Abstract
Mesenchymal stem cells (MSCs) are known to be capable of suppressing immune responses and offer therapeutic potential for achieving transplantation tolerance. This review will discuss the impacts of MSCs on transplant immunity and focus on the potential role of MSCs in protecting islet grafts from both rejection and autoimmune attack.
Keywords: Mesenchymal stem cells, Islet transplantation, Immunosuppression
Mesenchymal stem cells (MSCs) are a rare subset of cells within the bone marrow (BM) cavity, which serve as a reservoir for the continuous renewal of mesenchymal lineages under physiologic conditions. In addition to their extensive proliferation capacity and ability to differentiate into various kinds of mesodermal tissues, MSCs possess suppressive capabilities that may be exploited to control several subsets of immune cells, including naïve and memory T cells, B cells, dendritic cells, and natural killer cells, and to reduce inflammatory cytokine production (1–3).
It is well established that MSCs can exert immunosuppressive activity on T cells in response to stimulation by polyclonal mitogens, peptide major histocompatibility complexs (MHCs), and alloantigens (4), inhibiting both T-cell proliferation, cytotoxic T-lymphocyte activity and decreasing interferon-γ production. The characteristic of MSC-mediated suppression of T-cell responses is a block in responsiveness to interleukin (IL)-2, and although several soluble factors such as transforming growth factor-β, nitric oxide, indoleamine 2,3-dioxygenase, and heme oxygenase-1 have been implicated in this process, the precise role of many of these remains controversial (5). Furthermore, it has been recently shown that the immunosuppressive capacity of MSCs also seems to involve the cleavage of CD25, the α-chain of IL-2 receptor, from the surface of T cells by matrix metalloproteinase-2 and -9, thereby attenuating T-cell responses to IL-2 (6, 7).
MSCs have been shown to suppress autoreactive T-cell responses in models of autoimmunity such as experimental autoimmune encephalomyelitis (EAE) (8), collagen-induced arthritis (CIA) (9), and autoimmune enteropathy (10) (Table 1). For example, in EAE, a mouse model of multiple sclerosis, intravenous injection of MSCs in affected mice resulted in a profound delay in disease progression due to reduced inflammation and reduced demyelination (8). More recently, MSCs have been noted for their therapeutic benefit in the treatment of type 1 diabetes (T1DM) in nonobese diabetic (NOD) mice. Interestingly, although administration of BALB/c-MSC significantly delayed disease onset and resulted in sustained normoglycemia in almost 40% of animals treated, MSCs from NOD mice themselves were almost ineffective (11). This disparity seems to correlate closely with the in vitro suppressive ability of MSCs from these two strains where BALB/c-MSCs are significantly more effective in inhibiting T-cell proliferation. This study also indicated that the in vivo effects of MSCs in this model seemed to be related to the level of MSC expression of programmed death-ligand 1 (PD-L1), which can provide negative signals to T cells through PD-1, a pathway previously implicated in the development of hypoglycemia in NOD mice.
TABLE 1.
Use of MSCs in autoimmunity and transplantation
| Model | Source of MSCs | MSC treatment | Time of administration | Result | Reference |
|---|---|---|---|---|---|
| Autoimmunity | |||||
| Experimental autoimmune encephalomyelitis | Syngeneic BM-MSC | 1×106 IV, twice | Day 3 and 8 after EAE induction | Ameliorated disease progression | (8) |
| Collagen-induced arthritis | Allogeneic BM-MSC | 5×106, IP | Day 0 or 21 after CIA induction | Delayed and reduced severity of disease | (9) |
| Autoimmune enteropathy | BM-MSC | 3×105, IP | Diseased mice at 4 wk of age | Ameliorated disease progression | (10) |
| T1DM | Allogeneic BM-MSC | 5×105, IV, once a week for 4 wk | Nonobese diabetic mice at 10 wk of age | Delayed onset of diabetes | (11) |
| Alloresponses | |||||
| Skin transplantation | Allogeneic BM-MSC | 20×106/kg, IV | Day 0 | Prolonged survival of skin grafts | (12) |
| Heart transplantation | Semiallogeneic BM-MSC | 0.5×106, intraportal | Day 7 | Protected heart allografts | (13) |
| Islet transplantation | Syngeneic BM-MSC | 4×106, under RC | Day 0 | Prevented rejection of islet grafts | (6) |
T1DM, type 1 diabetes; BM, bone marrow; MSC, mesenchymal stem cell; IV, intravenous injection; IP, intraperitoneal injection; RC, renal capsule.
MSCs may also offer therapeutic opportunities in transplantation by directly targeting alloreactive T cells and thereby blunting the effector arm of the alloresponse. MSCs can suppress alloreactive T-cell responses both in vitro and in vivo. In vivo, MSCs have been shown to be capable of preventing the rejection of skin and heart allografts in animal transplant models (Table 1). In a baboon skin transplant model, a single intravenous administration of donor type MSCs into MHC-mismatched recipients resulted in nonspecific protective effects and prolonged graft survival to 11.8±1.4 days compared with 7.0±0 days in untreated controls (12). In a mouse transplant model, intraportal administration of MSCs extended heart allograft survival from 10 days in untreated controls to a median survival time of 40 days, with 33% of MSC-treated recipients showing long-term tolerance (13). The effectiveness of MSCs for the treatment of graft-versus-host disease (GVHD) in mouse experimental models is controversial, with some groups showing therapeutic efficacy and others observing no effect (14, 15). However, the treatment of patients with refractory acute GVHD after allogenic BM transplantation using haploidentical MSCs from third-party donors has resulted in reliable therapeutic efficacy, illustrating the potential of these populations to control alloreactivity (16). Thus, MSCs may have significant potential in clinical transplantation beyond the scope of BM transplantation.
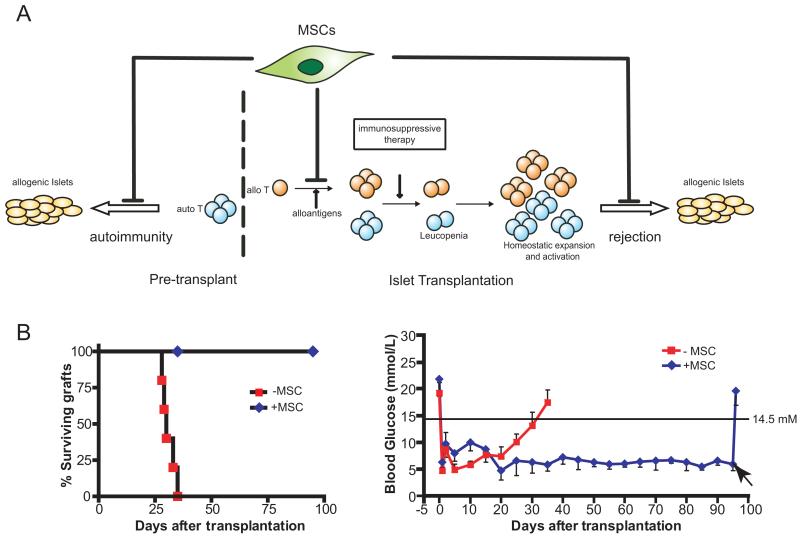
T1DM is one of the most prevalent autoimmune diseases of childhood. Recent predictions suggest an annual incidence of some 70,000 cases worldwide (www.eatlas.idf.org/index5c31.html), and historically, significantly higher frequencies are found in specific regions of the world such as Finland and Sardinia (17). The ultimate effector mechanisms of immune-mediated destruction of islet β-cells are diverse and complex, but an essential early event is the activation of islet cell antigen reactive T cells (Fig. 1A). Islet transplantation using the Edmonton protocol and its derivatives offers an attractive approach to treat certain patients with T1DM, and can lead to normal metabolic control of glucose levels and independence from exogenous insulin (18). However, these protocols do not seem to lead to long-term insulin independence, and most patients require exogenous insulin within 2 years posttransplant (19).
FIGURE 1.
(A) The therapeutic potential of mesenchymal stem cells (MSCs) in diabetic patients with islet transplantation. Diabetic patients mount autoimmune responses that target insulin-producing β cells. The intrinsic immunosuppressive capabilities of MSCs may protect islet grafts from both autoreactive and alloreactive immune responses. (B) In a mouse islet allograft model, MSCs protect allogenic islet grafts transplanted beneath the kidney capsule leading to long-term islet allograft survival whereas non-MSC control mice become profoundly diabetic within 30 to 35 days (left). Continued allograft function leads to stable normoglycemia (mean blood glucose 5–7 mM, right). Removal of the allogenic islets by nephrectomy results in a rapid return to hyperglycemia (arrow).
The failure of the allograft and reappearance of insulin dysfunction in islet transplant recipients can potentially involve both rejection resulting from the activation of alloreactive T cells and a reoccurrence of the original autoimmune disease (Fig. 1A). For example, the breakdown of immunologic tolerance that leads to primary islet insufficiency may result in a cross-reactive memory response against the transplanted islets, resulting in a loss of β-cell mass (20). In addition, it has been shown that leukopenia, which can result from intensive immunosuppressive therapy can favor the generation of islet-reactive T cells, leading to the destruction of islet allografts (21). Lymphopenia can induce homeostatic expansion and activation of remaining populations, particularly memory T cells and these seem to be somewhat refractory to conventional immunosuppressive therapy, potentially leading to enhanced responses to islet grafts (Fig. 1A).
In view of the fact that MSCs have the capacity to control autoimmune responses, it is possible that MSCs may protect transplanted allogeneic islets by negatively regulating persistent T-cell autoimmunity and by controlling the activation and effector function of alloreactive T cells. In addition, MSCs may also suppress the activation and proliferation of B cells and thereby impair the production of destructive autoantibodies and alloantibodies (22) and may prevent differentiation and maturation of dendritic cells (1), thus tipping the balance toward restoring immunologic tolerance in T1DM patients and preventing destruction of the transplanted islets.
To investigate the ability of MSCs to protect islet allografts from rejection, we have recently shown in a life-sustaining mouse islet allograft model that although allogeneic islets are rejected within 30 days, administration of MSCs prevents rejection and leads to long-term normoglycemia (Fig. 1B). Given the fact that MSCs were colocalized at the graft site, the efficacy of MSC treatment is likely related to the immunosuppressive milieu created by MSCs in the vicinity of the islet grafts, especially the local production of immunosuppressive matrix metalloproteinase-2 and -9, that impede the activation and expansion of alloreactive T cells (6). It is important to note that immunodeficient BALB/c mice were used as recipients in this study to examine the impact of MSC on defined effector T-cell populations in isolation from effects on other cell populations, but clearly, it will be necessary to conduct a further, rigorous evaluation in the much more challenging setting of immunocompetent recipients to establish the full potential of MSC in islet transplantation. However, if MSC-based strategies can be translated successfully from animal models to clinical islet transplantation, it may be possible to reduce overall levels of immunosuppression, thereby also reducing the risks associated with leucopenia-induced homeostatic proliferation.
One of the major questions concerning the therapeutic implications of MSCs in solid organ or tissue transplantation is the importance of the origin of MSCs, principally whether donor-, recipient- or third-party-derived MSCs should be used. Although the immunosuppressive capabilities of MSCs seem not to depend on MHC restriction, and both donor and recipient-derived MSCs have a comparable suppressive capacity in vitro, the relative efficacy of MSCs of different origin in terms of allograft protection is not clear. In the clinical setting, donor derived MSCs have been used in phase I and II trials to treat patients with steroid refractory GVHD after allogeneic BM transplantation (23). The advantage of using donor-derived MSCs in BM transplantation is that donor MSCs are relatively easy to obtain from BM aspirates during collection of donor hematopoietic stem cells, and because they are autologous with the BM that reconstitutes the recipient immune system, they are unlikely to be subject to rejection responses. The use of donor-derived rather than recipient-derived MSCs also avoids a potential contamination with malignant cells at the time of MSC collection. Although donor MSCs may have considerable theoretical advantages for the treatment of GVHD, in solid organ or tissue transplantation, particularly when organs or tissues are retrieved from deceased donors, it may be essentially impossible to obtain donor-derived MSCs rapidly enough in relation to the time of transplant for use as a cellular therapy. However, most notable from the results of a recent multicenter study where MSCs were used to treat 55 BM transplant patients with steroid-resistant GVHD was that third-party MSCs seemed to be as effective as human leukocyte antigen identical or haploidentical cells (23). Whether this effect will apply equally to solid organ or tissue transplantation is not clear, and indeed, experimental models seem to have yielded conflicting results. For example, in a rat heart transplantation model, recipient-strain MSCs prolonged graft survival, whereas third-party cells were ineffective (24). In contrast, in a baboon skin graft model, administration of donor MSCs led to the prolonged survival of both donor-specific and third-party allografts (12).
The decision of whether to use donor, recipient, or third-party cells in solid organ or tissue transplantation may ultimately depend on the theoretical benefits and on practicality. For example, if the intention is to use MSCs solely as an immunosuppressive population, then clearly, recipient-derived MSCs might be preferred but the likely difficulties of obtaining sufficient numbers of MSCs from patients with end-stage organ failure suggests that cells from a human leukocyte antigen-typed mesenchymal cell bank may be the most appropriate source. However, the greatest therapeutic effects might be obtained by using MSCs both to modulate immune responses and to induce some form of immunologic tolerance. There is a wealth of evidence from the transplant literature showing that operational tolerance can be induced by challenging the recipient immune system with donor cells in the context of immunocompromise (25) and that frequently, such strategies lead to the development of regulatory T cells. Thus, it might be possible to use donor-derived (or donor matched) MSCs to suppress alloreactive immune responses and drive the development of regulatory cells. Although this approach is attractive on a theoretical basis, a recent study has shown that far from being protolerogenic, MSCs have the capacity to evoke T-cell responses and drive the development of memory T cells (26). This suggests that if donor-derived or donor-compatible MSCs are to be used as a cellular therapy in the clinical setting, it will be necessary to deliver such populations in the context of transient immunosuppression. However, because it is unlikely that MSCs would be used in solid organ or tissue transplantation as a stand-alone therapy, and would be introduced in combination with accepted immunosuppressive regimens, it may indeed be possible to exploit the potential of this unique population of cells without the risk of sensitization.
ACKNOWLEDGMENTS
The authors thank Gang Feng and Karen English (Nuffield Department of Surgery, University of Oxford, Oxford) for expertise in mouse islet allograft models and instructive discussions.
This work was supported by The Wellcome Trust, the European Union through the OPTISTEM Integrated Project, and the Marie Curie Fellowship Programme.
REFERENCES
- 1.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 2.Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 3.Oh JY, Kim MK, Shin MS, et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 4.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 5.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 6.Ding Y, Xu D, Feng G, et al. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58:1797. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brusko TM. Mesenchymal stem cells: A potential border patrol for transplanted islets? Diabetes. 2009;58:1728. doi: 10.2337/db09-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 9.Augello A, Tasso R, Negrini SM, et al. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 10.Parekkadan B, Tilles AW, Yarmush ML. Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells. 2008;26:1913. doi: 10.1634/stemcells.2007-0790. [DOI] [PubMed] [Google Scholar]
- 11.Fiorina P, Jurewicz M, Augello A, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183:993. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 13.Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 14.Polchert D, Sobinsky J, Douglas G, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudres M, Norol F, Trenado A, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 16.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 17.DIAMOND Project Group Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 20.Huurman VA, Hilbrands R, Pinkse GG, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS ONE. 2008;3:e2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monti P, Scirpoli M, Maffi P, et al. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest. 2008;118:1806. doi: 10.1172/JCI35197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 23.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 24.Popp FC, Eggenhofer E, Renner P, et al. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20:55. doi: 10.1016/j.trim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Feng G, Chan T, Wood KJ, et al. Donor reactive regulatory T cells. Curr Opin Organ Transplant. 2009;14:432. doi: 10.1097/MOT.0b013e32832c58f1. [DOI] [PubMed] [Google Scholar]
- 26.Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]