3) | – | – | 1.000*5 | 1.43 (0.30– 6.75) |
| Referred by specialist (vs. family doctor) | n (%) | 122 (68.5) | 28 (77.8) | 94 (66.2) | 1.79 | – | 0.181 | 1.79 (0.77– 4.22) |
| Admitted from institution (vs. own home) | n (%) | 10 (5.6) | 6 (16.7) | 4 (2.8) | – | – | 0.005*4, 5 | 6.90 (1.83–25.97) |
| Psychological assessment | ||||||||
| MMSE <27 (vs. ≥ 27) | n (%) | 60 (33.7) | 25 (69.4) | 35 (24.6) | 25.79 | – | <0.001*4 | 6.95 (3.11–15.55) |
| Barthel Index < 85 (vs. ≥ 85) | n (%) | 43 (24.2) | 20 (55.6) | 23 (16.2) | 24.28 | – | <0.001*4 | 6.47 (2.92–14.32) |
| NOSGER | M (SD) | 38.4 (8.7) | 44.0 (9.4) | 37.0 (7.9) | 1322.50 | −4.49 | <0.001*3, 4 | −0.34 |
| MADRS | M (SD) | 6.6 (4.8) | 7.0 (3.9) | 6.4 (5.0) | 2312.00 | −0.89 | 0.373*3 | −0.07 |
| Medical characteristics | ||||||||
| BMI | M (SD) | 26.8 (4.3) | 26.8 (4.1) | 26.9 (4.3) | 2550.50 | −0.02 | 0.984*3 | −0.00 |
| Sleep*6 | M (SD) | 6.3 (1.3) | 5.9 (1.3) | 6.4 (1.2) | 1955.50 | −2.24 | 0.025*3, 4 | −0.17 |
| Infections*7 | n (%) | 37 (20.8) | 16 (44.4) | 21 (14.8) | 15.34 | – | <0.001*4 | 4.61 (2.06–10.30) |
| Cardiovascular problems*8 | n (%) | 145 (81.5) | 35 (97.2) | 110 (77.5) | 7.42 | – | 0.006*4 | 10.18 (1.34–77.24) |
| Anticholinergic medication*9 | n (%) | 89 (50.0) | 16 (44.4) | 73 (51.4) | 0.56 | – | 0.455 | 0.76 (0.36–1.58) |
| Metabolic disorder*10 | n (%) | 140 (78.7) | 33 (91.7) | 107 (75.4) | 4.55 | – | 0.033*4 | 3.60 (1.04–12.46) |
BMI, body mass index; PD, postoperative delirium; MMSE, Mini–Mental State examination; NOSGER, Nurses’ Observation Scale for Geriatric Patients; MADRS, Montgomery–Åsberg Depression Rating Scale; OR, odds ratio; M, mean; r, Z-value-based measurement of effect size; SD, standard deviation; U, U value; χ ², χ ² value; CI, confidence interval
*1Z value resulting from Mann-Whitney U test; *2 p- value, based on χ ² test unless otherwise indicated; *3 p- value, Mann-Whitney U test; *4 p- value <0.05;
*5p- value, Fisher’s exact test; *6 hours of sleep at night (measured by nursing staff); *7 predominantly urinary tract infections; *8 mostly coronary heart disease and arterial hypertension;
*9 medication with potential anticholinergic side effects; *10 predominantly glucose and thyroid metabolism disorders
The analysis performed to identify the effect of intervention included all patients in the study who were treated between September 2011 and June 2012. There were 53 patients in the control group (mean patient age 76.6 years, 47.2% [n = 25] were women) and 61 patients in the intervention group (mean patient age 77.8 years, 63.9% [n = 39] were women). The descriptive statistics of both groups are summarized in Table 4.
Table 4. Preoperative differences between the intervention group and the control group.
| Total (N = 114) | Intervention (n = 61) | Control (n = 53) | U or χ 2 | Z*1 | p*2 | ||
|---|---|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||||
| Age | M (SD) | 77.2 (5.7) | 77.8 (6.1) | 76.6 (5.3) | 1456.5 | −0.91 | 0.362*3 |
| Sex (female) | n (%) | 64 (56.1) | 39 (63.9) | 25 (47.2) | 3.24 | – | 0.072 |
| Education <8 years | n (%) | 64 (56.1) | 33 (54.1) | 31 (58.5) | 0.22 | – | 0.637 |
| Relatives (vs. no relatives) | n (%) | 89 (78.1) | 44 (72.1) | 45 (84.9) | 2.70 | – | 0.100 |
| Referred by specialist (vs. family doctor) | n (%) | 91 (79.8) | 52 (85.2) | 39 (73.6) | 2.39 | – | 0.122 |
| Admitted from institution (vs. own home) | n (%) | 6 (5.3) | 6 (9.8) | 0 (0.0) | – | – | 0.029*4, 5 |
| Psychological assessment | |||||||
| MMSE <27 (vs. ≥ 27) | n (%) | 30 (26.3) | 17 (27.9) | 13 (24.5) | 0.16 | – | 0.686 |
| Barthel Index <85 (vs. ≥ 85) | n (%) | 40 (35.1) | 34 (55.7) | 6 (11.3) | 24.57 | – | <0.001*5 |
| NOSGER | M (SD) | 38.5 (9.0) | 40.8 (10.4) | 35.7 (6.1) | 1218.5 | −2.28 | 0.023*3, 5 |
| MADRS | M (SD) | 7.9 (5.8) | 9.0 (6.3) | 6.6 (5.0) | 1235.5 | −2.19 | 0.029*3, 5 |
| Medical characteristics | |||||||
| BMI | M (SD) | 28.3 (6.7) | 29.4 (8.0) | 27.2 (4.7) | 1443.5 | −0.99 | 0.324*3 |
| Sleep*6 | M (SD) | 6.3 (1.6) | 6.3 (1.9) | 6.4 (1.3) | 1563.0 | −0.31 | 0.756*3 |
| Infections*7 | n (%) | 24 (21.1) | 16 (26.2) | 8 (15.1) | 2.12 | – | 0.146 |
| Cardiovascular problems*8 | n (%) | 96 (84.2) | 52 (85.2) | 44 (83.0) | 0.11 | – | 0.745 |
| Metabolic disorder*9 | n (%) | 90 (78.9) | 48 (78.7) | 42 (79.2) | 0.01 | – | 0.942 |
| Anticholinergic medication*10 | n (%) | 64 (56.1) | 39 (63.9) | 25 (47.2) | 3.24 | – | 0.072 |
BMI, body mass index; MMSE, Mini–Mental State Examination; NOSGER, Nurses’ Observation Scale for Geriatric Patients; MADRS, Montgomery–Åsberg Depression Rating Scale; U, U value; χ ², χ ² value; variables with p<0.10 were used in the calculation of the propensity score; SD, standard deviation; M, mean.
*1Z value resulting from Mann-Whitney U test; *2 p- value, based on χ ² test unless otherwise indicated;
*3 p- value, Mann-Whitney U test; *4 p- value, Fisher’s exact test; *5 p- value <0.05;
*6hours of sleep at night (measured by nursing staff); *7predominantly urinary tract infections; *8 mostly coronary heart disease and arterial hypertension;
*9 predominantly glucose and thyroid metabolism disorders; *10 medication with potential anticholinergic side effects
Intervention
During the intervention phase, on the intervention ward the preoperative screening was used to derive a delirium risk on the basis of general factors favorable to confusion or delirium: attention deficit, pre-existing cognitive deficits (demonstrated using the MMSE), and the duration and extent of the planned surgery. For example, femoral neck fractures are associated with a very high risk of delirium.
The delirium liaison nurse determined the delirium risk independently of the surgical ward staff. On the basis of the determined risk, an individual intervention plan was set up. The following goals were set:
Reorientation
Improved sleep
Cognition support
Structured daily schedule
Early mobilization
Involvement of relatives
Improved nutrition.
Table 2 shows the frequency with which the various interventions were actually used. For patients with postoperative delirium, an interventional support program adapted from the Hospital Elder Life Program (HELP) (31) (Box) was used.
Box. Hospital Elder Life Program.
The Hospital Elder Life Program (HELP) was developed by Sharon K. Inouye at the Yale University School of Medicine for the prevention, diagnosis, and treatment of delirium in general hospitals. HELP is an innovative approach to improving the hospital care of older patients through the following interventions:
Patients are activated and supported by trained volunteers.
Relatives are involved.
Patients are regularly screened for delirium.
Specialist nurses advise ward staff, and relatives.
In-hospital training is provided for nursing and medical staff.
Implementation of the program significantly reduces the development of delirium and functional decline in older patients.
Study goals
During the prevalence phase, we investigated how often postoperative delirium occurred in the patient population described above. During the subsequent intervention phase, we analyzed whether postoperative delirium occurred significantly less often on the intervention ward than on the control ward.
Statistical analysis
The statistical package SPSS 21 was used for statistical analysis.
Results
Prevalence of postoperative delirium
Over the whole of the study period, 20.2% of patients (n = 36; 95% confidence interval [CI]: 14.6 to 26.4) who received no specific intervention (n = 178) developed postoperative delirium.
Predictors of postoperative delirium
Descriptive statistics, test statistics, and effect sizes of the factors under consideration for patients with and those without postoperative delirium are listed in Table 3. Numerous variables show differences even preoperatively between those with and those without postoperative delirium.
In multivariate logistic regression analysis with forward selection, the variables age, MMSE, Barthel Index, and infections remained in the model. Only MMSE, age, and preoperative infections proved to be statistically significant (p<0.05). The individual results for these variables are summarized in Table 5.
Table 5. Predictors of postoperative delirium.
| Predictor | b | SE | Wald | df | p | OR (95-%-CI) |
|---|---|---|---|---|---|---|
| Age | 0.08 | 0.04 | 4.47 | 1 | 0.034* | 1.08 (1.01–1.16) |
| MMSE <27 | 1.43 | 0.46 | 9.88 | 1 | 0.002* | 4.18 (1.71–10.20) |
| Barthel Index <85 | 0.89 | 0.49 | 3.32 | 1 | 0.069 | 2.44 (0.93–6.37) |
| Infection | 1.15 | 0.49 | 5.51 | 1 | 0.019* | 3.16 (1.21–8.26) |
| Constant | –8.77 | 2.92 | 9.03 | 1 | 0.003 |
b, regression coefficient; df, degrees of freedom; MMSE, Mini–Mental State Examination; SE, standard error; OR, odds ratio; Wald, Wald statistic; 95% CI, 95% confidence interval
*p <0.05
Efficacy of intervention
Table 4 contains the descriptive statistics and test statistics of the preoperative characteristics in both groups. The intervention group had a higher proportion of patients with a Barthel Index <85 and higher preoperative NOSGER and MADRS scores. The proportion of patients who were admitted from an institution was higher in the intervention group than in the control group.
Overall, during the intervention phase of the study, 3 patients in the intervention group (4.9%; 95% CI: 0.0 to 11.5) and 11 patients in the control group (20.8%; 95% CI: 11.3–32.1) developed postoperative delirium. The χ 2 test showed the difference to be significant (χ 2= 6.60; N = 114; df = 1; p= 0.01). The model—adjusted using the “propensity score”—also showed a significant effect of which group a patient was in (odds ratio [OR] = 0.22; 95% CI: 0.05 to 0.98; p = 0.046). Patients in the intervention group had a lower risk of postoperative delirium than did patients in the control group.
Discussion
Delirium is a frequent condition in older patients on surgical or medical wards in general hospitals, and is associated with high consumption of health resources. The risk of postoperative delirium is particularly high in elderly patients with pre-existing cognitive deficits. Over the period of the present study, 20.2% of patients who received no intervention developed postoperative delirium. This agrees with figures published elsewhere (4).
Our results indicate that patients who develop postoperative delirium are different preoperatively to those who do not. Our data show that, especially, age, pre-existing infections, and the Mini–Mental State Examination score, which indicates existing cognitive deficits, are risk factors for postoperative delirium. The publications of Fischer and Assem-Hilger (32), Margiotta et al. (33), and Robinson et al. (9) support our findings.
Differences in preoperative functional status were also found between the patient groups, using NOSGER. In addition, it was noted that the risk of delirium was higher when patients had a preoperative Barthel Index <85, or lower level of education, or were admitted from an institution.
We obtained clear indications that consistently applied preoperative screening and simple interventions by a delirium liaison nurse—a nurse with many years’ experience of geriatric psychiatric nursing—can reduce the incidence of postoperative delirium. Andrews et al. (34), Holt et al. (35), and Milisen et al. (36) performed their studies under different conditions—in intensive care units or in older patients with femoral neck fractures—but they also showed that consistent monitoring of delirium risk performed by nurses can reduce this risk.
Whereas 20.8% of patients on the control ward during the intervention phase developed postoperative delirium, delirium was observed in only 4.9% of patients on the intervention ward (OR = 0.22; 95% CI: 0.05 to 0.98; p = 0.046). These results agree with those of Zaubler et al. (19), in whose study a HELP approach (31) was also followed, although this study was carried out in a medical ward. Unlike Zaubler et al. (19), who used only a historical control group for comparison, the present study included both an intervention and a (non-intervention) control ward during the intervention phase of the study, which increases the validity of its results. The present study also included validation, sleep improvement, cognitive activation, early mobilization, improved sensory stimulation, and nutritional and fluid intake during the investigation phase. We take this as a clear indication of the protective effect of such measures in preventing delirium.
This assessment also agrees with the findings of a recent meta-analysis of 14 intervention studies on non-drug prevention of delirium (37), which showed that multimodal non-drug delirium prophylaxis can reduce the incidence of delirium.
Some restrictions need to be observed when interpreting the present results. This was not a randomized, placebo-controlled study (RCT), but a service delivery study carried out in the setting of a general hospital.
All the calculated values show relatively wide confidence intervals, but this is probably because the patient population was quite small and only a moderate number of patients developed delirium.
Limitations
A limitation of this study is that, because of the low numbers involved (interventions undertaken in 65 of 320 patients) and because of the study design, it was not possible to analyze statistically the extent to which the various individual interventions can prevent postoperative delirium. In addition, it should be noted that the intervention group had a markedly higher percentage of patients with a low Barthel Index or a higher NOSGER score, indicating that the patients in this group already had greater physical and functional impairment. Despite the poorer initial conditions in the intervention group, intervention significantly reduced the incidence of postoperative delirium. During the intervention phase, a geriatric department was established in the hospital which was physically close to the control ward but not to the intervention ward. Despite this, however, the incidence of postoperative delirium was significantly reduced on the intervention ward compared to the control ward.
The present study focused on non-drug interventions for the prevention of postoperative delirium. Whether interventions based on the use of psychoactive drugs—e.g., preoperative administration of antipsychotics—have a preventive effect against postoperative delirium, remains unknown at present (10, 38, 39).
Summary
We believe that if the medical staff caring for the patient understand the risk factors, this can have a critical effect in reducing postoperative delirium. Establishing a delirium liaison nurse, who carries out screening for risk factors for postoperative delirium preoperatively and cognitive activation of the patient peri- and postoperatively, could be an important step towards improving treatment outcomes in older patients undergoing surgery in general hospitals.
Nursing staff in particular would be trained by a delirium liaison nurse in screening for risk factors and would learn simple methods and tools for delirium prophylaxis, e.g., cognitive activation or validation. Without this, lack of understanding or ignorance on the part of medical staff could lead to an increased incidence of delirium. This is why it is so important for specific treatment guidelines to be integrated into the hospital clinical routine for nurses and doctors in training. We believe that employing a delirium liaison nurse could be an important contribution toward this.
The delirium liaison nurse could be effective not just through his or her own immediate clinical activity, but as part of a multi-professional team on a surgical ward would have a considerable educational effect on all members of the team (learning by watching) (40).
In our view, further studies with larger patient numbers are needed to establish the part played by the individual interventions in the reduction of postoperative delirium.
Supplementary Material
A Prospective Intervention With Psychogeriatric Liaison on Surgical Wards in a General Hospital
Torsten Kratz, Manuel Heinrich, Eckehard Schlauß, Albert Diefenbacher
Materials and methods
Study design
This study was carried out on two general surgical wards of a hospital providing standard care. The Evangelische Krankenhaus Königin Elisabeth Herzberge (KEH) is an academic teaching hospital affiliated with? the Charité Faculty of Medicine at the University of Berlin, Germany. One ward was designated as the intervention ward, the other as the control ward. Two wards were chosen that treated similar patients with general, abdominal, and trauma surgery. However, the nursing and medical teams carrying out the treatment were different. No orthopedic procedures were performed. The patients were demographically similar. The original intention was to admit patients for general or abdominal surgery to the control ward and patients undergoing general or trauma surgery to the intervention ward, but this proved impossible to maintain consistently in the daily routine of patient care in a general hospital, and for this reason there was a mixture of surgical procedures in both wards at the time of the study.
There was no difference between groups in terms of the anesthesia used (local or regional vs. general). The duration of anesthesia during the procedures was up to 20% longer in about 10% of the patients in the control ward compared to the intervention ward. The incidence of postoperative treatment in an intensive care unit was similar in both groups.
Most patients were admitted to the wards after referral by their family doctor or a primary care surgeon, but some were emergency admissions or had been transferred from another ward in the same hospital. Admission to both wards—irrespective of indication or other factors—was according to bed availability.
The ward teams were made up of surgeons, surgical nursing staff, physiotherapists, and social workers. The two wards were similar in terms of numbers of surgeons and nurses per number of beds. The control ward had 16 beds under the care of 7.1 nurses and 6 physicians. Duration of stay was 5.5 days, with the ward at 90.5% capacity. The intervention ward had 25 beds under the care of 10.7 nurses and 8 physicians. Duration of stay was 5.1 days, with the ward at 86.5% capacity. There was no special training in geriatrics or geriatric psychiatry on either ward.
The first phase of the study (prevalence phase; March to August 2011) recorded on both wards the percentage of patients aged over 70 years who already showed cognitive impairment and confusion preoperatively. Patients’ socioeconomic data, diagnoses, physical diseases, and preoperative medication were documented. In addition, the following investigations were carried out and data recorded pre- and postoperatively:
Mini–Mental State Examination (MMSE) (22)
Activities of Daily Living (ADL) (23)
Confusion Assessment Method (CAM) (24)
Delirium Rating Scale (DRS) (25)
Nurses’ Observation Scale for Geriatric Patients (NOSGER scale) (26)
Barthel Index (27)
Montgomery–Åsberg Depression Rating Scale (MADRS) (28)
Body mass index (BMI)
Hours of sleep (Table 1).
In the subsequent intervention phase, which lasted 10 months (September 2011 to June 2012), one of the wards became the interventional ward, the other the control ward.
On the intervention ward, pre- and postoperative interventions were carried out by the geriatric psychiatric liaison nurses (delirium liaison nurses) in the areas of early mobilization, improved sensory stimulation, improved nutritional and fluid intake, non-drug improvement of sleep, and cognitive activation and validation, individualized to the needs of the patient (Table 2).
Geriatric psychiatric liaison nurse (delirium liaison nurse)
The delirium liaison nurse is a nurse with a specialist qualification in geriatric nursing. The nurse is experienced in dealing with older patients who are confused or are cognitively impaired on a geriatric psychiatric ward. The delirium liaison nurse is trained in dealing with abnormal behavior on the part of older persons with cognitive deficits and has knowledge of various intervention techniques such as the Feil validation method (29, 30). Before the start of the study, the delirium liaison nurse had already been advising the nursing staff on surgical and medical wards, at their request, on how to deal with postoperative delirium or disturbed behavior in patients with dementia (liaison nursing).
In preparation for the study, the delirium liaison nurse was trained in carrying out the CAM, MADRS, and MMSE screening procedures. The delirium liaison nurse was already familiar with the Barthel Index and NOSGER as standard instruments of care in everyday use. On average, the delirium liaison nurse needed 45 minutes for the preoperative assessment of each patient.
The following interventions were carried out:
Reorientation
Improved sleep
Cognition support
Structuring the daily routine
Rapid mobilization
Inclusion of relatives
Improved nutritional and fluid intake.
Since these measures are the same as those used in normal patient-centered care on a geriatric psychiatric ward, no additional training of the liaison nurse was needed.
Study participants
The study included all patients admitted to the two surgical wards between March 2011 and June 2012 who fulfilled the following criteria:
Age over 70 years
No clinically obvious signs of delirium
Able to understand the study and give consent to participating.
The following were criteria for exclusion of patients from the study:
Advanced dementia and inability to give consent
Severe delirium preoperatively
End-stage disease
Refusal to participate in the study.
The study design was approved by the hospital ethics committee on 7 October 2010.
A total of 320 patients were treated during the course of the study, and data from 239 of them were included in the analysis. In all, 81 patients (25.3%) were excluded on the basis of the criteria listed above. One patient was not included in the analysis because of missing data. The Figure shows the distribution of included and excluded patients in both study phases.
The analysis to determine the prevalence and preoperative predictors of postoperative delirium included all eligible patients admitted to the two surgical wards from March 2011 to June 2012. The study population consisted of 178 persons. The average age was 76.8 years, and 53.9% (n = 96) were women. The preoperative characteristics of the study population are summarized in Table 3.
The analysis to determine the effect of intervention included all 114 eligible patients admitted to the control ward and the intervention ward between September 2011 and June 2012. There were 53 patients in the control group (women: 47.2%; n = 25; mean age: 76.6 years) and 61 patients in the intervention group (women: 63.9%; n = 39; mean age: 77.8 years). The groups’ descriptive statistics are summarized in Table 4.
Intervention
During the intervention phase, on the intervention ward the preoperative screening was used to derive a delirium risk based on general confusion-promoting factors: attention disorders, existing cognitive deficits (demonstrated by MMSE), and the extent and duration of the planned surgery. For example, femoral neck fractures are associated with a very high risk of delirium. The delirium liaison nurse calculated the delirium risk without reference to the ward staff, and created individual intervention plans on the basis of this risk.
Various measures for reorientating the patient, improving the patient’s sleep, promoting cognition, and structuring the daily routine were suggested. In addition, patients were mobilized early, their relatives were involved, and their nutritional intake was improved. Table 2 shows the frequency with which the various intervention measures were actually used. Patients who gave evidence of a confused state after surgery received treatment with adapted interventions based on the Hospital Elder Life Program (HELP) (31) (Box).
Planning and implementing the intervention
The delirium liaison nurse applied an overall score based on the quantitative results of all the assessment instruments used, producing an estimated individual risk of delirium. The resulting individual intervention plan was thus based on the preoperative qualitative screening assessment and on observation of the patient’s behavior.
The observational baseline used was the initial cognitive and nursing status before surgery. The patient’s individual intervention plan was designed so that daily routine could be normalized as far as possible and as quickly as possible. The main focus was on the patient’s independence. The individual interventions chosen were orientated to the patient’s resources and those of his or her environment (relatives, social situation), and also to his or her interests and preferences. In this way, both over-stimulation and under-stimulation were avoided.
The following goals were set:
Reorientation
Improved sleep
Promotion of cognition
Structuring the day
Early mobilization
Involvement of relatives
Improved nutritional and fluid intake.
The intervention plan was individually adapted depending on how well the patient did postoperatively.
Study aims
During the prevalence phase, we investigated the prevalence of postoperative delirium in the patient population described. During the intervention phase, we analyzed whether postoperative delirium occurred statistically less often on the intervention ward than on the control ward. We also intended, if statistically warranted, to analyze which specific interventions help to prevent postoperative delirium.
Statistical analysis
To determine the incidence, we calculated the relative frequency with which patients who received no specific intervention developed postoperative delirium. Preoperative differences between patients with and those without postoperative delirium were tested for statistical significance using the Mann–Whitney U test or the χ 2 test. Odds ratios with 95% confidence intervals or Z-value-based effect sizes were calculated. Intergroup differences with a p-value <0.05 were regarded as statistically significant; those with p<0.2 were regarded as potentially of clinical relevance and were exploratively investigated for independent predictive value as predictors of postoperative delirium, using multivariate logistic regression with forward selection.
The efficacy of intervention was evaluated by, as a first step, testing preoperative differences between patients in the intervention and the control group for statistical significance using the Mann–Whitney U test or the χ 2 test. Differences between groups with a p-value <0.05 were regarded as statistically significant. In assessing the efficacy of the preventive measures, to allow for differences between the intervention group and the control group because of the lack of randomization, a “propensity score” was next calculated on the basis of all variables for which a preoperative difference with a p-value <0.10 was observed. Next, the logistic regression was calculated. In addition to treatment status (intervention: yes/no), the propensity score was included in the model as an additional variable. All tests were carried out as two-sided tests. The SPSS 21 package was used for data analysis.
Key Messages.
Postoperative delirium is a frequent complication in general hospitals.
Postoperative delirium is most often seen in older patients (over the age of 70) who have cognitive deficits.
Risk factors for developing postoperative delirium are increasing age, cognitive deficits, and preoperative infections, especially urinary tract infections.
Concepts for prevention of postoperative delirium already exist, e.g., the Hospital Elder Life Program (HELP).
Employing nursing staff with special training in geriatric psychiatry (“delirium liaison nurses”) could help to prevent postoperative delirium in older patients.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Torsten Kratz has received lecture fees from Janssen-Cilag, Pfizer, and Lilly.
Albert Diefenbacher has received lecture fees from Janssen-Cilag.
Manuel Heinrich and Eckehard Schlauß declare that no conflict of interest exists.
References
- 1.Dilling H, Mombour W, Schmidt MH. Klinisch-diagnostische Leitlinien nach Kapitel V (F) der ICD-10. 9th edition. Bern: Huber Verlag; 2014. Klassifikation psychischer Krankheiten. [Google Scholar]
- 2.Saß H, Wittchen H, Zaudig M. Göttingen: Hogrefe Verlag; 2000. Diagnostisches und Statistisches Manual psychiatrischer Störungen DSM-IV 3rd edition. [Google Scholar]
- 3.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5:210–220. doi: 10.1038/nrneurol.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickel H, Gradinger R, Kochs E, Förstl H. High risk of cognitive and functional decline after postoperative delirium: a three year prospective study. Dement Geriatr Cogn Disord. 2008;26:26–31. doi: 10.1159/000140804. [DOI] [PubMed] [Google Scholar]
- 5.Elie M, Cole MG, Primeau FJ, et al. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13:204–212. doi: 10.1046/j.1525-1497.1998.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons: predictive model and interrelationship with baseline vulnerability. J Am Med Assoc. 1996;275:852–857. [PubMed] [Google Scholar]
- 7.Fick D, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26:30–40. doi: 10.3928/0098-9134-20000101-09. [DOI] [PubMed] [Google Scholar]
- 8.Isaia G, Astengo MA, Tibaldi V, et al. Delirium in elderly home-treated patients: a prospective study with 6-month follow-up. Age. 2009;31:109–117. doi: 10.1007/s11357-009-9086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 10.AGS. www.archcare.org/static/files/pdf/ags-2014-clinical-practice-guideline-for-postop-delirium-in-older-adults.pdf. Clinical practice guideline for postoperative delirium in older adults. (last accessed on 7 January 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gemeinnützige Gesellschaft für soziale Projekte mbh. www.blickwechseldemenz.de/content/e964/e1583/ProjektBlickwechsel_A4.pdf. Projekt Blickwechsel - Nebendiagnose Demenz. (last accessed on 7 January 2015) [Google Scholar]
- 12.Caritasverband für die Diözese Münster e.V. www.caritas-muenster.de/service/dokumentationprojekte/demenzleben/demenzleben. Demenzleben. Gemeinsam für ein besseres Leben mit Demenz. (last accessed on 7 January 2015) [Google Scholar]
- 13.Gurlit S, Thiesemann R, Wolff B, Brommer J, Gogol M. Caring for people with dementia in general hospitals: an education curriculum from the Alzheimer’s Society of Lower Saxony. Z Gerontol Geriatr. 2013;46:222–225. doi: 10.1007/s00391-013-0479-7. [DOI] [PubMed] [Google Scholar]
- 14.Kirchen-Peters S, Diefenbacher A. Gerontopsychiatrische Konsiliar- und Liaisondienste - eine Antwort auf die Herausforderung Demenz? Z Gerontol Geriatr. 2014;47:595–604. doi: 10.1007/s00391-013-0561-1. [DOI] [PubMed] [Google Scholar]
- 15.Hibbeler B. Stationäre Behandlung: Der alte Patient wird zum Normalfall. Dtsch Arztebl. 2013;110:1036–1037. [Google Scholar]
- 16.Hibbeler B. Krankenhaus: OP gelungen, Patient Pflegefall. Dtsch Arztebl. 2014;111 [Google Scholar]
- 17.Kratz T. Delir bei Demenz. Z Gerontol Geriat. 2007;40:96–103. doi: 10.1007/s00391-007-0435-5. [DOI] [PubMed] [Google Scholar]
- 18.Kratz T, Diefenbacher A. Gerontopsychiatric consultation-liaison psychiatry. Z Psychiatr Psychol Psychother. 2008;56:39–45. [Google Scholar]
- 19.Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the hospital elder life program in a community hospital. Psychosomatics. 2013;54:219–226. doi: 10.1016/j.psym.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Hewer W, Rössler W. 2nd edition. München: Urban & Fischer Verlag/Elsevier GmbH; 2007. Akute psychische Erkrankungen - Management und Therapie. [Google Scholar]
- 21.Lorenzl S, Füsgen I, Noachtar S. Acute confusional states in the elderly—diagnosis and treatment. Dtsch Arztebl Int. 2012;109:391–400. doi: 10.3238/arztebl.2012.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. „Mini-Mental State“. A practical method for grading the state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe AW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 24.Hestermann U, Backenstrass M, Gekle I, et al. Validation of a German version of the confusion assessment method for delirium detection in a sample of acute geriatric patients with a high prevalence of dementia. Psychopathology. 2009;42:270–276. doi: 10.1159/000224151. [DOI] [PubMed] [Google Scholar]
- 25.Trzepacz PT, Dinesh Mittal, Torres R, Kanary K, Norton J, Jimmerson N. Validation of the delirium rating scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel R, Brunner C, Ermini-Fünfschilling D, et al. A new behavioral assessment scale for geriatric out- and in-patients: the NOSGER (Nurses’ Observation Scale for Geriatric Patients) J Am Geriatr Soc. 1991;39:339–347. doi: 10.1111/j.1532-5415.1991.tb02897.x. [DOI] [PubMed] [Google Scholar]
- 27.Mahoney FI, Barthel DW. Functional evaluation: the Barthel-Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 28.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 29.Feil N. Validation therapy. Geriatr Nurs. 1992;13:129–133. doi: 10.1016/s0197-4572(07)81021-4. [DOI] [PubMed] [Google Scholar]
- 30.Feil N, Altman R. Validation theory and the myth of the therapeutic lie. Am J Alzheimers Dis Other Demen. 2004;19:77–78. doi: 10.1177/153331750401900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strijbos MJ, Steunenberg B, van der Mast RC, Inouye SK, Schurmans MJ. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost-effectiveness in Dutch health care. BMC Geriatr. 2013;13 doi: 10.1186/1471-2318-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer P, Assem-Hilger E. Delir/Verwirrtheitszustand: Lehrbuch der Gerontopsychiatrie und -psychotherapie. In: Förstl H, editor. Grundlagen-Klinik-Therapie. Stuttgart: Thieme; 2003. pp. 394–440. [Google Scholar]
- 33.Margiotta A, Bianchetti, Ranieri P, Trabucchi M. Clinical characteristics and risk factors of delirium in demented and not demented elderly medical inpatients. J Nutr Health Aging. 2006;10:535–539. [PubMed] [Google Scholar]
- 34.Andrews L, Silva SG, Kaplan S, Zimbro K. Delirium monitoring and patient outcomes in a general intensive care unit. Am J Crit Care. 2015;24:48–56. doi: 10.4037/ajcc2015740. [DOI] [PubMed] [Google Scholar]
- 35.Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42:721–727. doi: 10.1093/ageing/aft120. [DOI] [PubMed] [Google Scholar]
- 36.Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49:523–532. doi: 10.1046/j.1532-5415.2001.49109.x. [DOI] [PubMed] [Google Scholar]
- 37.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175:512–520. doi: 10.1001/jamainternmed.2014.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teslyar P, Stock VM, Wilk CM, Camsari U, Ehrenreich MJ, Himelhoch S. Prophylaxis with antipsychotic medication reduces the risk of post-operative delirium in elderly patients: a meta-analysis. Psychosomatics. 2013;54:124–131. doi: 10.1016/j.psym.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Gilmore ML, Wolfe DL. Antipsychotic prophylaxis in surgical patients modestly decreases delirium incidence—but not duration—in high-incidence samples: ameta-analysis. Gen Hosp Psychiatry. 2013;35:370–375. doi: 10.1016/j.genhosppsych.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 40.National Institute for Health and Care Excellence. www.nice.org.uk/guidance/cg103/resources/guidance-delirium-pdf. Delirium. Diagnosis, prevention and management. NICE clinical guideline 103. (last accessed on 6 January 2015) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Prospective Intervention With Psychogeriatric Liaison on Surgical Wards in a General Hospital
Torsten Kratz, Manuel Heinrich, Eckehard Schlauß, Albert Diefenbacher
Materials and methods
Study design
This study was carried out on two general surgical wards of a hospital providing standard care. The Evangelische Krankenhaus Königin Elisabeth Herzberge (KEH) is an academic teaching hospital affiliated with? the Charité Faculty of Medicine at the University of Berlin, Germany. One ward was designated as the intervention ward, the other as the control ward. Two wards were chosen that treated similar patients with general, abdominal, and trauma surgery. However, the nursing and medical teams carrying out the treatment were different. No orthopedic procedures were performed. The patients were demographically similar. The original intention was to admit patients for general or abdominal surgery to the control ward and patients undergoing general or trauma surgery to the intervention ward, but this proved impossible to maintain consistently in the daily routine of patient care in a general hospital, and for this reason there was a mixture of surgical procedures in both wards at the time of the study.
There was no difference between groups in terms of the anesthesia used (local or regional vs. general). The duration of anesthesia during the procedures was up to 20% longer in about 10% of the patients in the control ward compared to the intervention ward. The incidence of postoperative treatment in an intensive care unit was similar in both groups.
Most patients were admitted to the wards after referral by their family doctor or a primary care surgeon, but some were emergency admissions or had been transferred from another ward in the same hospital. Admission to both wards—irrespective of indication or other factors—was according to bed availability.
The ward teams were made up of surgeons, surgical nursing staff, physiotherapists, and social workers. The two wards were similar in terms of numbers of surgeons and nurses per number of beds. The control ward had 16 beds under the care of 7.1 nurses and 6 physicians. Duration of stay was 5.5 days, with the ward at 90.5% capacity. The intervention ward had 25 beds under the care of 10.7 nurses and 8 physicians. Duration of stay was 5.1 days, with the ward at 86.5% capacity. There was no special training in geriatrics or geriatric psychiatry on either ward.
The first phase of the study (prevalence phase; March to August 2011) recorded on both wards the percentage of patients aged over 70 years who already showed cognitive impairment and confusion preoperatively. Patients’ socioeconomic data, diagnoses, physical diseases, and preoperative medication were documented. In addition, the following investigations were carried out and data recorded pre- and postoperatively:
Mini–Mental State Examination (MMSE) (22)
Activities of Daily Living (ADL) (23)
Confusion Assessment Method (CAM) (24)
Delirium Rating Scale (DRS) (25)
Nurses’ Observation Scale for Geriatric Patients (NOSGER scale) (26)
Barthel Index (27)
Montgomery–Åsberg Depression Rating Scale (MADRS) (28)
Body mass index (BMI)
Hours of sleep (Table 1).
In the subsequent intervention phase, which lasted 10 months (September 2011 to June 2012), one of the wards became the interventional ward, the other the control ward.
On the intervention ward, pre- and postoperative interventions were carried out by the geriatric psychiatric liaison nurses (delirium liaison nurses) in the areas of early mobilization, improved sensory stimulation, improved nutritional and fluid intake, non-drug improvement of sleep, and cognitive activation and validation, individualized to the needs of the patient (Table 2).
Geriatric psychiatric liaison nurse (delirium liaison nurse)
The delirium liaison nurse is a nurse with a specialist qualification in geriatric nursing. The nurse is experienced in dealing with older patients who are confused or are cognitively impaired on a geriatric psychiatric ward. The delirium liaison nurse is trained in dealing with abnormal behavior on the part of older persons with cognitive deficits and has knowledge of various intervention techniques such as the Feil validation method (29, 30). Before the start of the study, the delirium liaison nurse had already been advising the nursing staff on surgical and medical wards, at their request, on how to deal with postoperative delirium or disturbed behavior in patients with dementia (liaison nursing).
In preparation for the study, the delirium liaison nurse was trained in carrying out the CAM, MADRS, and MMSE screening procedures. The delirium liaison nurse was already familiar with the Barthel Index and NOSGER as standard instruments of care in everyday use. On average, the delirium liaison nurse needed 45 minutes for the preoperative assessment of each patient.
The following interventions were carried out:
Reorientation
Improved sleep
Cognition support
Structuring the daily routine
Rapid mobilization
Inclusion of relatives
Improved nutritional and fluid intake.
Since these measures are the same as those used in normal patient-centered care on a geriatric psychiatric ward, no additional training of the liaison nurse was needed.
Study participants
The study included all patients admitted to the two surgical wards between March 2011 and June 2012 who fulfilled the following criteria:
Age over 70 years
No clinically obvious signs of delirium
Able to understand the study and give consent to participating.
The following were criteria for exclusion of patients from the study:
Advanced dementia and inability to give consent
Severe delirium preoperatively
End-stage disease
Refusal to participate in the study.
The study design was approved by the hospital ethics committee on 7 October 2010.
A total of 320 patients were treated during the course of the study, and data from 239 of them were included in the analysis. In all, 81 patients (25.3%) were excluded on the basis of the criteria listed above. One patient was not included in the analysis because of missing data. The Figure shows the distribution of included and excluded patients in both study phases.
The analysis to determine the prevalence and preoperative predictors of postoperative delirium included all eligible patients admitted to the two surgical wards from March 2011 to June 2012. The study population consisted of 178 persons. The average age was 76.8 years, and 53.9% (n = 96) were women. The preoperative characteristics of the study population are summarized in Table 3.
The analysis to determine the effect of intervention included all 114 eligible patients admitted to the control ward and the intervention ward between September 2011 and June 2012. There were 53 patients in the control group (women: 47.2%; n = 25; mean age: 76.6 years) and 61 patients in the intervention group (women: 63.9%; n = 39; mean age: 77.8 years). The groups’ descriptive statistics are summarized in Table 4.
Intervention
During the intervention phase, on the intervention ward the preoperative screening was used to derive a delirium risk based on general confusion-promoting factors: attention disorders, existing cognitive deficits (demonstrated by MMSE), and the extent and duration of the planned surgery. For example, femoral neck fractures are associated with a very high risk of delirium. The delirium liaison nurse calculated the delirium risk without reference to the ward staff, and created individual intervention plans on the basis of this risk.
Various measures for reorientating the patient, improving the patient’s sleep, promoting cognition, and structuring the daily routine were suggested. In addition, patients were mobilized early, their relatives were involved, and their nutritional intake was improved. Table 2 shows the frequency with which the various intervention measures were actually used. Patients who gave evidence of a confused state after surgery received treatment with adapted interventions based on the Hospital Elder Life Program (HELP) (31) (Box).
Planning and implementing the intervention
The delirium liaison nurse applied an overall score based on the quantitative results of all the assessment instruments used, producing an estimated individual risk of delirium. The resulting individual intervention plan was thus based on the preoperative qualitative screening assessment and on observation of the patient’s behavior.
The observational baseline used was the initial cognitive and nursing status before surgery. The patient’s individual intervention plan was designed so that daily routine could be normalized as far as possible and as quickly as possible. The main focus was on the patient’s independence. The individual interventions chosen were orientated to the patient’s resources and those of his or her environment (relatives, social situation), and also to his or her interests and preferences. In this way, both over-stimulation and under-stimulation were avoided.
The following goals were set:
Reorientation
Improved sleep
Promotion of cognition
Structuring the day
Early mobilization
Involvement of relatives
Improved nutritional and fluid intake.
The intervention plan was individually adapted depending on how well the patient did postoperatively.
Study aims
During the prevalence phase, we investigated the prevalence of postoperative delirium in the patient population described. During the intervention phase, we analyzed whether postoperative delirium occurred statistically less often on the intervention ward than on the control ward. We also intended, if statistically warranted, to analyze which specific interventions help to prevent postoperative delirium.
Statistical analysis
To determine the incidence, we calculated the relative frequency with which patients who received no specific intervention developed postoperative delirium. Preoperative differences between patients with and those without postoperative delirium were tested for statistical significance using the Mann–Whitney U test or the χ 2 test. Odds ratios with 95% confidence intervals or Z-value-based effect sizes were calculated. Intergroup differences with a p-value <0.05 were regarded as statistically significant; those with p<0.2 were regarded as potentially of clinical relevance and were exploratively investigated for independent predictive value as predictors of postoperative delirium, using multivariate logistic regression with forward selection.
The efficacy of intervention was evaluated by, as a first step, testing preoperative differences between patients in the intervention and the control group for statistical significance using the Mann–Whitney U test or the χ 2 test. Differences between groups with a p-value <0.05 were regarded as statistically significant. In assessing the efficacy of the preventive measures, to allow for differences between the intervention group and the control group because of the lack of randomization, a “propensity score” was next calculated on the basis of all variables for which a preoperative difference with a p-value <0.10 was observed. Next, the logistic regression was calculated. In addition to treatment status (intervention: yes/no), the propensity score was included in the model as an additional variable. All tests were carried out as two-sided tests. The SPSS 21 package was used for data analysis.