Abstract
Prostate cancer (PCa) remains a leading cause of cancer-related death in the USA. While localized lesions are effectively treated through radical prostatectomy and/or radiation therapy, treatment for metastatic disease leverages the addiction of these tumors on the androgen receptor (AR) signaling axis for growth and disease progression. Though initially effective, tumors resistant to AR-directed therapeutics ultimately arise (a stage of the disease known as castration-resistant prostate cancer) and are responsible for PCa-specific mortality. Importantly, an abundance of clinical and preclinical evidence strongly implicates AR signaling cascades in the development of metastatic disease in both early and late stages, and thus a concerted effort has been made to delineate the AR-specific programs that facilitate progression to metastatic PCa. A multitude of downstream AR targets as well as critical AR cofactors have been identified which impinge upon both the AR pathway as well as associated metastatic phenotypes. This review will highlight the functional significance of these pathways to disseminated disease and define the molecular underpinnings behind these unique, AR-driven, metastatic signatures.
Keywords: Androgen, Androgen receptor (AR), Prostate cancer (PCa), Castration-resistant prostate cancer, CRPC, Metastasis, Cistrome
1 Overview: prostate cancer development, treatment, and progression
Prostate cancer (PCa) is a leading health concern in the USA with nearly 32,000 deaths attributed to the disease annually [1]. It remains the most common noncutaneous malignancy diagnosed in US men and is the second leading cause of cancer-related deaths [1]. A favorable prognosis is heavy reliant upon early detection of the primary tumor, where localized lesions are effectively treated by radical prostatectomy and/or radiation therapy [2]. Disseminated disease, however, proves much more of a clinical challenge. Upon radiographic evidence of metastatic lesions (most commonly found in local lymph nodes and bone at the earliest stages), patients undergo systemic therapy to thwart cancer growth and progression [3–6]. Unlike other solid tumors, prostatic adenocarcinoma, both at primary and metastatic sites, responds poorly to standard chemotherapeutic intervention [7–9]. Therefore, first-line treatment for this disseminated disease leverages the addiction of these tumors on androgens for both growth and survival [4, 10, 11].
Molecular modeling of PCa has demonstrated that the action of androgens is manifest through activation of the androgen receptor (AR), whose activity is critical for PCa development and progression at all stages of disease [4]. As such, patients with metastatic tumors are treated with androgen deprivation therapy to biochemically dampen AR signaling [5, 6]. Initially, this therapy is quite effective resulting in either stable disease or tumor regression as measured biochemically (via serum prostate-specific antigen (PSA)) or radiographically [6, 11]. However, within 2 to 3 years, progression is often noted despite suppression of testosterone, a stage of the disease known as castration-resistant prostate cancer (CRPC). Herein, the AR signaling axis has been inappropriately reactivated allowing for upregulation of growth and survival pathways necessary for tumor proliferation and metastatic progression [10, 12, 13]. This results in enhanced metastatic tumor burden, and ultimately patient mortality [14]. As a result, there is an intense effort to not only more effectively target AR in CRPC, but to define the molecular pathways governed by AR which are responsible for such lethal metastatic events.
2 Role of AR in disease progression
AR is a large ~110 kDa transcription factor which belongs to the steroid nuclear receptor family, which functions as a ligand-dependent transcription factor necessary for development and function of the prostate [15]. In the normal prostate, AR plays a critical role in the early formation of the prostatic epithelia, directing key developmental programs responsible for proper formation of epithelial architecture and differentiation [15]. Importantly in this context, AR also acts to limit prostatic epithelial growth, which is essential for the development and function of prostatic tissue.
As a function of tumorigenesis, the transcriptional targets of AR are altered, engaging pro-proliferative programs essential for growth of prostatic adenocarcinoma cells [16]. Activation of this pathway is heavily reliant upon circulating androgens, which enter epithelial cells and are rapidly converted to the potent AR ligand 5α-dihydrotestosterone (5α-DHT) by the enzyme 5α-reductase [17]. This facilitates the binding of 5α-DHT to the ligand binding domain (LBD) of AR, shifting the position of helix 12 of the LBD towards the N-terminus [18, 19]. Functionally, this results in enhanced stability of the protein and release from inhibitory heat shock proteins. Agonist-bound AR translocates to the nucleus where it dimerizes and binds to chromatin in a site- and cell type-specific manner [20]. Once bound, AR undergoes a multitude of posttranslational modifications (including phosphorylation, methylation, sumoylation, and acetylation) [21, 22] which facilitate co-factor recruitment and chromatin remodeling [23]. Ultimately, this leads to AR-specific programs critical for the survival and growth of PCa cells [16]. Clinically, the activity of AR can be monitored through the induction of the well-characterized AR target gene KLK3, which encodes a secreted protease PSA [6]. Levels of PSA can be monitored in patients serum and used a surrogate for AR activity and associated tumor burden [6]. As such, elevated or sustained increases in PSA levels are used (at least in part) as a marker of PCa development and progression.
2.1 Targeting AR in advanced disease
Work initiated by Huggins and Hodge in the early part of the twentieth century identified the first effective treatment for PCa, androgen deprivation therapy (ADT) [24–26]. To date, ADT still remains the most effective form of treatment for patients with disseminated disease. Standard of care options most often involve chemical castration through use of gonadotropin-releasing hormone (GnRH) agonists which, after an initial surge of testosterone production [27], suppress the secretion of leutinizing hormone (LH) and functionally inhibit testicular androgen synthesis [11, 28]. To prevent tumor growth in response to the testosterone flare, AR antagonists (e.g., biculatamide) are often given in combination with GnRH agonists, a therapeutic strategy known as total or maximum androgen blockade [11]. As a result, circulating testosterone levels often drop to levels seen in castrated males (<0.2 ng/dl [29]), which frequently correlate with a decline in serum PSA levels and stable or decreased tumor burden. Despite this, recurrence is common and within 2–3 years patients begin to experience a resurgence of tumor growth heralded by increases in serum PSA levels (biochemical recurrence), indicative of a reengagement of the AR signaling axis.
At this stage of the disease (metastatic CRPC), tumors have evolved mechanisms to reactivate AR despite continued ADT. As reviewed [4, 10, 30], a multitude of pathways have been described which contribute to inappropriate AR activity in CRPC, many of which involve manipulation of AR itself (amplification, alternative splicing, mutation, and post-translational modifications). Upstream of AR, however, recent evidence also suggests that tumors gain the ability to produce their own androgens, increasing the local androgen pool to sufficient levels to activate the AR axis [10, 31]. Microarray profiling of patient samples confirmed that key genes involved in the conversion of cholesterol derivatives to androgen precursors (CYP17A and HSDD3B2) are induced in response to ADT [32]. Consequently, direct inhibitors of CYP17 have been developed (orteronel (TAK-700) [13], galeterone (TOK-001) [14]) and currently, abiraterone acetate is approved for the treatment of metastatic CRPC patients who have disease progression despite ADT both pre- and postchemotherapy [33–37]. In clinical trials, administration of abiraterone acetate significantly increased time to biochemical progression and radiographic progression-free survival, indicating that metastatic tumors are still reliant on AR signaling at this stage [34, 35, 38]. As such, some clinical benefit has been seen with the introduction of novel anti-androgens (e.g., Enzalutamide), which extended patient survival by ~3 months [39, 40]. However, once resistant to hormone-directed therapies, taxanes are the next therapeutic option for patients, which, unfortunately only affords a 2–3 months survival advantage (with a high rate of patient morbidity) [8, 9, 41]. As a result, there is a critical need to better define AR-driven networks responsible for lethal tumor phenotypes to more accurately and effectively combat metastatic disease.
2.2 Current strategies for targeting metastatic CRPC
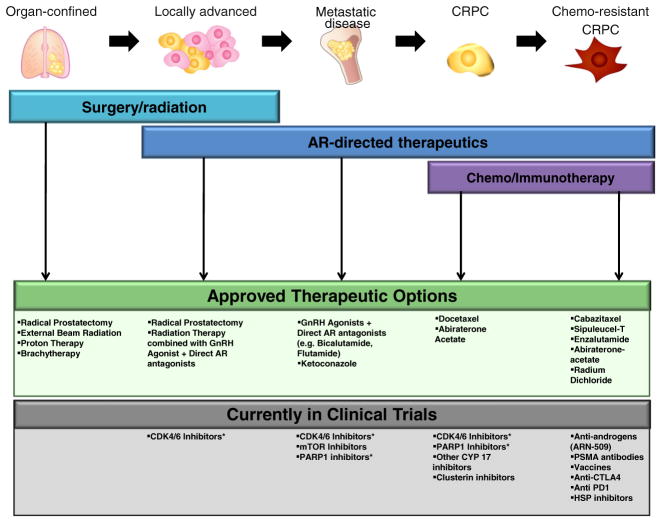
Therapeutic options for treating disease that progresses after hormone therapy are extremely limited (Fig. 1). Taxane-based chemotherapy has proven to be the most effective chemotherapeutic in the management of metastatic CRPC [9, 41]. Preclinical evidence suggests that taxanes inhibit tubulin polymerization, a critical cytoskeletal protein involved in maintenance of cellular integrity and architecture, mitosis, intracellular transport, cell signaling, and gene expression. Docetaxel, a semisynthetic taxane derived from the needles of Taxus baccata that reversibly binds to microtubules with high affinity, has been demonstrated to provide a 20–24 % improvement in survival for men with metastatic castrate-resistant prostate cancer [9, 41] and was the first Food and Drug Administration-approved agent for this patient population. Subsequent trials combining various chemotherapeutic or biologic agents to docetaxel have not yielded improved survival. However, preclinical work demonstrated that a second-line taxane, cabazitaxel, had cytotoxicity in cell lines and animal models both sensitive and resistant to docetaxel [36, 41, 42]. While the mechanism of overcoming docetaxel resistance is unclear, clinical evidence has validated the efficacy of this agent as it was shown to improve survival in CRPC patients who had received prior docetaxel [43]. Given the effectiveness in the docetaxel-pretreated patient population, there is currently an ongoing international randomized trial comparing first-line docetaxel versus cabazitaxel (trial #NCT01308567).
Fig. 1.
Therapeutic options for patients diagnosed with prostate cancer. Therapeutic options for localized PCa often involve surgery in combination with some form of radiation therapy. In contrast, advanced or metastatic disease leverages the requirement of androgens for tumor growth, utilizing systemic therapy to limit androgen production and directly inhibit AR activity. Once resistant to first-line hormonal therapy, options to limit tumor growth and progression are limited, and consist of second-line hormonal therapy or taxane-based chemotherapeutics. While there are several options which are providing benefit in the chemotherapy-resistant space, a number of clinical trials are currently ongoing which offer promise for earlier intervention to help thwart progression to lethal CRPC [34, 36, 38, 39, 125–129]. Trials can be found online at www.clinicalrials.gov: trial #NCT01171898, NCT01414283, NCT01695044, NCT00694551, NCT00705835, NCT01804465, NCT00113984, NCT01420965, NCT01078662, NCT00471432, NCT01548807, and NCT01546987. *Clinical trials are currently in the planning stages at the University of Michigan and Thomas Jefferson University
In addition to chemotherapy, a new agent was recently approved to treat CRPC of the bone. PCa primarily metastasizes to bone, where tumor growth leads to high fracture rates and is associated with patient morbidity [44, 45]. Clinically, high tumor burden at such metastatic sites correlates with decreased survival time [14], yet few therapeutic options are available which specifically target tumors at these sites. Currently, only Radium-223 dichloride (a radioactive α-emitting isotope which mimics the valence structure of calcium ions) has been developed as an effective treatment strategy for bone-specific metastases [36, 46]. A phase III clinical trial of CRPC patients demonstrated that treatment with Radium-223 significantly reduced first skeletal-related events and prolonged overall survival (~3 months) as compared to placebo [46]. Interestingly, however, it is unknown as to how effective Radium-223 would be in the hormone therapy (HT)-naive setting. Clinical data suggests that an intact AR signaling axis is critical for the progression of metastatic lesions. Thus it is tempting to speculate that combination ADT and Radium-223 would effectively inhibit PCa progression in hormone-sensitive metastatic disease. Future efforts will likely test this hypothesis to determine if such combinations could be leveraged for patient benefit. While Radium-223 represents a significant step forward in combating metastatic PCa progression, few strategies have been developed which specifically target metastatic programs unique to PCa.
3 Role of the AR in the development of metastatic disease
Given the poor prognosis associated with the development of metastatic prostate cancer and the known role of AR in promoting disease progression, a concerted effort was undertaken to identify the functions(s) by which AR facilitates the metastatic process and/or maintenance of metastatic disease. As will be reviewed herein, translational investigative studies revealed major roles for AR in promoting prometastatic events, through: (1) differential chemokine receptor/ligand function; (2) altered function of tumor-associated AR cofactors (e.g., FOXA1, cyclin D1b, and SWI/SNF); and (3) formation of prometastatic, AR-dependent gene fusions and downstream effectors (e.g., SOX9).
3.1 I. Chemokine and chemokine receptor dysregulation in PCa
Chemokine receptors belong to the G-protein coupled receptor (GPCR) family of proteins. There are over 20 known members of this family each of which contains a generally conserved structure consisting of a seven-pass transmembrane monomer and a soluble cytoplasmic tail [47, 48]. While expression is fairly ubiquitous, these receptors are known to have significant roles in the immune response, development, angiogenesis, and inflammation. Activation occurs upon ligand binding (chemokine family of soluble ligands) which initiates a series of signal transduction pathways responsible for mediating a multitude of cellular responses including actin skeleton rearrangements, calcium signaling, and survival (among others). Given the critical role these pathways play in cancer progression, chemokine receptors are often deregulated in human disease [47–49]. Importantly, as several of these receptors (and their ligands) have been shown to be regulated by AR [50, 51], there is growing interest in determining the relevance of these receptors in mediating metastatic phenotypes in both hormone therapy-sensitive and castration-resistant prostate cancer.
3.1.1 CXCR4
CXCR4 is a 352 amino acid GPCR which is activated though association with its soluble ligand SDF1 (also known as CXCL12) [47]. Upon activation, intramolecular signaling cascades (which vary with cell type) are transmitted through the cell, culminating in cytoskeletal rearrangements which promote migration towards the site of activation as well as transcriptional programs which regulate growth and survival [47]. The prostate gland is one of the few soft tissues which express detectable levels of CXCR4, though it is unclear if it plays a significant role in normal prostatic function [52]. Importantly, however, as a function of tumorigenesis, there is a marked induction of CXCR4 both at the transcript and protein levels [53]. Immunohistochemical analyses of PCa lesions confirm heightened expression of the receptor in both primary and metastatic disease (compared to benign control), with the highest expression detectable in bone metastases [53]. Of note, tissue-wide interrogation of SDF-1 expression indicated that bone expresses relatively high levels SDF-1 [54]; the bone representing a preferential site for PCa metastatic colonization. This supports the concept that induction of CXCR4 (as a consequence of tumorigenesis) functions to promote metastatic progression via attraction to the high SDF-1 gradient generated in tissues such as bone. While more clinical data is needed to confirm such conclusions, multiple clinically relevant models have demonstrated that CXCR4 is regulated as a function of androgen signaling, and it is likely that the interplay between the two promotes lethal metastatic events.
Multiple AR-positive PCa models have been used to describe enhanced migratory potential of CXCR4 expressing cells towards a gradient generated by SDF-1 [50]. Importantly however, such phenotypes were only observable in the presence of androgen, indicating that CXCR4-dependent metastatic phenotypes required an intact AR signaling axis [50]. Such conclusions are supported by transcriptional evidence which demonstrates robust induction of CXCR4 transcript in response to androgen stimulation [50], which can be reversed though either administration of ADT or direct AR antagonists. Exploration into the underlying mechanisms behind AR-induced CXCR4 expression revealed that the relationship was likely indirect. Chip sequencing analyses from multiple AR-positive cell lines lacked AR binding within a 50 kb window of the transcriptional start site [55, 56]. Such conclusions were confirmed wherein ligand bound AR was unable to promote CXCR4 induction in the presence of protein synthesis inhibitors. Subsequent analysis of AR-regulated transcription factors capable of fostering CXCR4 expression uncovered Kruppel-like-factor 5 (KLF5) as a potential candidate [50]. Indeed, transcript analyses from LNCaP cells revealed a robust and rapid induction of KLF5 transcript upon AR activation. Additionally, expression of exogenous KLF5 in a low-androgen setting was sufficient to promote CXCR4 expression and invasion toward an SDF-1 generated gradient [50]. Collectively, these data demonstrate a tumor-specific AR-driven pathway which promotes invasive phenotypes in vitro. While intriguing, it will be critical to determine if AR/KLF5-induced CXCR4 expression is sufficient to promote metastatic phenotypes in vivo, and whether or not such pathways are preserved in CRPC tumors. In addition, profiling of the ERG cistrome and transcriptome identified CXCR4 as a putative target of ERG signaling in fusion-positive disease [57]. Given the known metastatic functions of ERG, AR, and CXCR4, it will be important to determine how the interplay between these factors cooperate to promote metastatic disease, and if this pathway offers a novel therapeutic target to thwart metastatic progression in AR-positive, fusion-positive disease.
3.1.2 CX3CR1/fractalkine
While there is an abundance of literature examining tumor-associated androgen signaling and its role in mediating metastatic programs, there is a gap in knowledge as to the potential role of AR in facilitating metastatic events in distal tissues. Though AR is expressed in many tissues, its activity greatly influences bone development [58]. Congruent with this concept, a recent study has identified a novel role for androgen signaling in regulating the cleavage of fractalkine (CX3CL1), a membrane-bound cytokine which is expressed human bone marrow endothelial cells and possesses intrinsic cell adhesive properties [51, 59]. Previous studies utilizing human immune cells which express the cognate receptor for fractalkine, CX3CR1, showed direct cellular binding to endothelial cells, which likely aided cellular extravasation [60–62]. Analogous ex vivo studies utilizing PCa models demonstrated similar binding kinetics to bone marrow endothelial cells, which occurred in a fractalkine-dependent manner [51]. Further investigation determined that, though the induction of extracellular matrix-metalloproteases (e.g., ADAM17), AR signaling can promote the cleavage of fractalkine from bone cells but not bone marrow endothelium [59]. This supports a hypothesis whereby PCa cells bearing CX3CR1, adhere to endothelial bone walls expressing fractalkine, and extravasate towards the soluble fractalkine gradient generated by the AR signaling axis, ultimately resulting in the colonization of the bone microenvironment. Given that first-line therapy for metastatic disease involves androgen ablation, it will be interesting to determine how systemic hormonal therapy affects both fractalkine cleavage and metastatic growth within the bone. Furthermore, as a major mechanism through which PCa tumors evade ADT is through production of their own androgens [10, 13, 32], it would be useful to determine if local production of androgens within CPRC bone metastatic tumors further promotes fractalkine cleavage, bone homing, and subsequent metastatic tumor formation. Future efforts will likely define the clinical relevance of this metastatic cascade in both early and late stages of disease, and determine if targeting this pathway can be leveraged to limit metastatic tumor burden.
3.2 II. AR cofactor deregulation
The discovery of AR-regulated expression of chemokine receptors and ligands represent a significant advance in the search for AR-driven pathways, which could potentially be leveraged to limit metastatic progression. However, it is improbable that a single AR target is responsible for development of metastatic disease. More likely, disease-specific AR-driven networks are causal for invasive phenotypes and development of metastatic lesions. Indeed, recent studies uncovered multiple AR-associated cofactors capable of modulating and “reprogramming” genome-wide AR binding events. Such differential AR signatures have been associated with enhanced metastatic potential in vitro and in vivo, and have putatively significant implications for the development and progression of AR-driven metastatic events.
3.2.1 FOXA1 controls AR signaling and metastatic events in PCa
The ability of AR to localize to sites of transcriptional regulation upon activation is highly dependent on chromatin state and structure. Repressed, or closed, chromatin is often defined by repressive histone marks (such as H3K9me2) and lacks enrichment for transcriptional regulators, like AR [63–65]. Conversely, active chromatin structures are associated with mono- and dimethylation marks on H3K4 (H3K4me1 or H3K4me2), which are often coupled to sites of active transcription [63–65]. In the context of hormone-dependent tissues, such markers of active transcription are often enriched for binding of the FOXA family of transcription factors, also known as “pioneer factors” that facilitate site-specific chromatin remodeling and subsequent nuclear receptor binding [63, 64, 66–69]. Importantly, genome-wide analysis of the AR cistrome in PCa cells found a high degree of overlap with the FOXA family of proteins (nearly 70 % overlap) [68], consistent with the known role of FOXA factors serving as pioneer factors, which help to define both active and repressed chromatin states. More specifically, FOXA1 has been demonstrated to be a critical mediator of AR signaling in PCa, as loss of FOXA1 in AR-positive PCa cells, decreased both cell growth and AR signaling (as determined by AR target gene levels) [70, 71]. Moreover, analysis of the AR cistrome in the CRPC line LNCaP-Abl defined a critical role for FOXA1 in mediating AR recruitment to critical cell cycle genes (e.g., UBE2C), which facilitated castration-resistant growth [55]. Consistent with hormone-dependent AR signaling, loss of FOXA1 dramatically decreased growth at this stage indicating that FOXA1 is required for AR-driven proliferative events in both early and late stages of disease [55]. Contrary to what might be expected, knockdown of FOXA1 did not eliminate AR binding across the genome. In fact, depletion of FOXA1 levels resulted in an increase of total AR binding events genome wide (13,505 unique binding events with knockdown) [68, 70], indicating that tumor-associated elevation of FOXA1 levels dramatically restrict the AR cistrome, which likely results in AR-driven signatures responsible for both proliferative and metastatic events. Future efforts will aim to better define the functions of FOXA1 responsible for such divergent AR functions, and the downstream targets responsible for mediating aggressive phenotypes.
Given the knowledge above, it is not surprising that a number of studies have aimed to characterize the expression patterns of FOXA1 in lethal metastatic disease. Interestingly, however, FOXA1 levels have been reported as both elevated or diminished in PCa [71–74]. Such discrepancies could be attributed to potentially novel functions of FOXA1, independent of nuclear receptor signaling. Analysis of the FOXA1 cistrome in Abl cells demonstrated androgen-independent binding of FOXA1 to cyclin E2 regulatory loci, which resulted in increased expression of cyclin E2 and progression through cell cycle [71]. Loss of FOXA1 dramatically reduced cellular growth of CRPC cells, while ectopic expression of cyclin E2 restored proliferative capacity in this context [71], substantiating AR-independent roles of FOXA1 in CRPC-specific proliferation. Conversely, independent studies also reported decreased levels of FOXA1 from metastatic tissue, and demonstrated an association between FOXA1 loss and enhanced metastatic potential [73]. Chip sequencing analyses of the FOXA1 cistrome in LNCaP cells defined a prominent FOXA1 binding site within the intragenic region of the SNAI2(Slug) gene, which was associated with low expression of Slug and limited migratory potential [73]. FOXA1 depletion dramatically increased Slug expression, which enhanced the invasive potential of these cells and promoted a dedifferentiation signature [73]. Such data implicate divergent roles of FOXA1 in mediating aggressive phenotypes in a prostate-specific context, and suggest that the functions of FOXA1 in PCa appear to be complex and highly cell type specific. While elevated levels of FOXA1 appear necessary to maintain CRPC-specific growth, depletion of FOXA1 promotes lethal metastatic events associated with patient mortality. Furthermore, recent deep sequencing of patient samples has uncovered mutations within the coding regions of the FOXA1 gene in late stage disease, some of which have the potential to affect FOXA1/DNA interactions and/or function [75–77]. Future efforts will focus on elucidating the context-specific functions of FOXA1, and these newly identified mutants, in promoting aggressive tumor phenotypes, and better define the role of AR in such events.
3.2.2 BAF57 deregulation promotes AR-dependent metastatic events
While pioneer factors like FOXA1 have been shown to play critical roles in defining the AR cistrome, other classes of DNA-associated co-factors have also demonstrated an ability to modulate disease-associated AR binding events. The SWI/SNF complex represents such a class, and functions as a multisubunit chromatin remodeling oligomer, which facilitates context-dependent gene activation or repression. The complex itself consists of a core ATPase (either BRG1 or BRM) and a host of associated cofactors, which help to define genome-wide specificity and tissue-specific gene expression programs [78–80]. Alterations or changes in the subunits that comprise the complex can dramatically affect the genomic localization or targets of SWI/SNF culminating in aberrant transcriptional profiles and cellular function [80]. Consequently, deregulation of SWI/SNF subunits is common in many tumors, including PCa [79, 81, 82]. Clinical evidence suggests that BAF57, a nonessential accessory SWI/SNF subunit is dramatically elevated in metastatic PCa lesions [82]. Furthermore, there is substantial evidence to suggest that BAF57 functions as a direct AR cofactor, enhancing AR-dependent transcriptional regulation and associated growth [81–83]. Microarray profiling of BAF57 upregulation in hormone-dependent cell lines confirmed such conclusions, where BAF57 induction was sufficient to promote ligand-independent AR activity under conditions mimicking castration (as monitored by the clinically relevant targets (KLK3, TMPRSS2, and FKBP5)) [82]. Interestingly, in addition to classical AR targets, gene ontology analysis uncovered a subset of BAF57-regulated genes involved in migration and invasion, specifically integrin alpha 2 (ITGA2). Genome-wide interrogation of the ITGA2 locus uncovered a putative AR binding event in a distal intragenic region, which was co-occupied by both BAF57 and AR (in a hormone-independent fashion) [56, 82]. Further analysis of SWI/SNF occupancy at this site in the presence of elevated BAF57 identified enrichment of BRG1, indicating that SWI/SNF activity is likely required for the AR/BAF57-mediated induction of ITGA2 under castrate conditions. Consonantly, isogenic models of BAF57 deregulation confirmed elevated levels of integrin alpha-2 at the protein level, which was associated with enhanced migratory and invasive phenotypes in vitro[82]. Blocking integrin alpha-2 function at the cell surface with neutralizing antibodies dramatically inhibited the migratory potential of both control and BAF57-expressing cells, suggesting that integrin alpha-2 is required for basal and BAF57-induced phenotypes [82]. Collectively, these data implicate SWI/SNF perturbations in the pathobiology of AR-driven metastatic events in response to hormone therapy, and provide novel targets which could be leveraged for therapeutic benefit. It will be vital to define both the BAF57 and AR cistromes in models of BAF57 deregulation in order to more completely understand the molecular mechanisms that drive BAF57/AR-mediated metastatic phenotypes.
3.2.3 Cyclin D1b alters AR function to promote metastatic events
In addition to BAF57 and FOXA1, cell cycle-specific AR cofactors are also likely to play a role in the development of metastatic disease. Cyclin D1b is a tumor-specific splice variant of the well-characterized cell cycle regulator cyclin D1, which arises due to a failure to splice at the exon4/intron4 boundary of the pre-mRNA, resulting in the incorporation of intron 4 and the introduction of an early stop codon [84]. The resulting protein thus lacks all sequences encoded by the terminal exon (exon5) and harbors a unique 33 amino acid C-terminus encoded by intron 4 [84]. Numerous studies aiming to define the biological and biochemical consequences of cyclin D1b induction have uncovered novel functions for this splice variant in tumor biology, which differ dramatically from full-length cyclin D1 (cyclin D1a). For example, expression of cyclin D1b but not cyclin D1a was sufficient to promote tumor formation in fibroblasts [85], enhanced the migratory potential of bladder cancer cells [86], and was associated with increased resistance to ER antagonists in breast cancer models [87]. Investigation in PCa-specific models uncovered a unique role for D-type cyclins in modulating AR activity. Consistent with the known role of D-type cyclins as transcriptional modulators, previous reports have described cyclin D1a as a potent regulator of AR, capable of directly inhibiting AR transactivation and subsequent AR activity (as determined by PSA readout) [88–91]. Similar studies assessing the consequences of cyclin D1b expression on AR activity determined that while still able to functionally interact with AR, cyclin D1b was unable to negatively regulate AR function [92]. Such data are congruent with clinical observations aiming to define expression patterns of both cyclin D1a and cyclin D1b in primary disease. Only a small number of tumors scored positive for cyclin D1a (7 %), while nearly 30 % of all primary tumors demonstrated positive staining for cyclin D1b [93]. Further analyses of expression patterns across non-neoplastic and cancerous tissues determined that while there was no induction in cyclin D1a levels as a function of tumorigenesis, there was a marked increase in cyclin D1b levels in cancerous tissues [93]. These data implicate cyclin D1b as a clinically relevant oncogene in PCa, with distinct functions from that of cyclin D1a.
Given the established oncogenic functions of cyclin D1b and the induction of this splice variant in clinical samples, follow-up studies aimed to define the consequence of cyclin D1b on both tumor phenotypes as well as AR activity in PCa. Isogenic models of cyclin D1b induction demonstrated that cyclin D1b-expressing cells harbored enhanced invasive potential which was critically dependent on an intact AR signaling axis [94]. Subsequent microarray analyses defined a large transcriptional regulatory network uniquely regulated by cyclin D1b, which was enriched for genes associated with migration and invasion. Among the most prominently regulated was the transcriptional regulator SNAI2, a known AR-regulated gene implicated in metastatic progression PCa [73, 95, 96]. Importantly, induction of cyclin D1b resulted in occupancy of both cyclin D1b and AR at SNAI2 regulatory loci, which correlated with active marks of transcription (acetylated histone H4) and well as enhanced transcriptional output [94]. Notably, depletion of Slug dramatically inhibited all cyclin D1b-induced phenotypes, indicating that Slug is a critical downstream mediator of cyclin D1b/AR-driven phenotypes in vitro [94]. Parallel studies in vivo determined that enhanced Slug expression dramatically increased metastatic growth at distal soft tissues (liver and lung) [94], an event that is clinically associated with resistance to chemotherapeutics and a poor prognosis [97]. Finally, analysis of CRPC tumors samples uncovered a strong positive correlation between cyclin D1b and Slug expression, indicating that the cyclin D1b/AR/Slug axis is preserved in human tumors [94]. Taken together, these data suggest that cyclin D1b hijacks the AR signaling axis to promote expression of genes (SNAI2), responsible for metastatic progression and ultimately patient mortality. Critical next steps will aim to better define both the cyclin D1b and AR cistrome to better understand the molecular underpinnings of cyclin D1b action on AR-driven metastatic events.
3.3 III. AR-dependent gene fusions and downstream effectors
While there is substantial evidence to suggest that AR cofactors significantly influence AR signaling networks to promote metastatic phenotypes, little is known as to how such factors are induced or regulated in human disease. While future efforts will characterize these pathways to better define the oncogenic cascades responsible for their expression, a class of AR-driven genetic fusions has been identified clinically, and are associated with progression to metastatic disease. As will be discussed, expression of these fusion products and downstream effectors is regulated as a function of AR activity, which can feedback to after the AR cistrome, culminating in an AR transcriptome which fosters invasive disease.
3.3.1 AR-regulated TMPRSS2: ETS fusions
Few genetic aberrations have been associated with the early stages of PCa, which has hindered the ability to stratify prostate cancer into subtypes. However, a chromosomal fusion event, which occurs between the regulatory locus of an AR-responsive gene (TMPRSS2) and the coding region of known oncogenic factors (erythroblast transformation specific, ETS genes) was identified in human prostate cancer samples. While reports vary, it is estimated that nearly half of all primary PCas harbor some form of this genetic fusion [98–103], which in some studies was associated with early onset of disease and a more aggressive, androgen fueled, phenotype [104]. Genetic profiling obtained from primary tumors has identified a multitude of fusion events which occur between the ETS family of transcriptional regulators (ERG, ETV1, ETV4, ETV5, and FLI1) and the regulatory locus of TMPRSS2, the most common of which occurs between the regulatory locus of TMPRSS2 and coding sequence of ERG [102, 103, 105, 106]. While it is still unclear as to how these fusions arise, there is evidence to implicate the AR signaling pathway in their formation [107, 108]. In vitro models suggest that upon androgen stimulation, AR facilitates a bridging event between the TMPRSS2 and ETS loci, which fosters DNA recombination at low frequency [107, 108]. Importantly, while the mechanisms behind the fusions initiation are still under investigation, their clinical relevance has been suggested by clinical analyses. In a recent study where treatment involved watchful waiting, patients who were positive for the fusion had more aggressive disease and showed a worse prognosis as compared to fusion-negative patients [109]. Such data indicate that fusions may play a role in the pathogenesis and progression of PCa.
Multiple models of fusion-positive PCa (both in vitro and in vivo) have been generated in order to define the oncogenic pathways regulated by ERG, which promote aggressive disease. In vitro, the VCaP line (derived from a vertebral metastasis) harbors an endogenous fusion between TMPRSS2 and ERG, and is highly responsive to AR activation. While still dependent upon AR signaling for growth and survival, modulation of fusion expression did not affect growth, tumor formation, or survival, yet drastically affected the invasive capacity of these cells [110]. This is congruent with a number of in vivo studies which demonstrate that neither induction of ERG or ETV1 alone is not sufficient to promote neoplastic events in the prostate [57, 111]. However, in combination with PTEN deficiency (PTEN +/−) prostate-specific ERG induction promoted aggressive and invasive adenocarcinoma by 8 weeks, with some mice developing poorly differentiated tumors by 12 weeks [57]. Mechanistically, these aggressive phenotypes were attributed to changes in the AR cistrome, which promoted programs of migration and angiogenesis [57]. Furthermore, parallel studies aiming to define the unique AR cistromes between fusion-positive (VCaP) and fusion-negative (LNCaP) cells confirmed the concept that ERG alters the AR cistrome [112]. While there is some discrepancy between the signatures of these studies, both describe ERG-driven alterations of the AR cistrome, which are associated with enhanced invasive/metastatic potential. The latter also defines an important role for the histone methyltransferase EZH2 in ERG-mediated phenotypes [112]. EZH2 is well characterized for its role in maintaining stem cells in a dedifferentiated state, and has been shown to be elevated in metastatic/advanced disease [113, 114]. In PCa models, ERG-induced expression and activity of EZH2, which resulted in the repression of well-known EZH2 target genes (e.g., CDH1 and DAB2IP) and signatures representative of a dedifferentiated state [115]. Given that EZH2 has recently been implicated as an AR cofactor requisite for CRPC-specific AR signaling [115], it will be critical to determine how ERG, AR, and EZH2 function together to define a genome landscape that promotes lethal metastatic disease.
3.3.2 SOX9: effector of ERG function in PCa
SOX9 belongs to the SOX family of transcription factors that have well-documented roles in the regulation of developmental processes as well as in disease progression [116, 117]. In adult tissues (e.g., liver and pancreas), it contributes to the maintenance of stem-like progenitor cells, promoting programs of dedifferentiation and self-renewal [118–120]. In the developing prostate, SOX9 is required for proper ductal morphogenesis, and loss dramatically impairs prostatic architecture and function [121]. While both SOX9 and AR are required for proper development of the prostate, each is localized to different epithelial compartments in luminal tissue [121]. SOX9 expression is typically limited to the AR-negative/low basal cells, distinct from the AR-positive, SOX9 low, luminal cells [121]. Interestingly, however, AR and SOX9 are coexpressed in a subset of primary PCa [122, 123]. Analysis of a large number of primary PCa tumors identified elevated SOX9 expression in patients who harbored TMPRSS:ERG fusions, a signature which was further enriched in CRPC metastatic tumors [124]. Given the known roles of both ERG fusions and SOX9 in promoting metastatic phenotypes, it was likely that these pathways cooperated to enhance PCa progression and metastasis. Modeling this event using isogenic lines of fusion-positive disease confirmed that androgen-induced ERG expression promoted elevated levels of both SOX9 transcript and protein, while knockdown of either ERG or SOX9 was sufficient to inhibit invasive properties in vitro [124]. Importantly however, exogenous expression of SOX9 was unable to promote invasive phenotypes in the absence of androgen, indicating that additional androgen-regulated genes are required for ERG/SOX9-mediated metastatic events [124]. Furthermore, elegant mouse models utilizing prostate-specific inducible SOX9 constructs demonstrated that exogenous SOX9 was sufficient to promote PIN formation and localized invasion in hormone-proficient animals [124]. These phenotypes were further enhanced in a PTEN heterozygous background, indicating that ERG/SOX9-driven pathways cooperate with clinical relevant genetic alterations to promote both PCa progression and invasion. Such data demonstrate that SOX9 is a critical mediator of ERG-driven phenotypes in PCa.
While ERG expression was sufficient to drive SOX9 expression in vitro and in vivo, the underpinning mechanisms behind such induction remained unclear. ChIP-Seq studies analyzing the ERG cistrome in fusion-positive models uncovered an enrichment of ERG binding over a 2 kb region proximal to the 3′ UTR of the SOX9 [124]. Interestingly, analysis of the AR cistrome found strong localization of AR within this region, which was lost upon ERG knockdown. Co-occupancy of both AR and ERG was associated with active marks of transcription and enhanced SOX9 expression [124]. Conversely, in fusion-negative models, AR activity decreased expression of SOX9 transcript and protein, and was instead only recruited to an enhancer-like region upstream of the SOX9 gene, implicated in transcriptional repression [124]. These data indicate that ERG can rewire the AR cistrome to promote expression of genes associated with cancer progression and metastasis. Given the knowledge that SOX9-mediated phenotypes require additional AR targets, it will be critical to determine which parallel pathways are governed by AR and SOX9/ERG in order to better define networks which can be exploited for patient benefit.
4 Conclusions and key questions
There is an abundance of clinical data to substantiate the AR signaling axis as a critical driver of metastatic disease at both the hormone therapy-sensitive and castration-resistant states. The findings described herein illuminate multiple pathways governed by AR which are responsible for the induction of prometastatic events, and broaden our understanding of disease-associated AR signatures (Fig. 2). In most cases, expression of critical AR cofactors was responsible for mediating such signatures, in essence rewiring the AR signaling axis to promote programs of invasion, migration, survival, and growth. While identification of these unique programs represents a significant leap forward in the attempt to thwart progression to lethal metastatic disease, there are several gaps in knowledge that have yet to be addressed. First, despite the identification of numerous AR cofactors associated with metastatic disease (ERG, EZH2, cyclin D1b, BAF57, etc.) PCa still suffers from a lack of biomarkers which are effective at predicting metastatic events. While most of the cofactors described above have demonstrated the ability to promote prometastatic phenotypes in AR-positive cells, it remains unclear if these markers alone are sufficient to predict metastasis or therapeutic outcome in the clinical setting. Definition of the co-occurrence of each of these cofactors in human disease will be critical to refine clinical signatures which more accurately predict progression to metastatic disease. Second, how does expression of multiple AR cofactors within a given tumor affect the AR cistrome and downstream biological events? Given, the heterogeneity of PCa tumors, it is likely that multiple disease-associated AR cofactors are expressed within a given tumor/tumor cell. While current models have examined each of these factors in isolation, critical next steps will aim to build off of clinical knowledge to better define and model the AR interactome, and identify clinically relevant targets and downstream effectors. Third, what regulates the expression of AR cofactors which promote progression to lethal disease states? Outside the identification of AR-driven expression of genetic fusions, little is known as to how each of these proteins are regulated. While current emphasis has been directed toward elucidating the downstream consequences of their tumor-associated expression, identification of the upstream oncogenic pathways that mediate their induction could offer novel avenues for therapeutic intervention to curtail development of metastatic lesions. Fourth, are AR-driven metastatic signatures preserved between hormone therapy sensitive and CRPC tumors? Genome-wide studies strongly suggest that the AR cistrome changes during the transition to CRPC, yet few models have been established which have characterized the function of critical AR cofactors and downstream mediators on the AR cistrome in this stage of disease. Uncovering the function of the aforementioned factors in CRPC will help define disease state-specific AR signatures involved in metastatic events at this stage. Finally, it is time to directly treat the primary tumor in disseminated disease? It is uncommon for primary tumors to be resected after the detection of distant metastases. Consequently, it remains possible that future distant metastases also originated from this same primary site. Are these primary sites of CRPC tumors as indolent as expected, or are they in essence factories for disseminated tumor cells? While clinical trials directly testing these hypotheses have been proposed, little is known as to the role of primary tumors in advancing metastatic CRPC. Ultimately, elucidation of the sites responsible for such lethal metastatic events could provide novel avenues for intervention to limit the spread of lethal CRPC, dramatically improving patient morbidity and mortality.
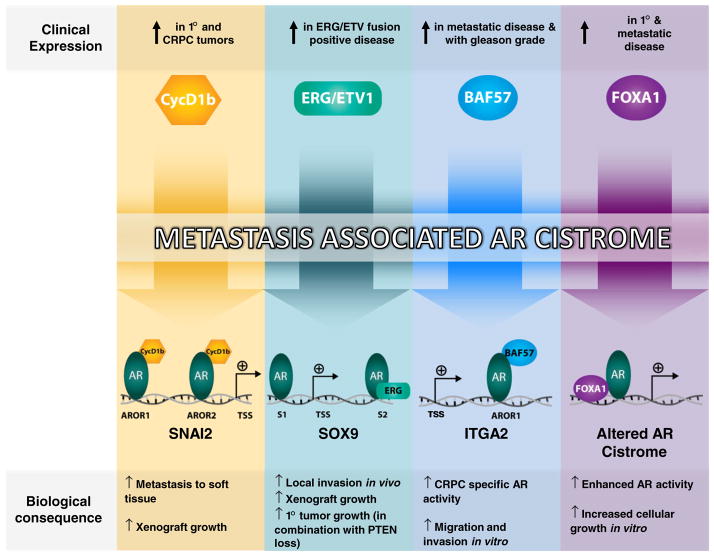
Fig. 2.
Cofactor-induced AR cistromes that promote metastatic phenotypes. A multitude of disease-associated AR cofactors have been identified in both primary and metastatic disease, which can impinge upon the AR cistrome to promote divergent AR binding events, culminating in transcriptional programs responsible for metastatic phenotypes. From left to right; (1) cyclin D1b co-occupied SNAI2 regulatory loci (Androgen Receptor Occupied Region 1—AROR1 and AROR2) with AR, promoting both AR occupancy and chromosomal looping, resulting in enhanced Slug expression and metastatic progression (Transcriptional Start Site—TSS) [94]; (2) in fusion-positive disease, ERG binding at a 3′ SOX9 enhancer region (site 2–S2) facilitates AR recruitment to this site, resulting in enhanced SOX9 expression and increased invasion and tumor growth in vivo [124]; (3) tumor-associated elevation of BAF57 levels promote AR occupancy within an intronic enhancer of the ITGA2 gene (AROR1) in a ligand-independent manner, recruiting necessary SWI/SNF subunits to promote ITGA2 transcription and facilitate invasive and migratory phenotypes in vitro [82]; (4) Elevated levels of FOXA1 localize to sites on chromatin and restrict the total number of AR binding events genome wide. AR binding events, in this context, are thus enriched for FOXA1 binding motifs, and are associated with tumor-specific programs of growth and survival [68]
In summary, is it clear that the AR pathway is critical for the development and progression to metastatic CRPC. Recent advances have uncovered dramatic changes in the AR cistrome as a function of disease progression, often attributed to altered expression of critical AR cofactors. Such changes have been demonstrated to not only promote the transition to CRPC, but also influence PCa-specific metastatic potential. Further investigation into the pathways downstream of both AR and these cofactors will likely uncover novel targets that can be targeted along side of AR to limit the development of lethal metastatic disease and ultimately enhance patient survival.
Acknowledgments
We thank the Knudsen Lab for insightful discussions; Dr. T. Mankanme, M. Urban, and C. McNair critical reading of the manuscript; W.K. Kelly for expert assistance and feedback on clinical topics; and E. Schade for expert graphical and technical support.
Footnotes
Disclosure The authors have no disclosures.
Contributor Information
Michael A. Augello, Email: Michael.augello@jefferson.edu, Department of Cancer Biology, Thomas Jefferson University, 233, 10th St., BLSB 1008, Philadelphia, PA 19107, USA. Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107, USA
Robert B. Den, Email: Robert.den@jefferson.edu, Department of Cancer Biology, Thomas Jefferson University, 233, 10th St., BLSB 1008, Philadelphia, PA 19107, USA. Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107, USA. Department of Radiation Oncology, Thomas Jefferson University, Philadelphia, PA 19107, USA
Karen E. Knudsen, Email: karen.knudsen@kimmelcancercenter.org, Department of Cancer Biology, Thomas Jefferson University, 233, 10th St., BLSB 1008, Philadelphia, PA 19107, USA. Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107, USA. Department of Urology, Thomas Jefferson University, Philadelphia, PA 19107, USA. Department of Radiation Oncology, Thomas Jefferson University, Philadelphia, PA 19107, USA
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Klein EA, Ciezki J, Kupelian PA, Mahadevan A. Outcomes for intermediate risk prostate cancer: are there advantages for surgery, external radiation, or brachytherapy? Urologic Oncology. 2009;27:67–71. doi: 10.1016/j.urolonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L. Combined androgen blockade: an update. The Urologic Clinics of North America. 2006;33:161–166. v–vi. doi: 10.1016/j.ucl.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clinical Cancer Research. 2009;15:4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. Journal of Clinical Oncology. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 6.Beekman KW, Hussain M. Hormonal approaches in prostate cancer: application in the contemporary prostate cancer patient. Urologic Oncology. 2008;26:415–419. doi: 10.1016/j.urolonc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Berthold DR, Sternberg CN, Tannock IF. Management of advanced prostate cancer after first-line chemotherapy. Journal of Clinical Oncology. 2005;23:8247–8252. doi: 10.1200/JCO.2005.03.1435. [DOI] [PubMed] [Google Scholar]
- 8.Petrylak DP. Chemotherapy for advanced hormone refractory prostate cancer. Urology. 1999;54:30–35. doi: 10.1016/s0090-4295(99)00452-5. [DOI] [PubMed] [Google Scholar]
- 9.Dreicer R. Chemotherapy for advanced prostate cancer: docetaxel and beyond. Hematology/Oncology Clinics of North America. 2006;20:935–946. x. doi: 10.1016/j.hoc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Knudsen KE, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends in Endocrinology and Metabolism. 2010;21:315–324. doi: 10.1016/j.tem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukka H, Waldron T, Klotz L, Winquist E, Trachtenberg J. Maximal androgen blockade for the treatment of metastatic prostate cancer—a systematic review. Current Oncology. 2006;13:81–93. doi: 10.3747/co.v13i3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urologic Oncology. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan X, Cai C, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. 2013 doi: 10.1038/onc.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennis ER, Jia X, Mezheritskiy IS, et al. Bone scan index: a quantitative treatment response biomarker for castration-resistant metastatic prostate cancer. Journal of Clinical Oncology. 2012;30:519–524. doi: 10.1200/JCO.2011.36.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha GR, Ricke W, Thomson A, et al. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. Journal of Steroid Biochemistry and Molecular Biology. 2004;92:221–236. doi: 10.1016/j.jsbmb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Schiewer MJ, Augello MA, Knudsen KE. The AR dependent cell cycle: mechanisms and cancer relevance. Molecular and Cellular Endocrinology. 2012;352:34–45. doi: 10.1016/j.mce.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penning TM, Jin Y, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Molecular and Cellular Endocrinology. 2008;281:1–8. doi: 10.1016/j.mce.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matias PM, Donner P, Coelho R, et al. Structural evidence for ligand specificity in the binding domain of the human androgen receptor. Implications for pathogenic gene mutations. Journal of Biological Chemistry. 2000;275:26164–26171. doi: 10.1074/jbc.M004571200. [DOI] [PubMed] [Google Scholar]
- 19.Sack JS, Kish KF, Wang C, et al. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4904–4909. doi: 10.1073/pnas.081565498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centenera MM, Harris JM, Tilley WD, Butler LM. The contribution of different androgen receptor domains to receptor dimerization and signaling. Molecular Endocrinology. 2008;22:2373–2382. doi: 10.1210/me.2008-0017. [DOI] [PubMed] [Google Scholar]
- 21.Gioeli D, Paschal BM. Post-translational modification of the androgen receptor. Molecular and Cellular Endocrinology. 2012;352:70–78. doi: 10.1016/j.mce.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Coffey K, Robson CN. Regulation of the androgen receptor by post-translational modifications. Journal of Endocrinology. 2012;215:221–237. doi: 10.1530/JOE-12-0238. [DOI] [PubMed] [Google Scholar]
- 23.Agoulnik IU, Weigel NL. Androgen receptor coactivators and prostate cancer. Advances in Experimental Medicine and Biology. 2008;617:245–255. doi: 10.1007/978-0-387-69080-3_23. [DOI] [PubMed] [Google Scholar]
- 24.Huggins C. Effect of orchiectomy and irradiation on cancer of the prostate. Annals of Surgery. 1942;115:1192–1200. doi: 10.1097/00000658-194206000-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huggins C. The diagnosis of cancer of the prostate including the interpretation of serum phosphatase values. Bulletin of the New York Academy of Medicine. 1943;19:195–200. [PMC free article] [PubMed] [Google Scholar]
- 26.Huggins C. The treatment of cancer of the prostate: (the 1943 address in surgery before the Royal College of Physicians and Surgeons of Canada) Canadian Medical Association Journal. 1944;50:301–307. [PMC free article] [PubMed] [Google Scholar]
- 27.van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001–1006. doi: 10.1016/j.urology.2007.12.070. [DOI] [PubMed] [Google Scholar]
- 28.Klotz L. Hormone therapy for patients with prostate carcinoma. Cancer. 2000;88:3009–3014. doi: 10.1002/1097-0142(20000615)88:12+<3009::aid-cncr17>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Oefelein MG. Time to normalization of serum testosterone after 3-month luteinizing hormone-releasing hormone agonist administered in the neoadjuvant setting: implications for dosing schedule and neoadjuvant study consideration. Journal of Urology. 1998;160:1685–1688. [PubMed] [Google Scholar]
- 30.Knudsen KE, Kelly WK. Outsmarting androgen receptor: creative approaches for targeting aberrant androgen signaling in advanced prostate cancer. Expert Review of Endocrinology and Metabolism. 2011;6:483–493. doi: 10.1586/eem.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Research. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Research. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attard G, Richards J, de Bono JS. New strategies in metastatic prostate cancer: targeting the androgen receptor signaling pathway. Clinical Cancer Research. 2011;17:1649–1657. doi: 10.1158/1078-0432.CCR-10-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. New England Journal of Medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. Journal of Clinical Oncology. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishr M, Saad F. Overview of the latest treatments for castration-resistant prostate cancer. Nature Reviews Urology. 2013;10(9):522–528. doi: 10.1038/nrurol.2013.137. [DOI] [PubMed] [Google Scholar]
- 37.Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clinical Cancer Research. 2011;17:3876–3883. doi: 10.1158/1078-0432.CCR-10-2815. [DOI] [PubMed] [Google Scholar]
- 38.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncology. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 39.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New England Journal of Medicine. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 40.Dhingra R, Sharma T, Singh S, et al. Enzalutamide: a novel anti-androgen with prolonged survival rate in CRPC patients. Mini Reviews Medicinal Chemistry. 2013;13:1475–1486. doi: 10.2174/13895575113139990003. [DOI] [PubMed] [Google Scholar]
- 41.Attard G, de Bono JS. Translating scientific advancement into clinical benefit for castration-resistant prostate cancer patients. Clinical Cancer Research. 2011;17:3867–3875. doi: 10.1158/1078-0432.CCR-11-0943. [DOI] [PubMed] [Google Scholar]
- 42.Bahl A, Oudard S, Tombal B, et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Annals of Oncology. 2013;24(9):2402–8. doi: 10.1093/annonc/mdt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 44.Berruti A, Dogliotti L, Bitossi R, et al. Incidence of skeletal complications in patients with bone metastatic prostate cancer and hormone refractory disease: predictive role of bone resorption and formation markers evaluated at baseline. Journal of Urology. 2000;164:1248–1253. [PubMed] [Google Scholar]
- 45.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Human Pathology. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 46.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. New England Journal of Medicine. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 47.Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathology International. 2010;60:497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Cheng G, Hao M, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer and Metastasis Reviews. 2010;29:709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vindrieux D, Escobar P, Lazennec G. Emerging roles of chemokines in prostate cancer. Endocrine-Related Cancer. 2009;16:663–673. doi: 10.1677/ERC-09-0109. [DOI] [PubMed] [Google Scholar]
- 50.Frigo DE, Sherk AB, Wittmann BM, et al. Induction of Kruppel-like factor 5 expression by androgens results in increased CXCR4-dependent migration of prostate cancer cells in vitro. Molecular Endocrinology. 2009;23:1385–1396. doi: 10.1210/me.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Research. 2004;64:4693–4698. doi: 10.1158/0008-5472.CAN-03-3437. [DOI] [PubMed] [Google Scholar]
- 52.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Developmental Biology. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 53.Sun YX, Wang J, Shelburne CE, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. Journal of Cellular Biochemistry. 2003;89:462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 54.Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. Journal of Bone and Mineral Research. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 55.Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massie CE, Lynch A, Ramos-Montoya A, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO Journal. 2011;30:2719–2733. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Chi P, Rockowitz S, et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nature Medicine. 2013;19:1023–1029. doi: 10.1038/nm.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeh S, Tsai MY, Xu Q, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamieson WL, Shimizu S, D’Ambrosio JA, Meucci O, Fatatis A. CX3CR1 is expressed by prostate epithelial cells and androgens regulate the levels of CX3CL1/fractalkine in the bone marrow: potential role in prostate cancer bone tropism. Cancer Research. 2008;68:1715–1722. doi: 10.1158/0008-5472.CAN-07-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fong AM, Robinson LA, Steeber DA, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. Journal of Experimental Medicine. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goda S, Imai T, Yoshie O, et al. CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. Journal of Immunology. 2000;164:4313–4320. doi: 10.4049/jimmunol.164.8.4313. [DOI] [PubMed] [Google Scholar]
- 62.Umehara H, Goda S, Imai T, et al. Fractalkine, a CX3C-chemokine, functions predominantly as an adhesion molecule in monocytic cell line THP-1. Immunology and Cell Biology. 2001;79:298–302. doi: 10.1046/j.1440-1711.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 63.Lupien M, Eeckhoute J, Meyer CA, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lupien M, Brown M. Cistromics of hormone-dependent cancer. Endocrine-Related Cancer. 2009;16:381–389. doi: 10.1677/ERC-09-0038. [DOI] [PubMed] [Google Scholar]
- 65.Serandour AA, Avner S, Percevault F, et al. Epigenetic switch involved in activation of pioneer factor FOXA1-dependent enhancers. Genome Research. 2011;21:555–565. doi: 10.1101/gr.111534.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lupien M, Eeckhoute J, Meyer CA, et al. Coactivator function defines the active estrogen receptor alpha cistrome. Molecular and Cellular Biology. 2009;29:3413–3423. doi: 10.1128/MCB.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson JL, Carroll JS. FoxA1 is a key mediator of hormonal response in breast and prostate cancer. Frontiers Endocrinology (Lausanne) 2012;3:68. doi: 10.3389/fendo.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahu B, Laakso M, Pihlajamaa P, et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Research. 2013;73:1570–1580. doi: 10.1158/0008-5472.CAN-12-2350. [DOI] [PubMed] [Google Scholar]
- 69.Sahu B, Laakso M, Ovaska K, et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO Journal. 2011;30:3962–3976. doi: 10.1038/emboj.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Augello MA, Hickey TE, Knudsen KE. FOXA1: master of steroid receptor function in cancer. EMBO Journal. 2011;30:3885–3894. doi: 10.1038/emboj.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang C, Wang L, Wu D, et al. Definition of a FoxA1 cistrome that is crucial for G1 to S-phase cell-cycle transit in castration-resistant prostate cancer. Cancer Research. 2011;71:6738–6748. doi: 10.1158/0008-5472.CAN-11-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerhardt J, Montani M, Wild P, et al. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. American Journal of Pathology. 2012;180:848–861. doi: 10.1016/j.ajpath.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 73.Jin HJ, Zhao JC, Ogden I, Bergan RC, Yu J. Androgen receptor-independent function of FoxA1 in prostate cancer metastasis. Cancer Research. 2013;73:3725–3736. doi: 10.1158/0008-5472.CAN-12-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain RK, Mehta RJ, Nakshatri H, Idrees MT, Badve SS. High-level expression of forkhead-box protein A1 in metastatic prostate cancer. Histopathology. 2011;58:766–772. doi: 10.1111/j.1365-2559.2011.03796.x. [DOI] [PubMed] [Google Scholar]
- 75.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature Genetics. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson JL, Holmes KA, Carroll JS. FOXA1 mutations in hormone-dependent cancers. Frontiers Oncology. 2013;3:20. doi: 10.3389/fonc.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu JI. Diverse functions of ATP-dependent chromatin remodeling complexes in development and cancer. Acta Biochimica Biophysica Sinica (Shanghai) 2012;44:54–69. doi: 10.1093/abbs/gmr099. [DOI] [PubMed] [Google Scholar]
- 79.Weissman B, Knudsen KE. Hijacking the chromatin remodeling machinery: impact of SWI/SNF perturbations in cancer. Cancer Research. 2009;69:8223–8230. doi: 10.1158/0008-5472.CAN-09-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu JI, Lessard J, Crabtree GR. Understanding the words of chromatin regulation. Cell. 2009;136:200–206. doi: 10.1016/j.cell.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Link KA, Balasubramaniam S, Sharma A, et al. Targeting the BAF57 SWI/SNF subunit in prostate cancer: a novel platform to control androgen receptor activity. Cancer Research. 2008;68:4551–4558. doi: 10.1158/0008-5472.CAN-07-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balasubramaniam S, Comstock CE, Ertel A, et al. Aberrant BAF57 signaling facilitates prometastatic phenotypes. Clinical Cancer Research. 2013;19:2657–2667. doi: 10.1158/1078-0432.CCR-12-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Link KA, Burd CJ, Williams E, et al. BAF57 governs androgen receptor action and androgen-dependent proliferation through SWI/SNF. Molecular and Cellular Biology. 2005;25:2200–2215. doi: 10.1128/MCB.25.6.2200-2215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knudsen KE. The cyclin D1b splice variant: an old oncogene learns new tricks. Cell Division. 2006;1:15. doi: 10.1186/1747-1028-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu F, Gladden AB, Diehl JA. An alternatively spliced cyclin D1 isoform, cyclin D1b, is a nuclear oncogene. Cancer Research. 2003;63:7056–7061. [PubMed] [Google Scholar]
- 86.Kim CJ, Nishi K, Isono T, et al. Cyclin D1b variant promotes cell invasiveness independent of binding to CDK4 in human bladder cancer cells. Molecular Carcinogenesis. 2009;48:953–964. doi: 10.1002/mc.20547. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Dean JL, Millar EK, et al. Cyclin D1b is aberrantly regulated in response to therapeutic challenge and promotes resistance to estrogen antagonists. Cancer Research. 2008;68:5628–5638. doi: 10.1158/0008-5472.CAN-07-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burd CJ, Petre CE, Moghadam H, Wilson EM, Knudsen KE. Cyclin D1 binding to the androgen receptor (AR) NH2-terminal domain inhibits activation function 2 association and reveals dual roles for AR corepression. Molecular Endocrinology. 2005;19:607–620. doi: 10.1210/me.2004-0266. [DOI] [PubMed] [Google Scholar]
- 89.Comstock CE, Augello MA, Schiewer MJ, et al. Cyclin D1 is a selective modifier of androgen-dependent signaling and androgen receptor function. Journal of Biological Chemistry. 2011;286:8117–8127. doi: 10.1074/jbc.M110.170720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petre CE, Wetherill YB, Danielsen M, Knudsen KE. Cyclin D1: mechanism and consequence of androgen receptor co-repressor activity. Journal of Biological Chemistry. 2002;277:2207–2215. doi: 10.1074/jbc.M106399200. [DOI] [PubMed] [Google Scholar]
- 91.Petre-Draviam CE, Williams EB, Burd CJ, et al. A central domain of cyclin D1 mediates nuclear receptor corepressor activity. Oncogene. 2005;24:431–444. doi: 10.1038/sj.onc.1208200. [DOI] [PubMed] [Google Scholar]
- 92.Burd CJ, Petre CE, Morey LM, et al. Cyclin D1b variant influences prostate cancer growth through aberrant androgen receptor regulation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2190–2195. doi: 10.1073/pnas.0506281103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene. 2006;25:1620–1628. doi: 10.1038/sj.onc.1209371. [DOI] [PubMed] [Google Scholar]
- 94.Augello MA, Burd CJ, Birbe R, et al. Convergence of oncogenic and hormone receptor pathways promotes metastatic phenotypes. Journal of Clinical Investigation. 2013;123:493–508. doi: 10.1172/JCI64750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Emadi Baygi M, Soheili ZS, Essmann F, et al. Slug/SNAI2 regulates cell proliferation and invasiveness of metastatic prostate cancer cell lines. Tumour Biology. 2010;31:297–307. doi: 10.1007/s13277-010-0037-5. [DOI] [PubMed] [Google Scholar]
- 96.Wu K, Gore C, Yang L, et al. Slug, a unique androgen-regulated transcription factor, coordinates androgen receptor to facilitate castration resistance in prostate cancer. Molecular Endocrinology. 2012;26:1496–1507. doi: 10.1210/me.2011-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kelly WK, HS, Carducci MA, et al. Liver metastases (LM) to predict for short overall survival (OS) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts). Journal of Clinical Oncology; 2012 ASCO Annual Meeting; Chicago, IL. 2012. p. Abstract 4655. [Google Scholar]
- 98.Barry M, Perner S, Demichelis F, Rubin MA. TMPRSS2-ERG fusion heterogeneity in multifocal prostate cancer: clinical and biologic implications. Urology. 2007;70:630–633. doi: 10.1016/j.urology.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehra R, Tomlins SA, Shen R, et al. Comprehensive assessment of TMPRSS2 and ETS family gene aberrations in clinically localized prostate cancer. Modern Pathology. 2007;20:538–544. doi: 10.1038/modpathol.3800769. [DOI] [PubMed] [Google Scholar]
- 101.Mosquera JM, Mehra R, Regan MM, et al. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clinical Cancer Research. 2009;15:4706–4711. doi: 10.1158/1078-0432.CCR-08-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tomlins SA, Bjartell A, Chinnaiyan AM, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. European Urology. 2009;56:275–286. doi: 10.1016/j.eururo.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 103.Tomlins SA, Laxman B, Dhanasekaran SM, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–599. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 104.Weischenfeldt J, Simon R, Feuerbach L, et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell. 2013;23:159–170. doi: 10.1016/j.ccr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 105.Helgeson BE, Tomlins SA, Shah N, et al. Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Research. 2008;68:73–80. doi: 10.1158/0008-5472.CAN-07-5352. [DOI] [PubMed] [Google Scholar]
- 106.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nature Reviews Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mani RS, Tomlins SA, Callahan K, et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science. 2009;326:1230. doi: 10.1126/science.1178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin C, Yang L, Tanasa B, et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell. 2009;139:1069–1083. doi: 10.1016/j.cell.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Demichelis F, Fall K, Perner S, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–4599. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 110.Tomlins SA, Laxman B, Varambally S, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.King JC, Xu J, Wongvipat J, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nature Genetics. 2009;41:524–526. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu J, Mani RS, Cao Q, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Varambally S, Dhanasekaran SM, Zhou M, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 114.Yu J, Rhodes DR, Tomlins SA, et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Research. 2007;67:10657–10663. doi: 10.1158/0008-5472.CAN-07-2498. [DOI] [PubMed] [Google Scholar]
- 115.Xu K, Wu ZJ, Groner AC, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Developmental Cell. 2002;3:167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- 117.Pritchett J, Athwal V, Roberts N, Hanley NA, Hanley KP. Understanding the role of SOX9 in acquired diseases: lessons from development. Trends in Molecular Medicine. 2011;17:166–174. doi: 10.1016/j.molmed.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 118.Seymour PA, Freude KK, Tran MN, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seymour PA, Shih HP, Patel NA, et al. A Sox9/Fgf feed-forward loop maintains pancreatic organ identity. Development. 2012;139:3363–3372. doi: 10.1242/dev.078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nature Genetics. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 121.Thomsen MK, Butler CM, Shen MM, Swain A. Sox9 is required for prostate development. Developmental Biology. 2008;316:302–311. doi: 10.1016/j.ydbio.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 122.Thomsen MK, Ambroisine L, Wynn S, et al. SOX9 elevation in the prostate promotes proliferation and cooperates with PTEN loss to drive tumor formation. Cancer Research. 2010;70:979–987. doi: 10.1158/0008-5472.CAN-09-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang H, Leav I, Ibaragi S, et al. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Research. 2008;68:1625–1630. doi: 10.1158/0008-5472.CAN-07-5915. [DOI] [PubMed] [Google Scholar]
- 124.Cai C, Wang H, He HH, et al. ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. Journal of Clinical Investigation. 2013;123:1109–1122. doi: 10.1172/JCI66666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garcia JA, Dreicer R. Immunotherapy in castration-resistant prostate cancer: integrating sipuleucel-T into our current treatment paradigm. Oncology (Williston Park) 2011;25:242–249. [PubMed] [Google Scholar]
- 126.Magi-Galluzzi C, Zhou M, Reuther AM, Dreicer R, Klein EA. Neoadjuvant docetaxel treatment for locally advanced prostate cancer: a clinicopathologic study. Cancer. 2007;110:1248–1254. doi: 10.1002/cncr.22897. [DOI] [PubMed] [Google Scholar]
- 127.Dreicer R, Agus DB, MacVicar GR, Wang J, MacLean D, Stadler WM. Safety, pharmacokinetics, and efficacy of TAK-700 in metastatic castration-resistant prostate cancer: a phase I/II, open-label study. Journal of Clinical Oncology. 2010;28(suppl):15s. abstr 3084. [Google Scholar]
- 128.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. New England Journal of Medicine. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pacey S, Wilson RH, Walton M, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clinical Cancer Research. 2011;17:1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]