Abstract
Background
Prostate-specific antigen (PSA) detected in vaginal fluid can be used in studies of HIV/sexually transmitted infection (STI) and pregnancy prevention as an alternative to relying on participant reports of exposure to semen. Optimal methods for collecting and storing specimens for this testing have not been determined.
Study Design
We conducted a controlled, in vitro experiment of 550 specimens spiked with semen to determine the effects of swab type (five types), storage conditions of the swabs (room temperature with or without desiccant or at −80°C without desiccant) and time from collection to testing (seven intervals over the course of 12 months) on the identification of PSA. We performed factorial analysis of variance to identify factors influencing PSA detection.
Results
Concentrations of PSA detected in the swabs declined with time of storage over the 1-year experiment (p<.01). The 1-mL, rayon-tipped swab stored immediately at −80°C following collection performed best.
Conclusions
If immediate testing or freezer storage is not feasible, investigators should use a swab with 1-mL capacity with processing and testing as soon as possible after specimen collection.
Keywords: Biomarker, In vitro experiment, Prostate-specific antigen
1. Introduction
Prostate-specific antigen (PSA) has long been used in sexual assault cases as a highly sensitive and specific method of determining the presence of semen [1–4]. More recently, the identification of PSA has been used in studies of HIV/ sexually transmitted infection (STI) and pregnancy prevention as an alternative to relying on participant reports of exposure to semen, which are not always accurate [5]. For example, the effectiveness of male and female condoms in protecting against exposure to semen can be evaluated by comparing levels of PSA detected in pre- and postcoital vaginal swabs [6–9]. This objective measurement of the woman’s exposure to semen represents a methodological improvement by reducing the potential for intentional or unintentional (e.g., from undetected condom malfunctions) misreporting of condom effectiveness. PSA also could be used to monitor changes in condom use following the introduction of interventions to prevent HIV (e.g., circumcision or microbicide use). Furthermore, studies on the role of hormonal contraception on HIV acquisition and transmission could test for the biomarker in order to identify differences between study arms in their underlying risk of HIV.
Evidence from forensic science suggests that PSA is a relatively stable protein; the marker has been detected in dried samples stored up to 30 years at room temperature [1]. Sensabaugh [4], though, did not detect PSA as consistently in vaginal washings as in semen stains on material, possibly because the overall concentration of PSA was much greater in stains than when it had been diluted in vaginal fluid. Given the greater flexibility in the collection and storage of specimens in clinical research than in forensic casework, determining the optimal procedures for storing vaginal specimens is feasible for improving the use of PSA as a biomarker in clinical trials.
2. Materials and methods
2.1. Overall
We conducted a 1-year, controlled, in vitro experiment to determine the ideal conditions for collecting and storing vaginal swabs for PSA testing. We evaluated the performance of five swabs: three that have been used in quantitative testing for PSA in research studies [Puritan,2 #808 (Fisher), Falcon Swube Collection and Transport System, #220210 (Becton Dickinson and Co.), BBL CultureSwabs, #220135 (Becton Dickinson)], one that has been used for testing for Y-chromosome deoxyribonucleic acid [Starswab II, #S09D (Starplex Scientific)] and a newer swab with a breathable filter [FAB-SWAB, #SW3B400 (Abacus Diagnostics)] (Table 1). We also evaluated the effect of duration of storage (i.e., 1 day, 1 or 2 weeks or 1, 3, 6 or 12 months) and temperature (i.e., room temperature with or without desiccant or at −80°C without desiccant). We spiked five swabs for each condition to be tested with pooled semen remnants from anonymous donors at a health clinic. We also tested five swabs of each type immediately after their preparation (Day 0). Thus, we prepared and tested a total of 550 swabs.
Table 1.
Types of swabs evaluated
| Swab | Product name (manufacturer) | Specifications | Studies using the swab |
|---|---|---|---|
| Puritan | Puritan, #808 (Fisher) | 1-mL, rayon-tipped swab used with a custom cardboard tampon-like applicator | [6–9] |
| “Swube” | Falcon Swube Collection and Transport System, #220210 (Becton Dickinson and Co.) | 150-μL cotton-tipped swab in a screw-cap tube | [15–19] |
| “CultureSwab” | BBL CultureSwabs, #220135 (Becton Dickinson) | Two 225-μL, polyester-tipped swabs on plastic applicators that fit into a single tube cap | [20] |
| “FAB-SWAB” | FAB-SWAB, #SW3B400 (Abacus Diagnostics) | 175-μL, cotton-tipped swab with a built-in Tyvek® breathable filter | |
| “Starswab” | Starswab II, #S09D (Starplex Scientific) | Two 125-μL rayon-tipped swabs on plastic applicators that fit into a single tube cap | [21–26] |
2.2. Laboratory procedures
A single technician performed all laboratory procedures. After allowing male semen stock to sit at room temperature until visibly thawed (about 10 min), we diluted it at a 1:1000 ratio with a 95:5 ratio mixture of phosphate buffer saline (PBS; 0.1M, pH 7.2) and polysorbate 20 (Tween 20). The resulting mixture had a mean of 569.8 ng/mL of total PSA. Using a calibrated pipette, we applied a sufficient quantity of the semen and buffer mixture to the swabs so that each swab received approximately the volume of its capacity. (For example, the Puritan swab received 1 mL of the semen mixture.) We placed each swab into its tube, which was then placed into an individual, sealable plastic bag. For the Puritan, CultureSwab, Starswab and FAB-SWAB swabs that were to be stored with desiccant, we placed two desiccant packets (MiniPax Sorbent; Multisorb technologies, Buffalo, NY, USA) into each plastic bag. For the Swube, we used a glass stirring rod to push a desiccant packet into the bottom of the tube before the swab was inserted. The sealed bags were stored in either a −80°C freezer or at room temperature for 1 day, 1 or 2 weeks or 1, 3, 6 or 12 months.
At the specified time for extraction and testing, we inserted the vaginal swab into a 15-mL Falcon conical tube containing PBS/Tween 20 (95:5 ratio) in an amount three times that of the capacity of the specific swab. After the swab contents eluted into the buffer at room temperature for 20–30 min, we vortexed the swab for about 10 s to dislodge any material from the swab tip and then vigorously rotated and pressed the swab against the side of the tube to maximize sample recovery. After vortexing the extraction tubes for a few seconds, we pipetted 300 μL of the resulting eluent into a sample cup. Following the manufacturer’s published protocol, we tested the specimens using ARCHITECT assays for total PSA and free PSA (Abbott Diagnostics, Abbott Park, IL, USA). Any samples that exceeded the detection limit of the assays for total and free PSA (100 and 30 ng of PSA per mL, respectively) were diluted with the PBS/Tween 20 buffer and retested to quantify concentrations above these levels. Because the differences in results between the assays for total and free PSA were slight and did not change any of the conclusions from the analysis of variance (ANOVA) analyses, we report only the total PSA.
We discarded the specimens after testing except for the eluent from the testing of the Puritan swabs at baseline, 1 day and 6 months. (The Puritan swab was the only swab with sufficient residual eluent to permit additional testing.) The residual eluents were stored in cryovials at −80° for rethawing and retesting at subsequent testing times (followed by immediate refreezing).
2.3. Statistical analysis
We performed factorial ANOVA to examine the effects of time, swab type, storage condition and their respective interactions on the quantitative measure of PSA. Because the PSA values had a skewed distribution, we used the logarithm of the values as the dependent outcome in the modeling. For factors with a significant F value (p<.05), we used the Tukey Range Test to evaluate whether the means of each level of the factor differed significantly from one another [10].
3. Results
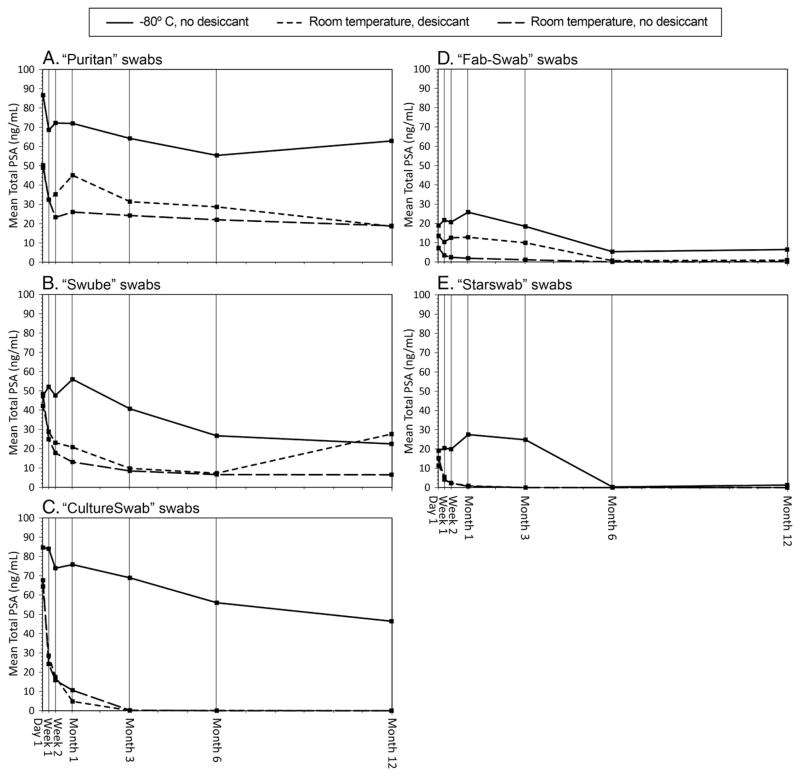
The effects of time, swab types, storage conditions and their respective interactions explained most of the variation in the total PSA detected (R2=.86, factorial ANOVA). The amount of PSA detected in the stored swabs declined over time irrespective of the swab type or the storage condition (p<.01; Table 2, Fig. 1A–1E). The swabs differed in their performances (p<.01) with the Puritan swab yielding the most total PSA (geometric mean, 38.8 ng/mL) followed by the Swube (22.0 ng/mL), the CultureSwab (4.3 ng/mL), the FAB-SWAB (3.7 ng/mL) and the Starswab (2.0 ng/mL). The performances of the five swabs were all statistically significantly different from each other except for the comparison between the CultureSwab and the FAB-SWAB.
Table 2.
Mean total PSA by swab type and storage condition
| Swab type | Storage temperature and desiccant use | Mean PSA in ng/mL
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Week 1 | Week 2 | Month 1 | Month 3 | Month 6 | Month 12 | ||
| Puritan | N/A | 91.4 | |||||||
| Room temperature, no desiccant | 50.3 | 32.4 | 23.3 | 26.0 | 24.2 | 22.0 | 18.9 | ||
| Room temperature, desiccant | 49.0 | 32.4 | 35.2 | 45.1 | 31.4 | 28.7 | 18.6 | ||
| −80°C, no desiccant | 86.6 | 68.6 | 72.2 | 72.0 | 64.2 | 55.4 | 62.9 | ||
| Swube | N/A | 67.9 | |||||||
| Room temperature, no desiccant | 48.4 | 24.9 | 17.8 | 13.1 | 8.5 | 6.6 | 6.5 | ||
| Room temperature, desiccant | 42.2 | 28.8 | 23.1 | 20.8 | 9.8 | 7.3 | 27.6 | ||
| −80°C, no desiccant | 47.5 | 52.1 | 47.6 | 56.0 | 40.7 | 26.7 | 22.5 | ||
| CultureSwab | N/A | 87.4 | |||||||
| Room temperature, no desiccant | 67.6 | 24.2 | 15.8 | 10.6 | 0.2 | 0.0 | 0.0 | ||
| Room temperature, desiccant | 64.4 | 28.5 | 17.5 | 4.8 | 0.1 | 0.1 | 0.0 | ||
| −80°C, no desiccant | 84.6 | 84.0 | 73.9 | 75.8 | 68.9 | 56.0 | 46.4 | ||
| FAB-SWAB | N/A | 30.0 | |||||||
| Room temperature, no desiccant | 7.2 | 3.4 | 2.4 | 1.9 | 1.1 | 0.0 | 0.1 | ||
| Room temperature, desiccant | 13.5 | 10.3 | 12.5 | 12.8 | 9.9 | 0.6 | 0.9 | ||
| −80°C, no desiccant | 18.8 | 21.7 | 20.6 | 25.8 | 18.4 | 5.3 | 6.4 | ||
| Starswab | N/A | 21.8 | |||||||
| Room temperature, no desiccant | 15.3 | 4.1 | 2.4 | 0.6 | 0.0 | 0.0 | 0.0 | ||
| Room temperature, desiccant | 11.4 | 5.5 | 2.3 | 0.9 | 0.0 | 0.0 | 0.0 | ||
| −80°C, no desiccant | 19.1 | 20.5 | 19.9 | 27.5 | 24.8 | 0.3 | 1.3 | ||
N/A=not applicable.
Fig. 1.
(A–E). Mean total PSA by swab type and storage condition.
The three storage conditions resulted in differences in the detection of total PSA (p<.01). The immediate storage of swabs at −80°C until the time of their processing and testing yielded the highest concentrations of the marker (geometric mean, 28.8 ng/mL). The use of desiccant resulted in higher PSA concentrations for the swabs stored at room temperature (geometric mean, 5.0 ng/mL vs. 3.2 ng/mL for those without desiccant).
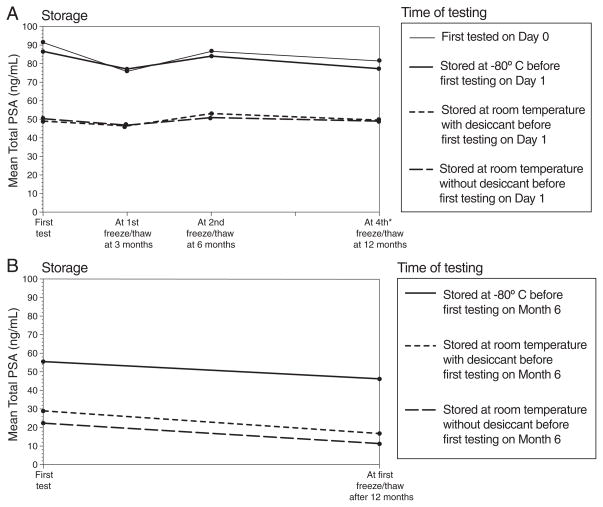
Repeated cycles of freezing the eluent followed by its thawing and retesting affected the ability to detect PSA in the Puritan swabs (Table 3; Fig. 2A–2B). Among the residual eluents stored in the freezer following their initial testing on Day 1, the concentrations of PSA differed between the thawing and retesting at 3 and 6 months (p<.03). The eluents stored in the freezer following their initial testing at 6 months resulted in lower concentrations of PSA detected at 12 months (p<.01). The swabs stored at room temperature (with or without desiccant) before their initial testing fared worse than those stored in the freezer before their initial testing (p<.01).
Table 3.
Mean total PSA detected from Puritan swabs after repeated freezing and thawing cycles
| Storage | Time of testing
|
|||
|---|---|---|---|---|
| First test | At first freeze/thaw at 3 months | At second freeze/thaw at 6 months | At fourth a freeze/thaw at 12 months | |
| First tested on Day 0 | 91.4 | 75.8 | 86.6 | 81.4 |
| Stored at room temperature without desiccant before first testing on Day 1 | 50.3 | 46.8 | 50.9 | 49.0 |
| Stored at room temperature with desiccant before first testing on Day 1 | 49.0 | 46.4 | 53.1 | 49.5 |
| Stored at −80°C before first testing on Day 1 | 86.6 | 77.1 | 84.0 | 77.3 |
| Storage | Time of testing
|
|
|---|---|---|
| First test | At first freeze/thaw after 12 months | |
| Stored at room temperature without desiccant before first testing on Month 6 | 22.0 | 11.1 |
| Stored at room temperature with desiccant before first testing on Month 6 | 28.7 | 16.6 |
| Stored at −80°C before first testing on Month 6 | 55.4 | 46.2 |
Data from third freeze/thaw cycle not available.
Fig. 2.
(A–B). Mean total PSA detected from “Puritan” swabs after repeated freezing and thawing cycles.
4. Discussion
Semen biomarkers could improve our ability to measure and control for sexual behavior in studies of contraception and HIV/STIs. The use of PSA in research would be improved by standardizing the methods for collecting and storing specimens for this testing. The present in vitro experiment revealed that the concentration of PSA detected declined over time, that the swab with the largest capacity performed best and that storage at −80°C was preferable over room temperature storage. This last finding is consistent with evidence suggesting that freezer is superior to room temperature storage for detection of free PSA in serum [11].
PSA exists in serum in several forms: complexed to alpha-1-anti-chymotrypsin, unbound (free PSA) and enveloped by alpha-2-macroglobulin (which is not detected by immunoassays) [12]. The Abbott assay for total PSA is optimized to detect a mixture of the first two forms. PSA in semen, though, is almost exclusively present in free form [13,14]. Consequently, the Abbott assay for total PSA (designed to detect PSA in blood) tended to result in lower concentrations for PSA than the assay for free PSA (data not reported). However, the differences between assays were slight, and testing for total PSA still could be preferred for several reasons including (a) its lower cost; (b) its higher limit of detection (which results in less laboratory burden required for the dilution and retesting of specimens) and (c) its use in previous studies of PSA detection for prevention of pregnancy or HIV/STIs [6–9], which facilitates comparisons across studies.
The Puritan swab may have performed better because of its greater 1-mL capacity compared to the other swabs, which have capacities ranging 150–225 μL. That is, the larger size of the Puritan swab head might have allowed it to capture more PSA when the specimen was prepared. The second largest swab tested, CultureSwab, performed about the same as the Puritan swab when stored in the freezer. In contrast to the effect of the swab capacity, the effect of the swab material on its performance was not evident. For example, while both the Puritan and the Starswab swabs are comprised of rayon, the Starswab resulted in the lowest average concentration of PSA detected.
The main limitation of the findings is that they are the result of in vitro research only using spiked swabs. Vaginal specimens collected from women following semen exposure might yield different PSA test results. However, we spiked the specimens with semen stock diluted with a buffer mixture in a ratio designed to approximate the dilution of semen that might occur in the vaginal cavity. The experiment also did not evaluate other vaginal products (e.g., lubricants or gels) or factors (e.g., menstruation or vaginal cleansing) that possibly could influence the clearance or detection of PSA in vaginal fluid specimens and could interact with swab types differently. Finally, other questions related to the laboratory processes remain unanswered. Because −20°C storage is more feasible for many clinics than −80°C storage, determining whether the two storage temperatures have a similar effect on the specimens would be helpful. In addition, it is unknown whether processing the specimens immediately and storing the eluent (rather than the swab) for later testing for PSA would yield similar results.
In summary, this in vitro study suggests that 1-mL swabs are optimal for PSA detection; swabs with lower capacities are not recommended. Swabs should be immediately tested or stored at −80°C following their collection. If neither of these is feasible, investigators should process and test the swabs as soon as possible.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Use of trade names is for identification only and does not imply endorsement by the US Department of Health and Human Services.
References
- 1.Hochmeister MN, Budowle B, Rudin O, et al. Evaluation of prostate-specific antigen (PSA) membrane test assays for the forensic identification of seminal fluid. J Forensic Sci. 1999;44:1057–60. [PubMed] [Google Scholar]
- 2.Johnson ED, Kotowski TM. Detection of prostate-specific antigen by ELISA. J Forensic Sci. 1993;38:250–8. [PubMed] [Google Scholar]
- 3.Rao D, Kashyap V. Dot blot immunoassay for detection of human semen. Immunoassay. 1992;13:537–44. doi: 10.1080/15321819208019834. [DOI] [PubMed] [Google Scholar]
- 4.Sensabaugh GF. Isolation and characterization of a semen-specific protein from human seminal plasma: a potential new marker for semen identification. J Forensic Sci. 1978;23:106–15. [PubMed] [Google Scholar]
- 5.Mauck CK, Doncel GF. Biomarkers of semen exposure clinical working group. Biomarkers of semen in the vagina: applications in clinical trials of contraception and prevention of sexually transmitted pathogens including HIV. Contraception. 2007;75:407–19. doi: 10.1016/j.contraception.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Macaluso M, Lawson ML, Hortin G, et al. Efficacy of the female condom as a barrier to semen during intercourse. Am J Epidemiol. 2003;157:289–97. doi: 10.1093/aje/kwf212. [DOI] [PubMed] [Google Scholar]
- 7.Macaluso M, Blackwell R, Jamieson DJ, et al. Efficacy of the male latex condom and of the female polyurethane condom as barriers to semen during intercourse: a randomized clinical trial. Am J Epidemiol. 2007;166:88–96. doi: 10.1093/aje/kwm046. [DOI] [PubMed] [Google Scholar]
- 8.Galvao LW, Oliveira LC, Diaz J, et al. Effectiveness of female and male condoms in preventing exposure to semen during vaginal intercourse: a randomized trial. Contraception. 2005;71:130–6. doi: 10.1016/j.contraception.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Macaluso M, Lawson L, Akers R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999;59:195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 10.Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied regression analysis and other multivariable methods. 3. New York: Duxbury Press; 1998. [Google Scholar]
- 11.Piironen T, Pettersson K, Suonpaa M, et al. In vitro stability of free prostate-specific antigen (PSA) and prostate-specific antigen (PSA) complexed to α(1)-antichymotrypsin in blood samples. Urology. 1996;48:81–7. doi: 10.1016/s0090-4295(96)00616-4. [DOI] [PubMed] [Google Scholar]
- 12.Lilja H, Christensson A, Dahlen U, et al. Prostate-specific antigen in serum occurs predominantly in complex with α(1)-antichymotrypsin. Clin Chem. 1991;37:1618–25. [PubMed] [Google Scholar]
- 13.Rittenhouse RG, Finlay JA, Mikolajczyk Partin AW. Human kallikrein 2 (hK2) and prostate-specific antigen (PSA): two closely related, but distinct, kallikreins in the prostate. Crit Rev Clin Lab Sci. 1998;35:275–368. doi: 10.1080/10408369891234219. [DOI] [PubMed] [Google Scholar]
- 14.Christensson A, Bjork T, Nilsson O, et al. Serum prostate specific antigen complexed to alpha-1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150:100–5. doi: 10.1016/s0022-5347(17)35408-3. [DOI] [PubMed] [Google Scholar]
- 15.Gallo MF, Behets FM, Steiner MJ, et al. Validity of self-reported ‘safe sex’ among female sex workers in Mombasa, Kenya—PSA analysis. Int J STD AIDS. 2007;18:33–8. doi: 10.1258/095646207779949899. [DOI] [PubMed] [Google Scholar]
- 16.Gallo MF, Behets FM, Steiner MJ, et al. Prostate-specific antigen to ascertain reliability of self-reported coital exposure to semen. Sex Transm Dis. 2006;33:476–9. doi: 10.1097/01.olq.0000231960.92850.75. [DOI] [PubMed] [Google Scholar]
- 17.Walsh TL, Frezieres RG, Peacock K, et al. Effectiveness of the male latex condom: combined results for three popular condom brands used as controls in randomized clinical trials. Contraception. 2004;70:407–13. doi: 10.1016/j.contraception.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Walsh TL, Frezieres RG, Peacock K, et al. Use of prostate-specific antigen (PSA) to measure semen exposure resulting from male condom failures: implications for contraceptive efficacy and the prevention of sexually transmitted disease. Contraception. 2003;67:139–50. doi: 10.1016/s0010-7824(02)00478-x. [DOI] [PubMed] [Google Scholar]
- 19.Walsh TL, Frezieres RG, Nelson AL, Wraxall BG, Clark VA. Evaluation of prostate-specific antigen as a quantifiable indicator of condom failure in clinical trials. Contraception. 1999;60:289–98. doi: 10.1016/s0010-7824(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 20.Anderson C, Gallo MF, Hylton-Kong T, et al. Randomized controlled trial on the effectiveness of counseling messages for avoiding unprotected sexual intercourse during STI and RTI treatment among female STI clinic patients. Sex Transm Dis. doi: 10.1097/OLQ.0b013e31827938a1. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brotman RM, Melendez JH, Smith TD, Galai N, Zenilman JM. Effect of menses on clearance of Y-chromosome in vaginal fluid: implications for a biomarker of recent sexual activity. Sex Transm Dis. 2010;37:1–4. doi: 10.1097/OLQ.0b013e3181b5f15d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose E, Diclemente RJ, Wingood GM, et al. The validity of teens’ and young adults’ self-reported condom use. Arch Pediatr Adolesc Med. 2009;163:61–4. doi: 10.1001/archpediatrics.2008.509. [DOI] [PubMed] [Google Scholar]
- 23.Ghanem KG, Melendez JH, McNeil-Solis C, et al. Condom use and vaginal Y-chromosome detection: the specificity of a potential biomarker. Sex Transm Dis. 2007;34:620–3. doi: 10.1097/01.olq.0000258318.99606.d9. [DOI] [PubMed] [Google Scholar]
- 24.Melendez JH, Giles JA, Yuenger JD, et al. Detection and quantification of Y-chromosomal sequences by real-time PCR using the LightCycler system. Sex Transm Dis. 2007;34:617–9. doi: 10.1097/01.olq.0000258336.65285.31. [DOI] [PubMed] [Google Scholar]
- 25.Jadack RA, Yuenger J, Ghanem KG, Zenilman J. Polymerase chain reaction detection of Y-chromosome sequences in vaginal fluid of women accessing a sexually transmitted disease clinic. Sex Transm Dis. 2006;33:22–5. doi: 10.1097/01.olq.0000194600.83825.81. [DOI] [PubMed] [Google Scholar]
- 26.Zenilman JM, Yuenger J, Galai N, Turner CF, Rogers SM. Polymerase chain reaction detection of Y chromosome sequences in vaginal fluid: preliminary studies of a potential biomarker for sexual behavior. Sex Transm Dis. 2005;32:90–4. doi: 10.1097/01.olq.0000149668.08740.91. [DOI] [PubMed] [Google Scholar]