Abstract
AMP-activated protein kinase has been shown to be a key regulator of energy homeostasis; it has also been identified as a tumor suppressor and is required for correct cell division and chromosome segregation during mitosis. The enzyme is a heterotrimer, with each subunit having more than one isoform, each encoded by a separate gene (two α, two β and three γ isoforms). In human endothelial cells, the activated kinase subunit of AMPK in the cytokinetic apparatus is α2, the minority α subunit, which co-localizes with β2 and γ2. This is the first demonstration of a trimeric complex of AMPK containing the γ2 regulatory subunit becoming selectively activated and being linked to mitotic processes. We also show that α1 and γ1, the predominant AMPK subunits, are almost exclusively localized in the cytoskeleton, while α2 and γ2 are present in all subcellular fractions, including the nuclei. These data suggest that pharmacological interventions targeted to specific AMPK subunit isoforms have the potential to modify selective functions of AMPK.
Keywords: AMP-activated protein kinase, isoforms, cytokinesis, subcellular localization, phosphorylation
Introduction
AMP-activated protein kinase (AMPK) is an evolutionarily conserved sensor and regulator of cellular energy balance (reviewed in ref. 1). It is widely accepted that AMPK exists as an αβγ heterotrimer. Phosphorylation of the catalytic α subunit by LKB1 or Ca2+-calmodulin-dependent protein kinase kinase β results in activation of AMPK. The β subunit binds glycogen and acts as a scaffold for the other subunits.2 Upon binding AMP when the AMP/ATP ratio increases, the regulatory γ subunit allosterically activates the kinase further.
Each AMPK subunit has more than one isoform, and each is encoded by a separate gene (α1, α2; β1, β2; γ1, γ2, γ3). Given the number of subunit isoforms, there are 12 different possible combinations of AMPK trimers. Evidence is emerging that particular trimeric combinations are preferentially activated and mediate specific roles. For example, α2/β2/γ3 is selectively activated during exercise in human skeletal muscle, and this complex is likely responsible for phosphorylation of acetyl-coA carboxylase.3 This may be the result of the distinct subcellular localization of this complex, and differential localization may also explain the other reported selective activations of α2-AMPK or β1-AMPK complexes.3-5 AMPK containing α2 was reported to have greater AMP dependence than α1-AMPK, and large proportion of α2, but not α1, was shown to be in the nucleus.6 There is some evidence that subcellular localization of AMPK may be dependent on the regulatory subunit, as γ1-containing complexes have been shown to be directed to the Z-line in differentiated myotubes via the binding of the cytoskeletal protein plectin.7 However, none of the other γ isoforms have yet been localized.
The expression profile of AMPK subunit isoforms has been investigated.8 Based on co-immunoprecipitation and subsequent activity measurements, the γ1 isoform seems to be the major regulatory subunit in all cells; γ3 is largely specific to skeletal muscle. Although γ2 is not the major regulatory subunit of AMPK, it is also widely expressed, and the allosteric activation of complexes containing γ2 is higher than that of γ1-AMPK.9 Mutations in PRKAG2, which encodes the γ2 subunit, cause hypertrophic cardiomyopathy with conduction disease;10 these mutations are exclusively found in the nucleotide-binding domains, and some of them directly involved in binding nucleotides, AMP or ATP.11
The regulatory role of AMPK in cellular energy homeostasis is not just the direct modulation of catabolic and anabolic processes; it also functions as a tumor suppressor via the inhibition of cell division, and it is implicated in the maintenance of cell polarity.1 AMPK activates myosin by phosphorylating the non-muscle myosin regulatory light chain,12 and non-muscle myosin II has been shown to be directly involved in karyokinesis.13 Activated AMPK, the phosphorylated form of the α subunits (P-α), was shown to be in transient association with several mitotic structures, including centrosomes, spindle poles, the central spindle midzone and the midbody during mitosis and cytokinesis.14 Moreover, during the G1/S-to-M-phase transition, AMPK activation resembles strikingly that of well-characterized “chromosomal passenger” proteins, such as Aurora B, INCENP or histone H3,15 and, therefore, the tumor suppressive activity of AMPK may manifest in the mitotic/cytokinetic phase of the cell cycle.16 AMPK has also been shown to regulate meiotic maturation of mouse oocytes17,18 and has been reported to activate directly stress-promoted transcription by phosphorylating histone H2B.19
In this paper, we have used isoform-specific antibodies to investigate AMPK subcellular localization by western blotting and immunofluorescence. We report that the activated AMPK complex linked to the mitotic apparatus is α2/β2/γ2, and we provide further evidence for the compartmentalization of different AMPK trimers, most likely directed by the regulatory γ subunit.
Results
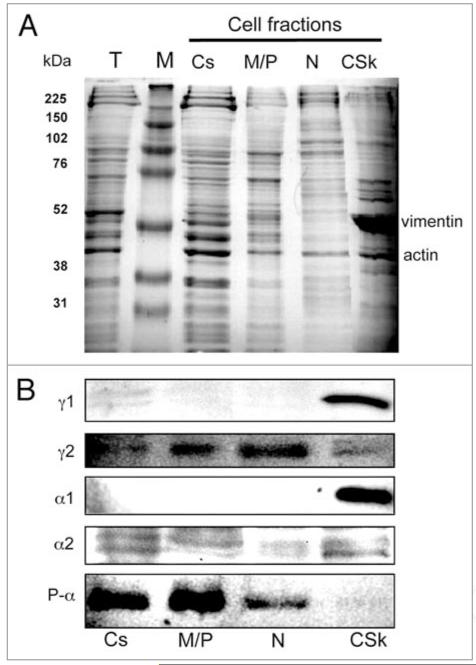
Cytosolic, membrane/particulate, nuclear and cytoskeletal fractions of HUVECs were prepared by sequential detergent extraction.20 The overall protein composition was analyzed by SDS-PAGE, which revealed the expected presence of vimentin (54 kDa) as a clear marker in the cytoskeleton (Fig. 1A). Western blots of the separated proteins of each fraction were probed with antibodies specific to AMPK subunit isoforms (Fig. 1B). The α1 subunit, which accounts for more than 80% of the total α protein in HUVEC,21 was exclusively detected in the cytoskeleton fraction by western blotting. The less abundant α subunit, α2, was detectable as a doublet at low levels in western blots of each fraction. The activated kinase subunit (P-α) was present predominantly in the cytosolic and the membrane/particulate fractions, with a lesser amount detected in the nuclear fraction. The γ1 subunit is the major regulatory subunit in HUVEC21 and was detected in the cytoskeleton. The minority regulatory subunit, γ2, was present in all fractions.
Figure 1.
Subcellular distribution of AMPK subunit isoforms. (A) SDS-PAGE was stained with Coomassie brilliant blue. (B) Western blots with antibodies α1 and α2 (Santa Cruz); p-α and γ1 (Cell Signaling); γ2 (Atlas antibodies). Approximately 107 cells used for cell fractionation and equal volumes of the fractions were loaded on the SDS-PAGE. T, total cell extract; M, molecular weight markers; CS, cytosol; MP, membrane/particulate; N, nuclear; CSk, cytoskeletal fractions.
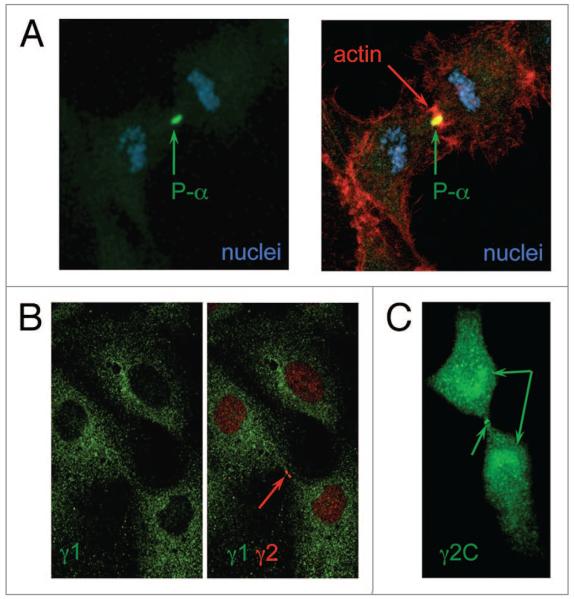
To gain information on the specific localization of AMPK subunits in HUVEC, we used confocal microscopy to examine cells stained with subunit-specific antibodies. Of particular interest was the localization of activated AMPK complexes during the cell cycle, as P-α has recently been shown to associate transiently in human cancer-derived epithelial cells with several mitotic structures including centrosomes, spindle poles, the central spindle midzone and the midbody during mitosis and cytokinesis.14 We found a similar pattern in dividing HUVEC and, in particular, detected strong staining with P-α antibody of the midbody and cleavage furrow (co-localization with actin) (Fig. 2A). As there is some evidence that the γ subunit may direct AMPK subcellular localization,7 we examined the distribution of the γ isoforms in these cells. In spite of the recent report of the nuclear presence of γ1,22 we did not observe γ1 in the nuclei, nor did we find co-localization with the activated AMPK during mitosis. In general, γ1 staining was dispersed throughout the cytoplasm (Fig. 2B). However, strong staining of the midbody, similar to P-α, was also shown for γ2, suggesting that activated AMPK at that site contains this regulatory subunit (Fig. 2B). The staining of the midbody was achieved with two different γ2 antibodies: one was raised against a short C-terminal peptide, γ2C (Fig. 2C); the other antibody was raised against a longer mid-segment of the protein (Fig. 2B). Specific co-localization of P-α and γ2 was not possible, as the P-α and both γ2 antibodies were raised in rabbit. Both γ2 antibodies also stain the nuclei (but not the nucleoli) and the condensed chromosomes (Figs. 2B, 3A and B), and we found intense staining of γ2 around the separating chromosomes (Fig. 2B). The staining around the condensed and segregating chromosomes suggests that AMPK containing γ2 may be associated with the kinetochore. Interestingly, no P-α was detected around the segregating chromosomes during mitosis.
Figure 2.
Co-localization of activated AMPK with γ2 in dividing HUVEC. Immunofluorescence localization was performed with antibodies raised against p-α, γ2 and a γ2 C-terminal peptide. Actin was stained with phalloidin-TRITC. The different subunits are labeled in appropriately colored text in each part.
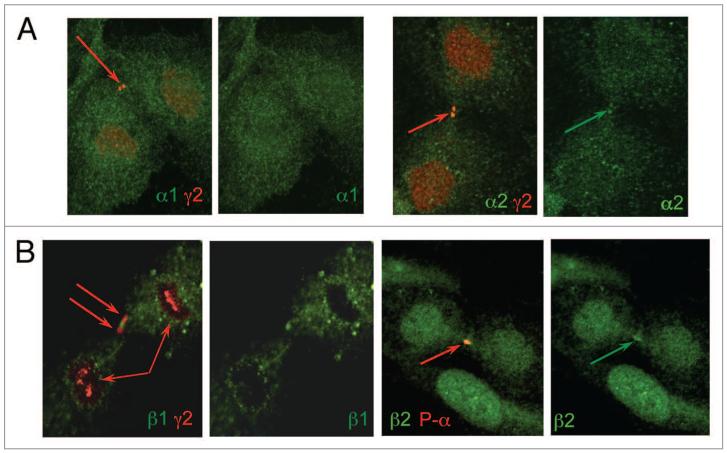
Figure 3.
Localization of AMPK subunits in HUVECs. (A) Localization of α1 and α2 along with γ2. (B) Localization of β1 and β2 along with γ2 and phospho-α. Immunofluorescence localization was performed using antibodies raised against p-α, γ2 and α1/α2, β1/β2. The different subunits are labeled in appropriately colored text in each part.
In order to determine the catalytic subunit present along with γ2 at the midbody, HUVEC were stained with antibodies to the α isoforms. The α1 subunit was found to be dispersed throughout the cell with little apparent nuclear staining (no co-localization with nuclear γ2) and did not concentrate at the midbody (Fig. 3A). In contrast, distinct midbody staining was shown for α2, with distinct co-localization with γ2 at this position (Fig. 3A); some α2 nuclear staining was also found in non-dividing cells (data not shown). Attempts to use the α antibodies with the P-α antibody were unsuccessful, presumably due to strong binding of the P-α antibody hindering the other α antibody.
The distribution of the β subunits in dividing HUVEC contrasted markedly. β1 was shown to be clearly extranuclear, with typically a granular appearance; in some instances, the staining coincided with the midbody, although did not precisely colocalize with γ2 (Fig. 3B). The β2 antibody was found to give nuclear and midbody staining, with the latter corresponding to the P-α localization. We conclude that the subunit composition of activated AMPK at the midbody is α2/β2/γ2.
Discussion
The specific localization of activated AMPK with several mitotic structures in human cancer-derived epithelial cells has recently been reported in reference 14. We have confirmed this in HUVEC and identified the subunit composition of activated AMPK at the midbody to be α2/β2/γ2. This is the first demonstration of AMPK containing the γ2 regulatory subunit becoming selectively activated and being linked to a specific cellular process. As non-muscle myosin II has been identified as an AMPK substrate and is known to be involved in scission during telophase, we suggest that one of the functions of the α2/β2/γ2 AMPK complex may be to regulate non-muscle myosin-II during cytokinesis directly or indirectly.12,13 In a recent publication, it was shown that AMPK directly phosphorylates several proteins which coordinate different aspects of mitosis.23 By phosphorylating protein phosphatase 1 regulatory subunit 12C and p21-activated protein kinase, AMPK indirectly regulates myosin regulatory light chain phosphorylation and, therefore, cytoskeleton rearrangement. This study was performed by overexpressing the α2 catalytic subunit co-expressed with β1/γ1; however, the modified α2 can form trimers with endogenous β2/γ2, which can be directed to the mitotic apparatus. On the other hand, NUAK2, a member of the AMPK family, also plays an important role in regulating the cytoskeleton and influencing cell proliferation and cell motility; aberration of both of these can result in tumorigenesis, and there may an interaction between the two pathways.24
AMPK activation can occur selectively in the nucleus. Polo-like kinase 1, the key regulator of cell cycle events, has been implicated in the nuclear activation of AMPK during mitosis.25 Furthermore, substantial P-α staining was detected first in the nuclei after radiation treatment in tumor cells, and the increase in cytoplasmic staining only occurred subsequently.26 The nuclear activation of AMPK by ionizing radiation was independent of LKB1 but, instead, dependent on ATM, the DNA damage sensor.
Given the extranuclear localization of the γ1 subunit, it is likely that other nuclear processes associated with AMPK (e.g., the activation stress-promoted transcription19) may also involve complexes containing γ2. In addition, γ2 being in the vicinity of the segregating chromosomes may indicate that γ2-containing AMPK is required for the formation of kinetochores. AMPK involvement in faithful chromosome segregation has been reported in reference 14. The kinase subunit in this case might be α1, as this catalytic subunit was shown to associate with the condensed chromatin during meiotic maturation in mouse oocytes,17 and activated α1 also appears to translocate into the nucleus during the development of Huntington disease.27 We only detected α1 in the cytoskeletal fraction of HUVECs, while P-α and α2 both were present in the nuclear fraction (Fig. 1B). An earlier study showed that a significant proportion of α2 but not α1 was found in the nucleus and also found that AMP activated the α2-containing complexes to a greater extent than α1-AMPK.6 Our current work suggests these α2-containing complexes would have γ2 as their regulatory subunit and, as AMPK containing γ2 is more sensitive to changes of AMP concentrations,9 this is the likely explanation of their greater activation.
It is unclear whether α2/β2/γ2 AMPK shuttles between the cytoplasm and the nucleus. Nuclear entry of α2/β2/γ2 complexes may be directed by α2, which has a nuclear localization signal,28 and/or by γ2, which, unlike the γ1 isoform, has both a strong atypical bipartite nuclear localization signal (9-KKK KDV SSP GGS GGK KNA SQK RR-31) and a nuclear exit signal (546-LSD ILQ ALI L-556). If some γ2 is permanently in the nucleus (Figs. 2 and 3), it could plausibly act by recruiting activated and translocated α2/β2 subunits into distinct positions at different stages of mitosis. Only a weak interaction was found between the β and the γ subunits, whereas the binding between α-β was reported to be very strong.29 It has been reported that in human skeletal muscle biopsies, γ2 is present but does not appear to participate in any AMPK complex.30 Therefore the low contributions (less than 10%) of γ2-containing complexes to the overall AMPK pool8 may be the result of γ2’s transient participation in an AMPK complex; hence, the majority of γ2 may be not in complex and its cellular presence underestimated.
Based on our observations, we think that AMPK activation and function depends on its subcellular localization, and that it may be largely dependent on the regulatory γ subunit in the complex. We only detected γ1 in the cytoskeleton, and the majority of α1 is also in this fraction (Fig. 1B). Deletion of either α1 or γ1 causes premature death of erythrocytes in mouse, and that seems to be the only phenotype.31 AMPK has been shown to regulate cell structure,12 and the loss of either α1 or γ1 is detrimental after the erythrocytes become enucleated. However, selective activation that depends on other AMPK subunits has also been reported. The α1-containing AMPK is preferentially phosphorylated in muscle cells upon metformin treatment, and fatty acid metabolism is inhibited, while AICAR preferentially activates α2-containing complexes.32 These observations would suggest that AMPK activation is dependent on the kinase subunit. On the other hand, that may be simply the consequence of locating the α1-AMPK or α2-AMPK to certain positions in the cell and predisposing them for selective activation.
The identification of a specific AMPK trimer that is specifically localized and activated during cytokinesis raises the question whether this complex may be selectively targeted pharmacologically to specifically modulate mitosis. While thienopyridone compounds target β1-containing complexes,33 most of the commonly used AMPK activators or inhibitors are not selective. Metformin, a potent activator of AMPK originally used to treat hyperglycemia and type 2 diabetes, also has anticancer properties; the latter is partly due to impaired cytokinesis.16 The other axis of cancer treatment is the AMPK-FoxO3A pathway, also activated by metformin; AMPK via the transcription factor FoxO3A modulates not only cell metabolism, but also stress resistance, autophagy and cell death.34 There are a number of possible targets of AMPK. If the γ subunits are the chief mediator of AMPK localization, and, as a consequence, the compartmentalized complex is assigned to a specific signaling pathway, targeting the γ subunits could be a profitable direction for new, selective drug design for the manipulation of specific AMPK functions.
Experimental Procedures
Cell lines
Primary HUVEC (Invitrogen) were cultured in Medium 200 (Invitrogen) supplemented with Low-Serum Growth Supplement (Invitrogen). Cells were used between passages 2–5.
Reagents
The FractionPREP™ cell fractionation kit was purchased from BioViosion and used according to manufacturer’s instructions.
Primary antibodies used were as follows: Rabbit mAB anti-P-AMPKαThr172 (clone 40H9) and polyclonal rabbit AMPKγ1 antibody (Cell Signaling Technology); rabbit polyclonal γ2 anti-body—anti-PRKAG2 antibody (Atlas Antibodies); rabbit polyclonal C-terminal γ2 antibody (gift from D. Carling); goat polyclonal AMPKγ1 (T-20) (sc-19138), goat polyclonal AMPKα1 (C-20) (sc-19128), polyclonal AMPKα2 (A-20) (sc-19129), polyclonal AMPKβ1 (N-18) (sc-19132) and polyclonal AMPKβ2 (E-20) (sc-19136) were all purchased from Santa Cruz Biotechnology, Inc.
For fluorescence detection of immunostaining, donkey anti-goat Alexa Fluor 488 and donkey anti-Rabbit Alexa Fluor 594 (both Invitrogen) were used. The actin network was visualized by Phalloidin-Tetramethylrhodamine B isothiocyanate (Phalloidin-TRITC) (Sigma) staining.
Immunofluorescence staining and confocal imaging
HUVEC were grown on poly-D-Lysine-coated coverslips in 12-well plates. Cells were fixed in 4% PFA, permeabilized in 0.2% Triton® X-100, blocked with 3% BSA in PBS then incubated with the appropriate primary and secondary antibodies.
Cells were mounted with SlowFade® Gold antifade reagent with DAPI (Invitrogen) and imaging was performed with a Leica TCS SP5 confocal laser-scanning microscope with a 63×, 1.4NA objective.
Acknowledgments
This work was funded by the British Heart Foundation
Footnotes
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
References
- 1.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. http://dx.doi.org/10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 2.Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, et al. AMPKbeta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–71. doi: 10.1016/s0960-9822(03)00292-6. http://dx.doi.org/10.1016/S0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 3.Birk JB, Wojtaszewski JF. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577:1021–32. doi: 10.1113/jphysiol.2006.120972. http://dx.doi.org/10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGee SL, Howlett KF, Starkie RL, Cameron-Smith D, Kemp BE, Hargreaves M. Exercise increases nuclear AMPKalpha2 in human skeletal muscle. Diabetes. 2003;52:926–8. doi: 10.2337/diabetes.52.4.926. http://dx.doi.org/10.2337/diabetes.52.4.926. [DOI] [PubMed] [Google Scholar]
- 5.Treebak JT, Birk JB, Hansen BF, Olsen GS, Wojtaszewski JF. A-769662 activates AMPK beta1-containing complexes but induces glucose uptake through a PI3-kinase-dependent pathway in mouse skeletal muscle. Am J Physiol Cell Physiol. 2009;297:1041–52. doi: 10.1152/ajpcell.00051.2009. http://dx.doi.org/10.1152/ajpcell.00051.2009. [DOI] [PubMed] [Google Scholar]
- 6.Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, et al. AMP-activated protein kinase: greater AMP dependence and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998;334:177–87. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregor M, Zeöld A, Oehler S, Marobela KA, Fuchs P, Weigel G, et al. Plectin scaffolds recruit energy-controlling AMP-activated protein kinase (AMPK) in differentiated myofibres. J Cell Sci. 2006;119:1864–75. doi: 10.1242/jcs.02891. http://dx.doi.org/10.1242/jcs.02891. [DOI] [PubMed] [Google Scholar]
- 8.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–69. http://dx.doi.org/10.1042/0264-6021:3460659. [PMC free article] [PubMed] [Google Scholar]
- 9.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–84. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, et al. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–20. doi: 10.1093/hmg/10.11.1215. http://dx.doi.org/10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 11.Oakhill JS, Scott JW, Kemp BE. Structure and function of AMP-activated protein kinase. Acta Physiol (Oxf) 2009;196:3–14. doi: 10.1111/j.1748-1716.2009.01977.x. http://dx.doi.org/10.1111/j.1748-16.2009.01977.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–20. doi: 10.1038/nature05828. http://dx.doi.org/10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 13.Ma X, Jana SS, Conti MA, Kawamoto S, Claycomb WC, Adelstein RS. Ablation of nonmuscle myosin II-B and II-C reveals a role for nonmuscle myosin II in cardiac myocyte karyokinesis. Mol Biol Cell. 2010;21:3952–62. doi: 10.1091/mbc.E10-04-0293. http://dx.doi.org/10.1091/mbc.E10-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle. 2009;8:2385–98. doi: 10.4161/cc.8.15.9082. http://dx.doi.org/10.4161/cc.8.15.9082. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez-Martin A, López-Bonet E, Oliveras-Ferraros C, Pérez-Martínez MC, Bernadó L, Menendez JA. Mitotic kinase dynamics of the active form of AMPK (phospho-AMPKalphaThr172) in human cancer cells. Cell Cycle. 2009;8:788–91. doi: 10.4161/cc.8.5.7787. http://dx.doi.org/10.4161/cc.8.5.7787. [DOI] [PubMed] [Google Scholar]
- 16.Vazquez-Martin A, Oliveras-Ferraros C, Lopez-Bonet E, Menendez JA. AMPK: Evidence for an energy-sensing cytokinetic tumor suppressor. Cell Cycle. 2009;8:3679–83. doi: 10.4161/cc.8.22.9905. http://dx.doi.org/10.4161/cc.8.22.9905. [DOI] [PubMed] [Google Scholar]
- 17.Downs SM, Ya R, Davis CC. Role of AMPK throughout meiotic maturation in the mouse oocyte: evidence for promotion of polar body formation and suppression of premature activation. Mol Reprod Dev. 2010;77:888–99. doi: 10.1002/mrd.21229. http://dx.doi.org/10.1002/mrd.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano A, Kato H, Watanabe T, Min KD, Yamazaki S, Asano Y, et al. AMPK controls the speed of micro-tubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12:583–90. doi: 10.1038/ncb2060. http://dx.doi.org/10.1038/ncb2060. [DOI] [PubMed] [Google Scholar]
- 19.Bungard D, Fuerth BJ, Zeng PY, Faubert B, Maas NL, Viollet B, et al. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science. 2010;329:1201–5. doi: 10.1126/science.1191241. http://dx.doi.org/10.1126/science.1191241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsby ML, Makowski GS, Khairallah EA. Differential detergent fractionation of isolated hepatocytes: biochemical, immunochemical and two-dimensional gel electrophoresis characterization of cytoskeletal and noncytoskeletal compartments. Electrophoresis. 1994;15:265–77. doi: 10.1002/elps.1150150146. http://dx.doi.org/10.1002/elps.1150150146. [DOI] [PubMed] [Google Scholar]
- 21.Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421:163–9. doi: 10.1042/BJ20090613. http://dx.doi.org/10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27:4317–27. doi: 10.1128/MCB.02222-06. http://dx.doi.org/10.1128/MCB.02222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, et al. Chemical Genetic Screen for AMPKα2 Substrates Uncovers a Network of Proteins Involved in Mitosis. Mol Cell. 2011;44:878–92. doi: 10.1016/j.molcel.2011.11.005. http://dx.doi.org/10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Namiki T, Coelho SG, Hearing VJ. NUAK2: an emerging acral melanoma oncogene. Oncotarget. 2011;2:695–704. doi: 10.18632/oncotarget.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Menendez JA. Polo-like kinase 1 regulates activation of AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell Cycle. 2011;10:1295–302. doi: 10.4161/cc.10.8.15342. http://dx.doi.org/10.4161/cc.10.8.15342. [DOI] [PubMed] [Google Scholar]
- 26.Sanli T, Rashid A, Liu C, Harding S, Bristow RG, Cutz JC, et al. Ionizing radiation activates AMP-activated kinase (AMPK): a target for radiosensitization of human cancer cells. Int J Radiat Oncol Biol Phys. 2010;78:221–9. doi: 10.1016/j.ijrobp.2010.03.005. http://dx.doi.org/10.1016/j.ijrobp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Ju TC, Chen HM, Lin JT, Chang CP, Chang WC, Kang JJ, et al. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington’s disease. J Cell Biol. 2011;194:209–27. doi: 10.1083/jcb.201105010. http://dx.doi.org/10.1083/jcb.201105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazgan N, Williams T, Forsberg LJ, Brenman JE. Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase. Mol Biol Cell. 2010;21:3433–42. doi: 10.1091/mbc.E10-04-0347. http://dx.doi.org/10.1091/mbc.E10-04-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townley R, Shapiro L. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science. 2007;315:1726–9. doi: 10.1126/science.1137503. http://dx.doi.org/10.1126/science.1137503. [DOI] [PubMed] [Google Scholar]
- 30.Wojtaszewski JF, Birk JB, Frøsig C, Holten M, Pilegaard H, Dela F. 5′AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. J Physiol. 2005;564:563–73. doi: 10.1113/jphysiol.2005.082669. http://dx.doi.org/10.1113/jphysiol.2005.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foretz M, Hébrard S, Guihard S, Leclerc J, Do Cruzeiro M, Hamard G, et al. The AMPKγ1 subunit plays an essential role in erythrocyte membrane elasticity, and its genetic inactivation induces splenomegaly and anemia. FASEB J. 2011;25:337–47. doi: 10.1096/fj.10-169383. http://dx.doi.org/10.1096/fj.10-169383. [DOI] [PubMed] [Google Scholar]
- 32.Bogachus LD, Turcotte LP. Genetic downregulation of AMPK-alpha isoforms uncovers the mechanism by which metformin decreases FA uptake and oxidation in skeletal muscle cells. Am J Physiol Cell Physiol. 2010;299:1549–61. doi: 10.1152/ajpcell.00279.2010. http://dx.doi.org/10.1152/ajpcell.00279.2010. [DOI] [PubMed] [Google Scholar]
- 33.Scott JW, van Denderen BJ, Jorgensen SB, Honeyman JE, Steinberg GR, Oakhill JS, et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol. 2008;15:1220–30. doi: 10.1016/j.chembiol.2008.10.005. http://dx.doi.org/10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Chiacchiera F, Simone C. The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle. 2010;9:1091–6. doi: 10.4161/cc.9.6.11035. http://dx.doi.org/10.4161/cc.9.6.11035. [DOI] [PubMed] [Google Scholar]