Abstract
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), provide a new cell source for regenerative medicine, disease modeling, drug discovery, and preclinical toxicity screening. Understanding of the onset and the sequential process of hematopoietic cells from differentiated hPSCs will enable the achievement of personalized medicine and provide an in vitro platform for studying of human hematopoietic development and disease. During embryogenesis, hemogenic endothelial cells, a specified subset of endothelial cells in embryonic endothelium, are the primary source of multipotent hematopoietic stem cells. In this review, we discuss current status in the generation of multipotent hematopoietic stem and progenitor cells from hPSCs via hemogenic endothelial cells. We also review the achievements in direct reprogramming from non-hematopoietic cells to hematopoietic stem and progenitor cells. Further characterization of hematopoietic differentiation in hPSCs will improve our understanding of blood development and expedite the development of hPSC-derived blood products for therapeutic purpose. This article is protected by copyright. All rights reserved
Keywords: Human pluripotent stem cells, Hemogenic endothelial cells, Hematopoietic stem cells
1. Introduction
Hematopoietic stem cell (HSC) transplantation is the most widely used stem cell therapy in the clinic to treat many blood diseases, such as leukemia. However, the limitation on the source of HSCs, including bone marrow, peripheral blood and umbilical cord blood, and the lack of method for HSC expansion restrict HSC based clinic application. Research and development of new technology for the production of transplantable HSCs in vitro or ex vivo are essential for future clinical application development. The successful derivation of human PSCs provoked tremendous interest in using hPSCs as a source to generate unlimited blood cells from therapeutic purpose. Human blastocyst-derived pluripotent cell lines, human embryonic stem cells (hESCs), were first generated in 1998 (Thomson et al. 1998). Thereafter, the successful differentiation of hESCs into cell types spanning the three germ layers, such as neural progenitors of endoderm (Ben-Hur et al. 2004; Schulz et al. 2004), cardiomyocytes and endothelial cells of mesoderm (Levenberg et al. 2002; Laflamme et al. 2007), and hepatocytes and pancreatic cells of ectoderm (Cai et al. 2007; Shim et al. 2007; Wang et al. 2011), demonstrated their pluripotent capabilities. Reprogramming of somatic cells to generate human induced pluripotent stem cells (hiPSCs) (Takahashi et al. 2007; Yu et al. 2007) provides an unprecedented opportunity for disease modeling, patient-specific drug-selection, and novel approaches of regenerative therapy based on immunologically compatible patient-specific cells (Guha et al. 2013). Both hESCs and hiPSCs are human pluripotent stem cells (hPSCs) with similar gene expression pattern, and similar developmental potential to generate functional mature cells, including multilineage blood cells.
Since the first study of hESC differentiation into hematopoietic cells by Dan Kaufman and colleagues (Kaufman et al. 2001), numerous studies have been conducted that led to successful derivation of a broad spectrum of blood cell lineages from hESCs and hiPSCs (Park et al. 2005; Galic et al. 2006; Kennedy et al. 2007; Martin et al. 2008; Su et al. 2008; Choi et al. 2009; Lu et al. 2010; Lu et al. 2011), promising future development of clinical applications based on hPSC for transfusions, hematopoietic stem cell (HSC) transplantation and cellular immunotherapy. Efforts to study the onset and hierarchy structure of hPSC-derived hematopoietic differentiation revealed that hematopoietic differentiation from PSC recapitulate embryogenesis process, and knowledge gained from hPSC differentiation study will greatly facilitate the advance of technical evolvement for clinical application.
Recent studies have led to a better understanding of the developmental relationship between hematopoietic and endothelial lineages. The putative common progenitor of both hematopoietic and endothelial lineages, the hemangioblast, has been studied in vitro and in vivo in invertebrate and vertebrate systems (Park et al. 2005). A comparable hemangioblast population derived from hESC was demonstrated by their capacity to generate blast colony-forming cells (BL-CFCs), which displayed both hematopoietic and vascular potential (Kennedy et al. 2007). Although the nature of hemangioblasts is still debatable, increasing evidences indicate that hemogenic endothelial (HE) cells are transient intermediates that contribute to de novo production of multipotent HSCs during embryogenesis. The molecular mechanisms underlying hematopoietic and HE development are still largely unknown.
2. Onset of embryonic hematopoiesis
During embryonic development, hematopoiesis occurs in spatially and temporally distinct sites. Parallel development of blood vessels and blood cells (Figure 1A) establishes a functional circulatory system for the supply of nutrients and oxygen, and the removal of metabolic wastes (Hirschi 2012). The origin of vascular and blood cells may be different depending on the stage of development and the maturation of hematopoiesis (Table 1, 2).
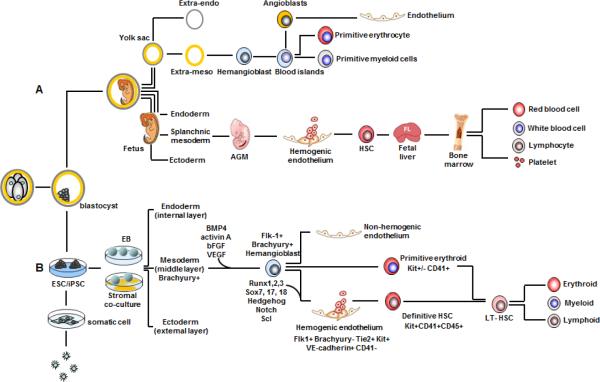
Figure 1. Schematic hematopoietic differentiation of human PSC and normal human embryonic hematopoiesis.
A. Human embryonic hematopoiesis occurs in extra-mesoderm to generate primitive erythroid and myeloid cells which is termed as extraembryonic hematopoiesis. Intraembryonic hematopoiesis originates from splanchnic mesoderm to form AGM region. Bi-potent progenitor of hematopoietic and endothelial lineages, including hemangioblast and hemogenic endothelium, are responsible for definitive hematopoiesis and give rise to mature blood cells in a stepwise timeline. B. Human ESCs or iPSCs form embryo bodies (hEB) which recalculate human yolk sac differentiation. Hemangioblast, hemogenic endothelium, and primitive or definitive blood cells are generated in a sequential process.
Table 1.
Comparison of hemangioblasts and hemogenic endothelial cells in mouse
| location | features | hemangioblasts extraembryo | hemogenic endothelial cell | references | |
|---|---|---|---|---|---|
| extraembryo intraembryo | |||||
| In vivo | time | E7.0 - E7.75 mid-streak stage of gastrulation | E8.25 lumen wall of YK capillaries | E10.5 - E11.5 ventral wall of the dorsal aortic endothelium | [Adamo et al., 2009; Huber et al., 2004; Iacovino et al., 2011; Kim et al., 2013; Liu et al., 2013; Marcelo et al., 2013; Tsunoda et al., 2010; Yue et al., 2012] |
| phenotype | brachyury+ Flk-1+ | Flk1+c-Kit+ CD45–Aldh+ SP cells | Flk-1+c-Kit+CD31+CD45–CD34+AA4.1+/VEC+Aldh+ SP cells | ||
| differentiation potential | primitive hematopoiesis and endothelium smooth muscle | definitive hematopoiesis and endothelium | definitive hematopoiesis endothelium | ||
| transcript factors | Flk1, Hhex,Mixl1, Smad1, Scl,Runx1, Gata1,lmo2, ZFAT | Scl/TAL1, Hhex, Runx1,Gata2, Gata3,c-Myb, Gfi1,Gfi1b | Sox17 ,HoxA3, Scl, Runx1,Gata2, Gfi1, Gfi1b, Fli1 | ||
| signaling pathways | ER71, VEGF/Flk1, FGF, BMP, Notch, Wnt, Hedgehog (Hh), Spry1 | RA/c-kit/Notch1/p27 | RA, Notch1, Wnt, BMPs, Hedgehog (Hh), NO signaling, VEGF, IL-1, IL-3 | ||
| In vitro (ESC) | time phenotype differentiation potential | 3.25-4 days of EB Flk-1+ BL-CFU smooth muscle cardiomyocyte mural cells | after 36-48 hrs cultured hemangioblasts Tie2+c-Kit+ CD41- definitive hematopoiesis endothelium | [Adamo et al., 2009; Choi et al., 1998; Faloon et al., 2000; Goldie et al., 2008; Kim et al., 2013; Lancrin et al., 2009; Liu et al., 2013; Marcelo et al., 2013; Pearson et al., 2008; Pimanda et al., 2007; Yue et al., 2012] | |
| transcript factors | FGF2, activin A, Flk1, Scl, Runx1, Gata1,Gata2,Lmo2, Hhex,Mixl1, Smad1, Lycat | Scl/TAL1,HoxA3,Runx1, GATA2, LMO2, Gfi1 and Gfi1b | |||
| signaling pathways | ER71,VEGF/Flk1,FGFR1, BMP, Notch, Wnt, Hedgehog (Hh), phosphatidylinositol 3-kinase | Hedgehog(Hh), Notch-1, NO signaling, F2r-RhoA/ROCK | |||
Table 2.
Comparison of human hemangioblasts and hemogenic endothelial cells in vitro
| features | hemangioblasts ESC | hemogenic endothelial cells | references | |
|---|---|---|---|---|
| ESC | cord blood | |||
| time | day 3–4 from EB | day 4 in a chemically defined medium | 30 days in MH-CM culture | [Kennedy et al., 2012b; Kennedy et al., 2007; Lu et al., 2008; Pelosi et al., 2012; Wang et al., 2012; Yu et al., 2012] |
| phenotype | KDR+CD31+CD3 4+CD45-VE-cadherin+PDGFRa+Ac-LDL+vWF+ | KDR+VE-Cad+CD31+CD34+CD45-CD43- | CD144+CD105+CD146+ CD31+ CD45- | |
| differentiation potential | blast colony-forming cells (BL-CFU) smooth muscle-like cells | definitive hematopoiesis endothelium | definitive hematopoiesis endothelium | |
| transcript factors | Mixl1+APLNR+, FLT1, NF-E2, EKLF, ICAM-4, glycophorins, EGR1, GFI1, SCL, LMO2, GATA-1, GATA2, MYC, LYL1, MYB, SOX18, RUNX1 | RUNX1, C-MYB, GATA2, GATA1, SCL, IKAROS, PU.1 | ||
| signaling pathways | FGF2, Activin/Nodal, BMP-4, VEGF | TGFβ, VEGF, bFGF, RA | ||
2.1 Extraembryonic hematopoiesis
The earliest hematopoietic and endothelial cells arise in the extraembryonic yolk sac (YS) that functions as placenta determining and controlling uptake, translocation, and maternofetal transportation in humans and in mice. YS is a bilayer structure of mesoderm- and endoderm-derived cell layers composing of trophoblast and a single layer of hypoblast (Jollie 1990). The endoderm layer forms an epithelium that functions as liver and intestines later, whereas the mesoderm layer gives the birth to the first visible blood cell in the YS, which is a large nucleated primitive erythroblast (Palis et al. 2001).
Blood islands in YS are recognized as structures comprising of an outer luminal layer of angioblasts (endothelial progenitor cells) and a loose inner mass of embryonic primitive hematopoietic cells that initiate primitive hematopoiesis and constitute the first circulation. In addition to primitive nucleated erythrocytes, megakaryocytes and macrophages are also identified in primitive hematopoiesis (Palis et al. 1999; Lichanska et al. 2000). Whether embryonic primitive hematopoietic cells contribute to fetal and adult hematopoiesis remains a subject of longstanding debate (Cumano et al. 2001; Palis et al. 2001; Baron 2013). In contrast to the adult hematopoiesis that initiate from HSCs, the primitive hematopoietic cells are derived from putative common bi-potent progenitors, hemangioblasts, that also give rise to angioblasts to form primitive vasculatures (Auerbach et al. 1996). Hemangioblasts are mesodermal precursors characterized by expression of transcription factor brachyury and Flk1 (KDR or VEGFR-2) (Ferkowicz et al. 2005). Indirect tracing of Flk1+ cells in the YS reveals that relatively few progenitors are detected, suggesting a very brief existence of hemangioblasts prior to rapid differentiation and expansion into restricted hematopoietic and vascular progenitors as they egress from the primitive streak (Huber et al. 2004). It is still unclear whether primitive erythrocytes, megakaryocytes, and macrophages directly arise from hemangioblasts. Transplantation of YS cells from murine E9.0 or E10.0 into neonatal livers contributes to adult lymphoid and myeloid, suggesting that YS in a late stage of development has a potential to generate definitive hematopoietic cells as well (Palis et al. 1999; Lux et al. 2008). However, further evidences are required to confirm whether the extraembryonic YS contributes to adult-type blood cells in nature.
2.2 Intraembryonic hematopoiesis
An early study from chicken-quail chimeric embryos demonstrated that, although extraembryonic hematopoiesis occurs early in development, it is rather transient and regional, suggesting that intraembryonic hematopoietic sources take more important role in giving the birth of multilineage hematopoietic cells for embryonic circulation (Dieterlen-Lievre 1975). About 100 years ago, “hematopoietic clusters” were postulated to arise from the vascular endothelium of the dorsal aorta (Jordan 1917). In recent years, studies in animal models have provided compelling evidences to demonstrate that hemogenic endothelial (HE) cells, a specified subset of endothelial cells in embryonic endothelium, give rise to multipotent definitive HSCs (Medvinsky et al. 1996; Taoudi et al. 2008; Eilken et al. 2009; Yoshimoto et al. 2009; Tavian et al. 2010; Hirschi 2012; Swiers et al. 2013). Endothelium in the aortic-gonado-mesonephros (AGM) region is a primary site to generate definitive HSCs (Zovein et al. 2008; Tavian et al. 2010; Rafii et al. 2013). In addition, HSCs are also characterized from endothelium in other tissues, such as the placenta (Rhodes et al. 2008), arterial vessels (Gordon-Keylock et al. 2013), endocardium of embryonic heart (Nakano et al. 2013), and embryonic head (Li et al. 2012). Multiple lines of evidence have revealed that hematopoietic stem and progenitor cells (HSPCs) emerge from HE cells through a process termed as endothelial-to-hematopoietic transition (EHT). Jaffredo et al. showed that the hematopoietic cluster-bearing floor of the aorta is CD45+ and Flk1-, while the rest of the aortic endothelium is CD45- and Flk1+. The newly emerged hematopoietic cells contain traces of Dil-conjugated low-density lipoproteins (LDL) that are used to label endothelial cells of the aorta (Jaffredo et al. 1998). Genetic tracing and imaging of HSPC budding from embryonic aortic endothelium provided direct evidence that HE cells undergo EHT to differentiate into multipotent HSPCs (Zovein et al. 2008; Bertrand et al. 2010; Boisset et al. 2010; Kissa et al. 2010). HE cells exist transiently during embryogenesis, and EHT occurs only in defined locations and at a specific time window. It is largely unknown what specific niche cues at critical times are essential for HE cell generation and differentiation into HSCs. It has been shown that HSCs derived from HE cells migrate to the fetal liver and then to adult bone marrow (BM) (Cumano et al. 2001; Baron 2013; Clements et al. 2013). Although it is not a hemogenic site generating de novo HSCs, the fetal liver plays an important role in HSC self-renewal by supporting external HSCs colonization (Zovein et al. 2008).
3. Hematopoietic differentiation of hPSCs
Hematopoietic development has been studied in vivo with cutting-edge technologies largely based on animal models, such as gene modification in mice and zebrafish. Because of the difficulty to access human tissues during early development, the study of regulation of hematopoietic development in human must take alternative strategies. Differentiation of hPSCs provides a great model for the study of early embryo events in vitro (Figure 1B). Three dimensional embryoid bodies (EBs) of hPSC differentiation mimic in vivo embryonic development of the three germ layers. Taking this advantage, several methods have been developed for hematopoietic differentiation in hPSCs through EB formation, including spontaneous EB formation (Zambidis et al. 2005), hanging-drop EB formation (Bai et al. 2013), and spin-EB formation (Civin et al. 1996; Yahata et al. 2002; Ye et al. 2009). The mesoderm-derived hematopoietic and vascular lineages within the cystic EBs demonstrated that the sequence of hematopoietic development in these experimental systems is similar to that in embryo, including hematopoietic development from hemangioblasts and HE cells. To understand the regulatory mechanisms and to promote hematopoietic differentiation in hPSCs, a variety of co-culture strategies intended to mimic niche cues have also been developed, that include co-culture selected cell populations on OP9 stromal cells (Vodyanik et al. 2005; Timmermans et al. 2009), on stromal cells derived from AGM region (Weisel et al. 2006; Ledran et al. 2008), on fetal liver-derived stromal cells (Ledran et al. 2008; Ma et al. 2008), and on S17 BM-derived stromal cells (Kaufman et al. 2001; Tian et al. 2006). Through these studies, tremendous progress has been made in the last decade in understanding of human blood cell development, intermediate identification, and unraveling regulatory mechanism. However, generation of hPSC-derived HSCs with features of engraftment and multilineage potential remains a challenge (Slukvin 2013), largely due to the difficulty of direct application of knowledge obtained from animal models to cell culture systems, and due to many differences between mouse and human hematopoietic differentiation systems.
3.1 Hematopoietic stem and progenitor cells derived from hPSCs
HSCs are rare cell population in adult BM, and possess the capability of self-renewal and the potential to differentiate to a full spectrum of blood cells. While the identification and evaluation of HSCs is still heavily relied on functional approach, much of the progress has been made in recent years to correlate molecular or phenotypic signatures with HSC functionality to facilitate HSC identification and isolation and to gain insights into HSC development. The long-term HSCs (LT-HSCs) from human primary tissue have been defined as CD34+CD38−CD90+CD45RA−Lin− population (Majeti et al. 2007). However, the immunophenotypes of hPSC-derived HSCs have not been well documented because hPSC-derived hematopoietic cells often have an incomparable immunophenotypes to that of adult hematopoietic cells and exhibit low efficient engraftment in immunedeficient mice. It has been recognized that HSCs exhibit high level of heterogeneity that reflect their discrete developmental stages. (Michael R. Copley Cell Stem Cell 2012) The inability to generate highly potent engraftable HSCs from hPSCs could be attributed to a lack of understanding in restricted temporal window for conductive factors to act and demanding cellular microenvironments for the derivation and maturation of HSCs from the differentiated hPSCs.
Several reports have presented encouraging results regarding the generation of putative HSCs with long-term multilineage engraftment potential from pluripotent stem cells. For example, engraftable HSCs are promoted by ectopic expression of HoxB4 in mouse embryonic stem cells (mESCs) followed by co-culture on OP9 stromal cells (Kyba et al. 2002); Notably, the effect of the enforced HoxB4 expression on human HSC generation from hPSCs are limited and controversial. A study by Bhatia and colleagues demonstrated that ectopic expression of HoxB4 has no effect on repopulation capacity of hESC-derived hematopoietic cells (Wang et al. 2005), whereas a study by Bowles et al. demonstrated that enforced-expression of HoxB4 promotes hESC differentiation into hematopoietic cells in vitro (how about engraftment test result??) (Bowles et al. 2006). Nevertheless, while the effect of the enforced HoxB4 expression on HSC remain to be evaluated, the significance of this strategy on clinical applications is questionable because of the risk of leukemia after HoxB4 retroviral transduction (Zhang et al. 2008).
However, the nature of the niche signals that direct hPSC differentiation into HSCs and the regulation mechanisms are yet to be revealed. In the effort to infer and resemble the natural niche signals required for HSC generation, stromal cells derived from mouse AGM region (cell name) and fetal liver (cell name) were used as feeder cells in PSCs differential culture. Indeed, this strategy yield hematopoietic cells that are capable of engraftment into immunedeficient mice if xxxcells are co-injected with stromal cells (Ledran et al. 2008), suggesting that cells derived from embryonic tissues in the vicinity of HSC-origin are able to provide microenvironment cues for functional HSC development from hPSCs. It is conceivable that, stromal cells from the AGM and from the fetal liver may provide different inductive signals to promote the development of HSCs from hPSCs, since in embryo AGM gives rise to de novo HSCs via EHT whereas the fetal liver is the site for external HSCs self-renew (Zovein et al. 2008). In another study aimed at generating engraftable HSCs from hPSCs, hPSCs were directly implanted into immunodeficient mice to form teratomas, which contain differentiated tissues of all three germ layers and provide embryonic microenvironment for hematopoietic cell development (Amabile et al. 2013; Suzuki et al. 2013). This approach again suggests a crucial role of niche signals in functional HSPC development and circumvents the difficulty in reconstitution of the required microenvironment, although it is unsuitable for cell therapies due to tumorigenesis of teratomas. Advanced understanding of molecular and cellular mechanisms involved in multipotent HSC development should provide insights into the generation of patient-specific blood cells from hPSCs.
3.2 Hemogenic endothelial (HE) cells from hPSC and HET
Distinguishing between hemangioblasts and HE cells from cell identity perspective is challenging because both of them are bi-potential cells that give rise to hematopoietic and endothelial cells. Hemangioblasts are characterized as capable of formation of blast colony–forming cells (BL-CFCs) and expression of Flk1 (KDR or VEGFR2) (Kennedy et al. 1997; Choi et al. 1998). Many hematopoietic and endothelial-related genes, including Flk1, Tie2, GATA2, Runx1, Scl, Sca-1 and CD34, are expressed in hemangioblasts of mouse ES cells (mESCs) (Orkin 1992; Millauer et al. 1993; Kallianpur et al. 1994; Yamaguchi et al. 1994; Young et al. 1995; North et al. 1999). A CD45-CD31+CD144+KDR+ population is consistent with endothelial and hematopoietic competency. These cells exhibit hematopoietic characteristics by expression of CD45 and the capability of hematopoietic colony formation in hematopoietic culture condition, whereas in endothelial cell culture condition, these cells possess endothelial properties including Dil-Ac-LDL uptake, expression of CD31 (PECAM-1) and CD144 (VE-cadherin), and being adherent (Wang et al. 2004). Lu et al identified a different type of hESC-derived blast cells (BC), which are negative for CD34, CD31 and KDR, also has hemangioblast potential to give rise to hematopoietic and endothelial cells (Lu et al. 2007). Interestingly, hESC-BCs are capable of differentiating into smooth muscle cells and mesenchymal stem cells, suggesting that they contain mesodermal precursors (Ito et al. 1996). Compared to hESCs, hiPSC-derived BCs have a reduced capability of expansion and hematopoietic colony formation. This phenomenon may be explained by the observation that more than half of hiPSC-BCs expressed β-galactosidase, indicating that hiPSC-BCs senesce in early growth phase (Feng et al. 2010). However, other studies demonstrated that early senesce of hiPSC-derived BCs may occur in selected hiPSC lines (Gokoh et al. 2011).
A study of BL-CFCs from mESCs by Lancrin et al. has demonstrated that hemangioblasts generate hematopoietic cells through the formation of a HE intermediate. The endothelial nature of HE cells is charaterized by their expression of CD34, CD31, Flk1, endoglin, and CD144. When Tie2+c-kit+CD41− HE cells isolated from BL-CFCs or mouse embryos are cultured on OP9 stromal cells, they give rise to CD41+ and CD45+ hematopoietic progenitor cells (Lancrin et al. 2009). Eilken et al. has taken a different approach to investigate de novo generation of hematopoietic cells from mECSs by tracking the differentiation of Flk1+E-cadmesodermal precursors at single-cell level. When the mesodermal precursors are induced to hematopoietic differentiation on OP9 stromal cells, they form an endothelial cell sheet prior to emergent of hematopoietic cells. The development of the endothelium and the budding of hematopoietic progenitor cells from the hemogenic endothelium occur in restricted time windows (Eilken et al. 2009). The HE cells are developed from committed endothelial cells, integrated into endothelial sheets by tight junction, and have a determined fate to give rise exclusively to hematopoietic progeny (Eilken et al. 2009).
The process of HE cells giving rise to hematopoietic cells via EHT is also demonstrated in hPSCs (Rafii et al. 2013). By using distinct fluorescent reporters that are specifically expressed in endothelial cells (CD144+) and in hematopoietic progenitor cells (CD41+), Rafii and colleagues have demonstrated that in the presence of vascular niche (E4ORF1+ ECs) and growth factors (BMP4, FGF2, and VEGF), hESCs differentiate into CD144+ endothelial cells, and then CD41+ hematopoietic progenitor cells (Seandel et al. 2008; Rafii et al. 2013). It was found that, in hESC-derived endothelial cells, the CD144+CD31+CD34+CD73− subpopulation contain the highest level of hemogenic potential (the frequency of 1 in 353 to 506 cells). In this study, the EHT is confirmed by imaging of direct conversion of CD144+CD41− endothelial cells to CD144+CD41+ hematopoietic progenitor cells at a single-cell level. In addition to erythrocytes (CD71+CD235a+), megakaryocytes (CD41+CD42+CD61+), granulocyte (CD15+CD33dim) and monocytes (CD14+CD33brightCD45+), hESC-derived lineagehematopoietic progenitor cells (CD15−CD41−CD45dimCD71−CD235a−) have a potential to generate both myeloid and erythro-megakaryocytic cells (Rafii et al. 2013). The hemogenic activity of CD144+CD31+CD34+CD73− endothelial cells has also been reported (Choi et al. 2012). Notably, hematopoietic progenitor cells derived from HE cells in these reports lack lymphoid potential (Rafii et al. 2013). To track the definitive hematopoietic development in hPSCs, Kennedy et al. have established a serum-free and stromal-free system for hPSC differentiation and monitored T lymphocyte development from HE cells as an indicator of definitive hematopoiesis (Kennedy et al. 2012). They demonstrated that the pattern of T lymphocyte development of hPSCs on OP9-DL4 is similar to that from cord blood-derived HSCs (Awong et al. 2009; Kennedy et al. 2012).
3.3. Signaling pathways regulating the development of HSCs and HE cells in hPSCs
Many studies of molecular mechanisms underlying HE cell and HSC development are animal models or mESCs based. A broad range of transcription factors and signaling pathways have been implicated in regulating HSC generation from HE cells, including c-kit, Runx1, Scl, Gata2, Lmo2, Sox 17, Notch, retinoic acid (RA) and TGFβ (Dzierzak et al. 2008; Antas et al. 2013). While transcription factor Scl is indispensable for the development of HE cells, Runx1 plays a critical role in regulating the onset of HET in HE cells (Lancrin et al. 2009). The sequential roles of Scl and Runx1 in de novo hematopoietic development are consistent with a study of HE cells in AGM of zebrafish embryos (Zhen et al. 2013). VEGF, FGF2, TGFβ, and RA signals have been demonstrated to regulate transition from hESC-derived CD31+ endothelial cells into CD43+ hematopoietic cells. Inhibition of FGF2 and TGFβ reduce production of CD43+ hematopoietic cells from hESC-derived CD31+ HE cells (Wang et al. 2012). Furthermore, manipulating Activin and Nodal signaling alters primitive and definitive hematopoietic development in hPSCs (Kennedy et al. 2012). While addition of TGFβ family members, Activin and Nodal, induces the generation of primitive erythrocytes, inhibition of Activin and Nodal signaling by SB 431542 inhibitor promotes definitive T lymphocyte development from CD34+CD43− progenitor cells from hPSCs (Kennedy et al. 2012). In the presence of BMP4, FGF2 and VEGF, inhibition of TGFβ signaling by SB 431542 promotes HE development in hPSCs (Bai et al. 2013; Rafii et al. 2013). However, TGFβ signaling has a dual effect on hematopoietic and endothelial differentiation in hPSCs. It enhances early mesodermal development, whereas after mesoderm induction, TGFβ signaling exhibits a negative effect on generation of hPSC-derived CD34+CD31+CD144+ hematopoietic and endothelial progenitors (Bai et al. 2013). The studies of hPSCs demonstrate that overexpression of Notch ligand Dll4 in vascular feeder cells enhances myeloid differentiation in hESCs (Rafii et al. 2013), whereas overexpression of Notch ligand Dll4 in OP9 enhances T lymphocyte differentiation in hPSCs (Schmitt et al. 2004; Kennedy et al. 2012). These data indicate that a signaling pathway may play a different role in hematopoietic differentiation in hPSCs when it combines with different niche cues.
A recent study of transcriptional diversity of human HSCs and their progeny demonstrated that gene alternative splicing may alter the function of genes in hematopoiesis (Challen et al. 2010). Runx1 plays a critical role in regulating the generation of hematopoietic cells from HE cells (Lancrin et al. 2009; Lam et al. 2010). Runx1 (also known as AML1, CBFA2, or PEBP2aB) is essential for the establishment of the definitive hematopoietic cells during embryogenesis (Okuda et al. 1996; Wang et al. 1996; Chen et al. 2009). The human Runx1 gene has 12 exons, and its expression is controlled by 2 promoters that generate 3 major protein isoforms (Runx1a, 1b and 1c) (Michelson 1996; Sturgeon et al. 2014). It has been reported that Runx1 isoforms show differential expression patterns during hematopoietic development, but have no discernible functional difference in mouse hematopoietic stem and progenitor cells (HSPCs) (Sturgeon et al. 2014). A study of Runx1 function in hPSCs has showed that though the expression of the 3 isoforms of Runx1are up-regulated during EB differentiation, enforced-expression of Runx1a enhances hPSC-derived hematopoietic differentiation with β-globin production and multilineage hematopoietic reconstitution in the intra-bone transplantation model (Ran et al. 2013). The specific role of Runx1b/c in regulating hematopoietic differentiation in hPSCs needs to be further elucidated.
4. Direct reprogramming from non-hematopoietic cells to hematopoietic stem and progenitor cells
Identification and derivation of hPSCs-derived HSCs that are capable of long-term engraftment and differentiation to multilineage hematopoietic cells remain significant challenge. Therefore, HSCs derived from hPSCs remain out of reach as one of the alternative sources for therapeutic HSCs. Rafii and colleagues have reported that lineage hematopoietic progenitor cells derived from HE cells effectively differentiate to erythrocytes, megakaryocytes and myeloid cells, but they are unable to differentiate to lymphoid cells in vitro or to engraft in immunedeficient NSG mice in vivo (Rafii et al. 2013). The same group has taken a different approach to generate engraftable hematopoietic cells by directly reprogramming human endothelial cells to hematopoietic progenitor cells (Sandler et al. 2014). By ectopic expression of transcription factors, FOSB, GFI1, RUNX1, and SPI1 (FGRS), human primary endothelial cells are reprogrammed into engraftable hematopoietic cells in an environment of vascular niche platform (Sandler et al. 2014). This direct reprogramming approach not only presents a plausible way to generate engraftable HSPCs, but also offers a platform to investigate the roles of transcription factors and microenvironment cues in hematopoietic development. Induction of hemogenic reprogramming is also done in mouse fibroblasts by ectopic expression of transcription factors, GATA2, GFI1, cFOS, and ETV6 (Pereira et al. 2013). However, the engraftment potential of fibroblast-reprogramming derived hematopoietic progenitor cells has not been demonstrated in vivo (Pereira et al. 2013). The human fibroblast reprogramming to hematopoietic progenitor cells has been reported (Cheng et al. 2000). Ectopic expression of Oct 4, a master transcription factor for the pluripotency of ES cells and iPS cells, in human dermal fibroblasts induces the generation of CD45+ hematopoietic progenitor cells that give rise to granulocytic, monocytic, megakaryocytic and erythroid lineages, and demonstrate in vivo engraftment capacity (Cheng et al. 2000). Oct 4, Sox 2, Klf4 and c-Myc are capable to reprogram fibroblasts to iPS cells (Takahashi et al. 2006). It is unclear whether overexpression of Oct 4 in fibroblasts induces plastic intermediates that can be selected for hematopoietic progenitor cells in appropriate culture condition. Nevertheless, directly reprogramming non-hematopoietic cells into engraftable human hematopoietic stem and progenitor cells (or specific lineage-committed cells) is an exciting research area.
5. Future prospective of hPSC hematopoietic differentiation for translational application
Hematopoietic capability of hESCs and hiPSCs provides a novel opportunity for regenerative medicine and pathogenic modeling, though many hurdles need to be overcome before therapeutic application. The low efficient production of hematopoietic cells is one of major hurdles in hPSC hematopoietic application. To efficiently generate hematopoietic cells, not only technical innovation is required, but also a better understanding of molecular relevance connecting to hematopoietic development is needed. Dependent on the developmental stages, some molecules exert positive and negative effects on the differentiation of HE cells. Dissecting molecular events during hPSC hematopoietic differentiation should provide insight for generating hematopoietic cells with high efficiency.
Identification of transplantable hematopoietic cells is another critical factor for clinical applications. During hPSCs differentiation, hematopoietic progenitor cells emerge from HE cells, suggesting a sequential developmental order. However, in vitro differentiation may not exactly reproduce embryo events in vivo, such as the timing differences in vitro and in vivo (Figure 2). Whether hPSC-derived HE cells represent primitive or definitive hematopoietic precursors is still debatable. Therefore, the generation of engraftable HSCs from hPSC-HE cells needs further exploration by comparing genotypes and phenotypes of HE-derived HSCs with adult HSCs. Recapitulations of the complex in vivo regulating hPSC-derived products and microenvironments are crucial for determining the implanted cellular fate. The new era will start at a successful stride to translate hPSC-derived hematopoietic cells into clinical therapeutic progress.
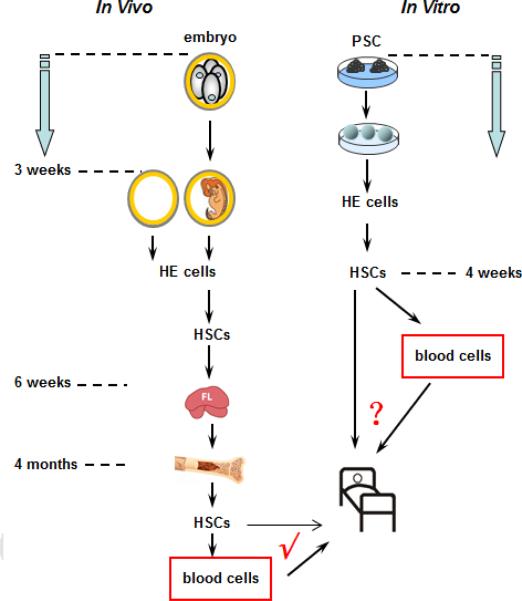
Figure 2. Timeline of hematopoietic events in in vitro hPSCs differentiation and in vivo embryo development.
Time required for hematopoietic differentiation in hPSCs model (in vitro) and in normal embryo development (in vivo). Both hemangioblast and hemogenic endothelium (HE) have been recognized as two stages of hematopoietic progenitors to give rise to mature blood cells. However, the biological similarity of blood cells from the in vitro hPSC model and from normal embryo development need further evidence to determine future translational application.
Acknowledgements
This study was supported by National Natural Science Foundation of China (31371480) and Foundation from Science and Technology Commission of Shanghai Municipality (13JC1406404) and by Maryland Stem Cell Research Fund (2012-MSCRFII-0124) and NIH/NHLBI U01 HL107446.
Grant sponsor: National Natural Science Foundation of China; Grant number: 31371480;
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jcb.25097]
Disclosures
The authors indicate no potential conflict of interest.
References
- Amabile G, Welner RS, Nombela-Arrieta C, D'Alise AM, Di Ruscio A, Ebralidze AK, Kraytsberg Y, Ye M, Kocher O, Neuberg DS, Khrapko K, Silberstein LE, Tenen DG. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121(8):1255–1264. doi: 10.1182/blood-2012-06-434407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antas VI, Al-Drees MA, Prudence AJ, Sugiyama D, Fraser ST. Hemogenic endothelium: a vessel for blood production. Int J Biochem Cell Biol. 2013;45(3):692–695. doi: 10.1016/j.biocel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Huang H, Lu L. Hematopoietic stem cells in the mouse embryonic yolk sac. Stem Cells. 1996;14(3):269–280. doi: 10.1002/stem.140269. [DOI] [PubMed] [Google Scholar]
- Awong G, Herer E, Surh CD, Dick JE, La Motte-Mohs RN, Zuniga-Pflucker JC. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114(5):972–982. doi: 10.1182/blood-2008-10-187013. [DOI] [PubMed] [Google Scholar]
- Bai H, Xie YL, Gao YX, Cheng T, Wang ZZ. The Balance of Positive and Negative Effects of TGF-beta Signaling Regulates the Development of Hematopoietic and Endothelial Progenitors in Human Pluripotent Stem Cells. Stem Cells Dev. 2013;22(20):2765–2776. doi: 10.1089/scd.2013.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Xie YL, Gao YX, Cheng T, Wang ZZ. The Balance of Positive and Negative Effects of TGF-ss Signaling Regulates the Development of Hematopoietic and Endothelial progenitors in Human Pluripotent Stem Cells. Stem Cells Dev. 2013 doi: 10.1089/scd.2013.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron MH. Concise Review: Early Embryonic Erythropoiesis: Not so Primitive After All. Stem Cells. 2013;31(5):849–856. doi: 10.1002/stem.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22(7):1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464(7285):116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Bowles KM, Vallier L, Smith JR, Alexander MR, Pedersen RA. HOXB4 overexpression promotes hematopoietic development by human embryonic stem cells. Stem Cells. 2006;24(5):1359–1369. doi: 10.1634/stemcells.2005-0210. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45(5):1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6(3):265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Qasba P, Vanguri P, Thiede MA. Human mesenchymal stem cells support megakaryocyte and pro-platelet formation from CD34(+) hematopoietic progenitor cells. J Cell Physiol. 2000;184(1):58–69. doi: 10.1002/(SICI)1097-4652(200007)184:1<58::AID-JCP6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Choi KD, Vodyanik MA, Slukvin Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119(9):2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Vodyanik MA, Togarrati PP, Suknuntha K, Kumar A, Samarjeet F, Probasco MD, Tian S, Stewart R, Thomson JA, Slukvin Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2(3):553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civin CI, Almeida-Porada G, Lee MJ, Olweus J, Terstappen LW, Zanjani ED. Sustained, retransplantable, multilineage engraftment of highly purified adult human bone marrow stem cells in vivo. Blood. 1996;88(11):4102–4109. [PubMed] [Google Scholar]
- Clements WK, Traver D. Signalling pathways that control vertebrate haematopoietic stem cell specification. Nat Rev Immunol. 2013;13(5):336–348. doi: 10.1038/nri3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15(3):477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol. 1975;33(3):607–619. [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9(2):129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Feng Q, Lu SJ, Klimanskaya I, Gomes I, Kim D, Chung Y, Honig GR, Kim KS, Lanza R. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28(4):704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33(9):1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Galic Z, Kitchen SG, Kacena A, Subramanian A, Burke B, Cortado R, Zack JA. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(31):11742–11747. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokoh M, Nishio M, Nakamura N, Matsuyama S, Nakahara M, Suzuki S, Mitsumoto M, Akutsu H, Umezawa A, Yasuda K, Yuo A, Saeki K. Early senescence is not an inevitable fate of human-induced pluripotent stem-derived cells. Cell Reprogram. 2011;13(4):361–370. doi: 10.1089/cell.2011.0004. [DOI] [PubMed] [Google Scholar]
- Gordon-Keylock S, Sobiesiak M, Rybtsov S, Moore K, Medvinsky A. Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood. 2013;122(14):2338–2345. doi: 10.1182/blood-2012-12-470971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12(4):407–412. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Hirschi KK. Hemogenic endothelium during development and beyond. Blood. 2012;119(21):4823–4827. doi: 10.1182/blood-2011-12-353466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK. Hemogenic endothelium during development and beyond. Blood. 2012;119(21):4823–4827. doi: 10.1182/blood-2011-12-353466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432(7017):625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- Ito T, Ishida Y, Kashiwagi R, Kuriya S. Recombinant human c-Mpl ligand is not a direct stimulator of proplatelet formation in mature human megakaryocytes. Br J Haematol. 1996;94(2):387–390. doi: 10.1046/j.1365-2141.1996.d01-1813.x. [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125(22):4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- Jollie WP. Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology. 1990;41(4):361–381. doi: 10.1002/tera.1420410403. [DOI] [PubMed] [Google Scholar]
- Jordan HE. Aortic Cell Clusters in Vertebrate Embryos. Proc Natl Acad Sci U S A. 1917;3(3):149–156. doi: 10.1073/pnas.3.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur AR, Jordan JE, Brandt SJ. The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood. 1994;83(5):1200–1208. [PubMed] [Google Scholar]
- Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98(19):10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M, Awong G, Sturgeon CM, Ditadi A, Lamotte-Mohs R, Zuniga-Pflucker JC, Keller G. T Lymphocyte Potential Marks the Emergence of Definitive Hematopoietic Progenitors in Human Pluripotent Stem Cell Differentiation Cultures. Cell Rep. 2012 doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Kennedy M, D'Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109(7):2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386(6624):488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109(1):29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood. 2010;116(6):909–914. doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457(7231):892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledran MH, Krassowska A, Armstrong L, Dimmick I, Renstrom J, Lang R, Yung S, Santibanez-Coref M, Dzierzak E, Stojkovic M, Oostendorp RA, Forrester L, Lako M. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3(1):85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99(7):4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lan Y, He W, Chen D, Wang J, Zhou F, Wang Y, Sun H, Chen X, Xu C, Li S, Pang Y, Zhang G, Yang L, Zhu L, Fan M, Shang A, Ju Z, Luo L, Ding Y, Guo W, Yuan W, Yang X, Liu B. Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell. 2012;11(5):663–675. doi: 10.1016/j.stem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Lichanska AM, Hume DA. Origins and functions of phagocytes in the embryo. Exp Hematol. 2000;28(6):601–611. doi: 10.1016/s0301-472x(00)00157-0. [DOI] [PubMed] [Google Scholar]
- Lu SJ, Feng Q, Park JS, Lanza R. Directed differentiation of red blood cells from human embryonic stem cells. Methods Mol Biol. 2010;636:105–121. doi: 10.1007/978-1-60761-691-7_7. [DOI] [PubMed] [Google Scholar]
- Lu SJ, Hipp JA, Feng Q, Hipp JD, Lanza R, Atala A. GeneChip analysis of human embryonic stem cell differentiation into hemangioblasts: an in silico dissection of mixed phenotypes. Genome Biol. 2007;8(11):R240. doi: 10.1186/gb-2007-8-11-r240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SJ, Li F, Yin H, Feng Q, Kimbrel EA, Hahm E, Thon JN, Wang W, Italiano JE, Cho J, Lanza R. Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Res. 2011;21(3):530–545. doi: 10.1038/cr.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111(7):3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Ebihara Y, Umeda K, Sakai H, Hanada S, Zhang H, Zaike Y, Tsuchida E, Nakahata T, Nakauchi H, Tsuji K. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci U S A. 2008;105(35):13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CH, Woll PS, Ni Z, Zuniga-Pflucker JC, Kaufman DS. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood. 2008;112(7):2730–2737. doi: 10.1182/blood-2008-01-133801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Michelson AD. Flow cytometry: a clinical test of platelet function. Blood. 1996;87(12):4925–4936. [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72(6):835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Nakano H, Liu X, Arshi A, Nakashima Y, van Handel B, Sasidharan R, Harmon AW, Shin JH, Schwartz RJ, Conway SJ, Harvey RP, Pashmforoush M, Mikkola HK, Nakano A. Haemogenic endocardium contributes to transient definitive haematopoiesis. Nat Commun. 2013;4:1564. doi: 10.1038/ncomms2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126(11):2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84(2):321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Orkin SH. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80(3):575–581. [PubMed] [Google Scholar]
- Palis J, Chan RJ, Koniski A, Patel R, Starr M, Yoder MC. Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc Natl Acad Sci U S A. 2001;98(8):4528–4533. doi: 10.1073/pnas.071002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001;29(8):927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- Park C, Ma YD, Choi K. Evidence for the hemangioblast. Exp Hematol. 2005;33(9):965–970. doi: 10.1016/j.exphem.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Pereira CF, Chang B, Qiu J, Niu X, Papatsenko D, Hendry CE, Clark NR, Nomura-Kitabayashi A, Kovacic JC, Ma'ayan A, Schaniel C, Lemischka IR, Moore K. Induction of a hemogenic program in mouse fibroblasts. Cell Stem Cell. 2013;13(2):205–218. doi: 10.1016/j.stem.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Kloss CC, Butler JM, Ginsberg M, Gars E, Lis R, Zhan Q, Josipovic P, Ding BS, Xiang J, Elemento O, Zaninovic N, Rosenwaks Z, Sadelain M, Rafii JA, James D. Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood. 2013;121(5):770–780. doi: 10.1182/blood-2012-07-444208. [DOI] [PubMed] [Google Scholar]
- Ran D, Shia WJ, Lo MC, Fan JB, Knorr DA, Ferrell PI, Ye Z, Yan M, Cheng L, Kaufman DS, Zhang DE. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013;121(15):2882–2890. doi: 10.1182/blood-2012-08-451641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2(3):252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler VM, Lis R, Liu Y, Kedem A, James D, Elemento O, Butler JM, Scandura JM, Rafii S. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014;511(7509):312–318. doi: 10.1038/nature13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5(4):410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- Schulz TC, Noggle SA, Palmarini GM, Weiler DA, Lyons IG, Pensa KA, Meedeniya AC, Davidson BP, Lambert NA, Condie BG. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22(7):1218–1238. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, Zhang F, Vertes EL, Kobayashi M, Zhang Y, Shmelkov SV, Hackett NR, Rabbany S, Boyer JL, Rafii S. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A. 2008;105(49):19288–19293. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R, Kim JH. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50(6):1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- Slukvin Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013;122(25):4035–4046. doi: 10.1182/blood-2013-07-474825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014;32(6):554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Frye C, Bae KM, Kelley V, Vieweg J. Differentiation of human embryonic stem cells into immunostimulatory dendritic cells under feeder-free culture conditions. Clin Cancer Res. 2008;14(19):6207–6217. doi: 10.1158/1078-0432.CCR-08-0309. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yamazaki S, Yamaguchi T, Okabe M, Masaki H, Takaki S, Otsu M, Nakauchi H. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther. 2013;21(7):1424–1431. doi: 10.1038/mt.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiers G, Baumann C, O'Rourke J, Giannoulatou E, Taylor S, Joshi A, Moignard V, Pina C, Bee T, Kokkaliaris KD, Yoshimoto M, Yoder MC, Frampton J, Schroeder T, Enver T, Gottgens B, de Bruijn MF. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nat Commun. 2013;4:2924. doi: 10.1038/ncomms3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Taoudi S, Gonneau C, Moore K, Sheridan JM, Blackburn CC, Taylor E, Medvinsky A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3(1):99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Tavian M, Biasch K, Sinka L, Vallet J, Peault B. Embryonic origin of human hematopoiesis. Int J Dev Biol. 2010;54(6-7):1061–1065. doi: 10.1387/ijdb.103097mt. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tian X, Woll PS, Morris JK, Linehan JL, Kaufman DS. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24(5):1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- Timmermans F, Velghe I, Vanwalleghem L, De Smedt M, Van Coppernolle S, Taghon T, Moore HD, Leclercq G, Langerak AW, Kerre T, Plum J, Vandekerckhove B. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol. 2009;182(11):6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- Vodyanik MA, Bork JA, Thomson JA, Slukvin Human embryonic stem cell-derived CD34+ cells: efficient production in the co-culture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105(2):617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- Wang C, Tang X, Sun X, Miao Z, Lv Y, Yang Y, Zhang H, Zhang P, Liu Y, Du L, Gao Y, Yin M, Ding M, Deng H. TGFbeta inhibition enhances the generation of hematopoietic progenitors from human ES cell-derived hemogenic endothelial cells using a stepwise strategy. Cell Res. 2012;22(1):194–207. doi: 10.1038/cr.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li L, Shojaei F, Levac K, Cerdan C, Menendez P, Martin T, Rouleau A, Bhatia M. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21(1):31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang L, Menendez P, Shojaei F, Li L, Mazurier F, Dick JE, Cerdan C, Levac K, Bhatia M. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201(10):1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Rodriguez RT, Wang J, Ghodasara A, Kim SK. Targeting SOX17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell. 2011;8(3):335–346. doi: 10.1016/j.stem.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A. 1996;93(8):3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel KC, Gao Y, Shieh JH, Moore MA. Stromal cell lines from the aorta-gonado-mesonephros region are potent supporters of murine and human hematopoiesis. Exp Hematol. 2006;34(11):1505–1516. doi: 10.1016/j.exphem.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Yahata T, Ando K, Nakamura Y, Ueyama Y, Shimamura K, Tamaoki N, Kato S, Hotta T. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor gamma null mice. J Immunol. 2002;169(1):204–209. doi: 10.4049/jimmunol.169.1.204. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8(24):3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang YY, Dang CV, Spivak JL, Moliterno AR, Cheng L. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114(27):5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M, Yoder MC. Developmental biology: Birth of the blood cell. Nature. 2009;457(7231):801–803. doi: 10.1038/457801a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PE, Baumhueter S, Lasky LA. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood. 1995;85(1):96–105. [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106(3):860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XB, Beard BC, Trobridge GD, Wood BL, Sale GE, Sud R, Humphries RK, Kiem HP. High incidence of leukemia in large animals after stem cell gene therapy with a HOXB4-expressing retroviral vector. The Journal of clinical investigation. 2008;118(4):1502–1510. doi: 10.1172/JCI34371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen F, Lan Y, Yan B, Zhang W, Wen Z. Hemogenic endothelium specification and hematopoietic stem cell maintenance employ distinct Scl isoforms. Development. 2013;140(19):3977–3985. doi: 10.1242/dev.097071. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3(6):625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]