Abstract
Desmoplastic small round cell tumor (DSRCT) is an extremely rare, highly aggressive, malignant tumor of undetermined histogenesis. Adolescent males are primarily affected with a typically abdominal or pelvic mass. Diagnosis is based on histologic analysis of biopsy and cytogenetic studies. Owing to the rarity of the tumor and the unusually aggressive presentation, treatment is challenging and has not been standardized. DSRCT has a very poor prognosis, with a median survival range of 17 to 25 months. In this work, we report a case of DSRCT in the transverse colon, which has never before been reported in the literature. Our case study is of a 30-year-old Chinese female who presented with a history of 6 months abdominal discomfort and fatigue. A palpable, hardly mobile mass was detected in the right lower quadrant abdomen by physical examination. Abdominal and pelvic magnetic resonance imaging revealed an 8.0 × 10.5 × 11.1-cm mass with no pulmonary and hepatic metastasis. The patient underwent exploratory laparotomy and transverse colectomy, which revealed a mass in the transverse colon and no metastasis in the peritoneum, greater omentum, or mesentery. Immunohistochemistry findings revealed positive staining for epithelial, mesenchymal, and neural markers, which confirmed the presentation of DSRCT. A 6-month postoperative follow-up failed to find any recurrence. DSRCT is a highly aggressive, malignant, mesenchymal tumor with a very poor prognosis. No consensus has been reached for the treatment protocol of DSRCT. However, debulking surgery with postoperative chemotherapy or radiotherapy might promise more optimistic results on survival.
Key words: Desmoplastic small round cell tumor, Transverse colon
Desmoplastic small round cell tumor (DSRCT) is a rare and highly aggressive malignant mesenchymal tumor that was first described by Gerald and Rosai in 1989.1 Fewer than 200 case reports have been documented in the literature so far.2 It is considered a childhood cancer that predominantly strikes male adolescents and young adults, with a male to female ratio of 4:1.3 DSRCT typically develops in the abdominal cavity and usually presents with diffuse multiple peritoneal implants.4 Organ involvement is inconstant and secondary, with liver and lung as two common sites for metastatic disease beyond the peritoneum.
DSRCT is characterized by nests of small, round, undifferentiated cells surrounded by plentiful desmoplastic fibrous stroma. DSRCT is associated with a characteristic chromosomal translocation, t(11;22)(p13;q12), which fuses the N-terminus of the Ewing sarcoma (EWS) gene to the C-terminus of the Wilms tumor (WT-1) gene. The presence of this translocation provides definitive diagnosis of DSRCT.5
Owing to the rarity of this tumor, there is no clear consensus on the optimal treatment. Current therapeutic options include aggressive debulking surgery, whole-abdominal radiation and high-dose chemotherapy.6 The addition of hyperthermic intraperitoneal chemotherapy in the multimodal approach has been reported in very few cases, and the effect on survival is controversial.7,8 DSRCT has a very poor prognosis, and its median survival range is from 17 to 25 months, with fewer than 20% of patients achieving 5-year survival.9
Herein, we report a case of a 30-year-old female who presented with a DSRCT in the transverse colon and was treated with a transverse colectomy and right hemicolectomy.
Case Presentation
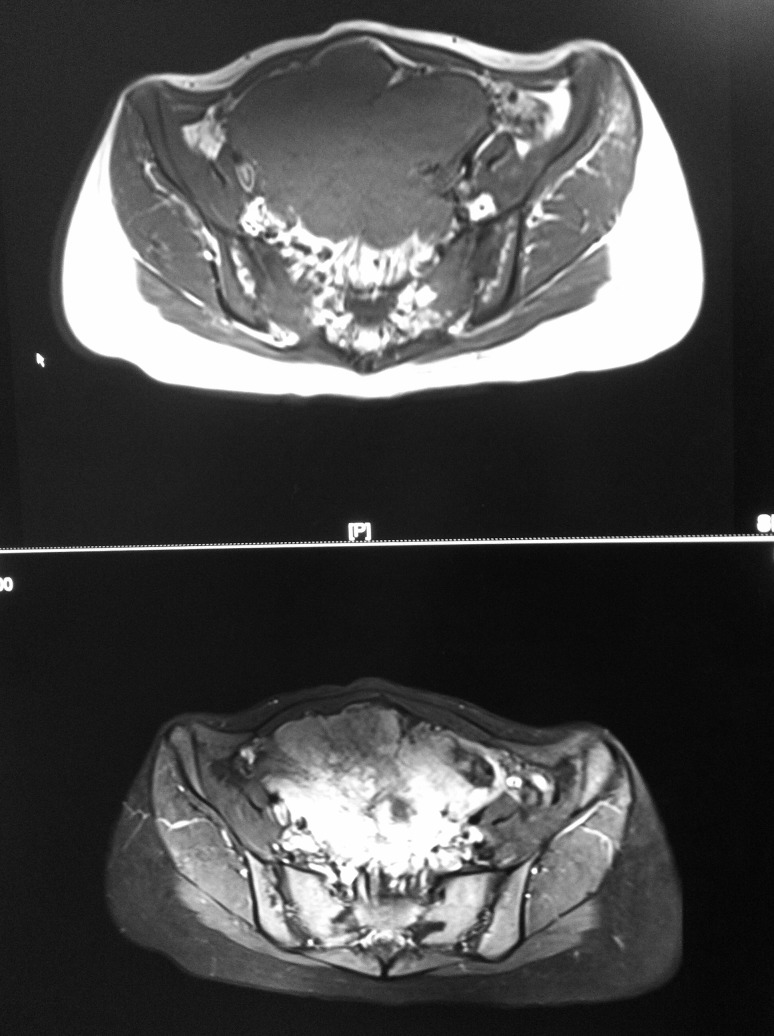
A 30-year-old, Chinese, healthy female patient presented to Shuguang Hospital with a 6-month history of abdominal discomfort and fatigue. There was no other significant medical history other than cesarean section. Physical examination revealed a palpable, hardly mobile mass, approximately 12 cm in diameter, in the right lower quadrant of the abdomen. All laboratory tests including complete blood count, hepatic and renal function, tumor markers (carcinoembryonic antigen [CEA], Alpha-fetoprotein (AFP), CA19-9, CA72-4) were normal. Only the tumor marker CA125 was slightly high at 53.9 U/mL (normal <35 U/mL). There was no hepatic or pulmonary metastasis found in computed tomography (CT) scans. Abdominal and pelvic magnetic resonance imaging (MRI) revealed an 8.0 × 10.5 × 11.1-cm mass, anterior to the uterus, with no evidence of enlarged lymph nodes or ascites. The mass was inhomogeneous isointense on T1WI and high-low mixed signal on T2WI (Fig. 1).
Fig. 1.
Abdominal and pelvic MRI revealed an 8.0 × 10.5 × 11.1-cm mass with no evidence of enlarged lymph nodes or ascites.
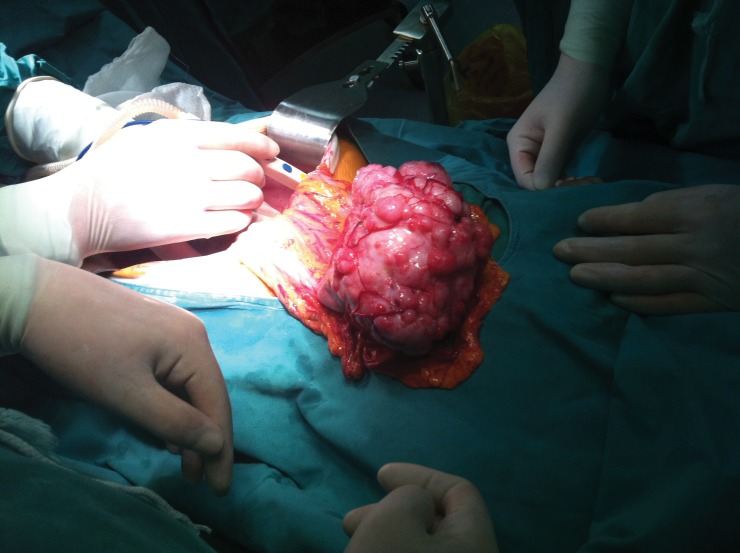
The patient underwent exploratory laparotomy. The transverse colon was located in the pelvis with a large mass in the middle part. No metastasis was found in the peritoneum, greater omentum, or mesentery. Radical transverse colectomy and right hemicolectomy were performed as the tumor had no local infiltration (Fig. 2).
Fig. 2.
The tumor had no local infiltration.
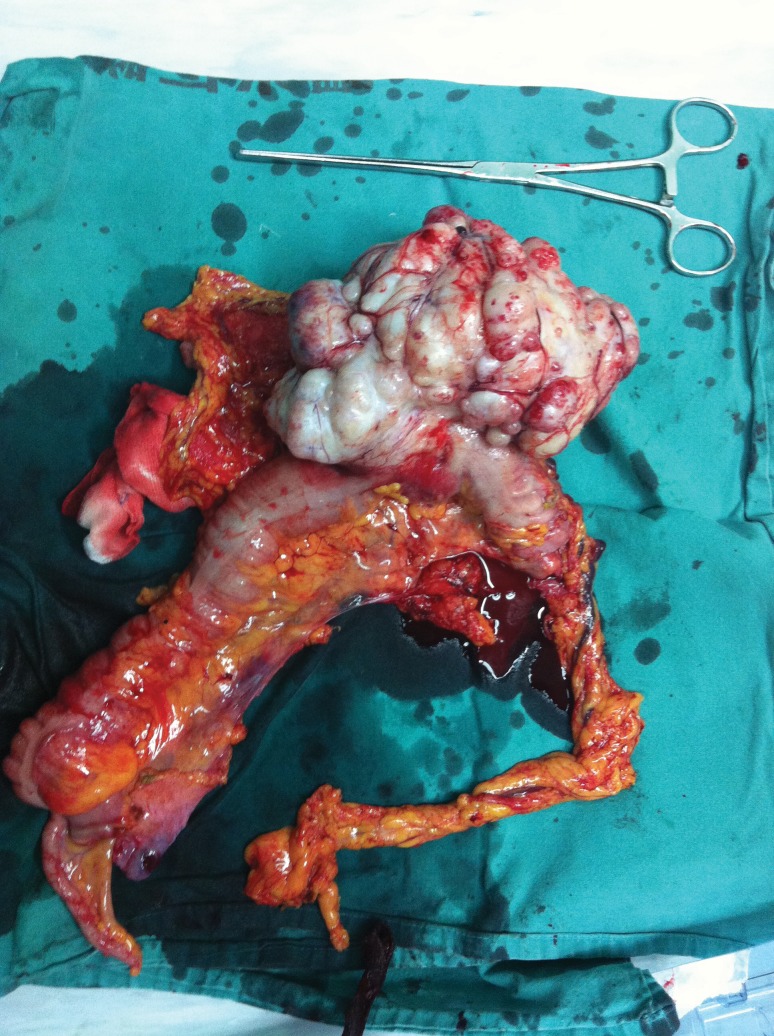
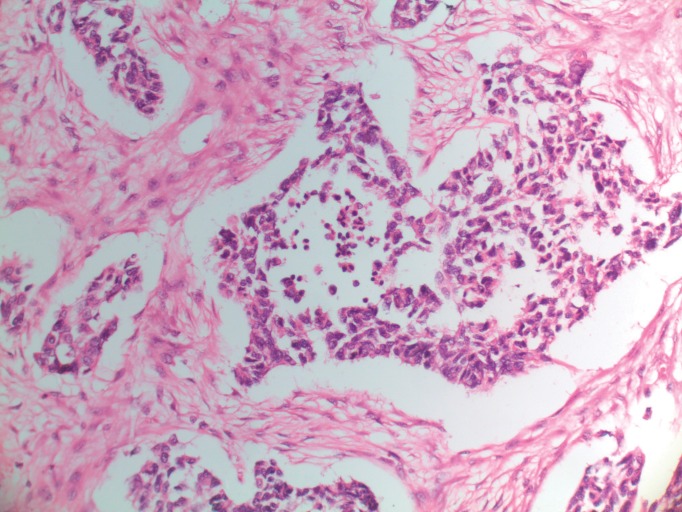
Macroscopically, the resected specimen was white, nodular, and firm, measuring 16 × 12 × 10 cm (Fig. 3). All 34 lymph nodes were negative. Microscopically, the tumor was composed of large sheets of small round blue cells distributed within abundant desmoplastic stroma. The round or oval nuclei were small, hyperchromatic, with inconspicuous nucleoli and irregular nuclear membrane (Fig. 4).
Fig. 3.
The resected specimen.
Fig. 4.
Histologic appearance of DSRCT, H&E (×200).
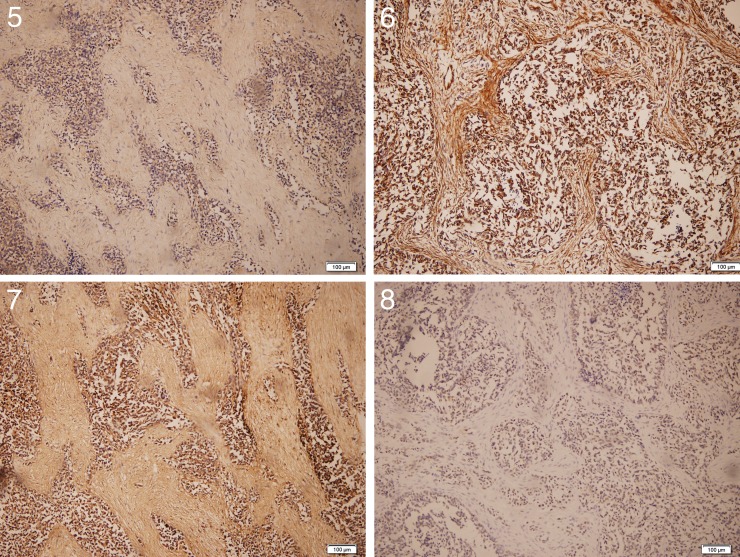
The immunohistochemical study included cytokeratin (CK), CEA, vimentin, desmin, MyoD1, smooth muscle actin (SMA), myoglobin, S-100 protein, human hematopoietic progenitor cell antigen (CD34), WT-1, leucocyte common antigen (LCA), and neuron-specific enolase (NSE). The immunohistochemical results revealed positive reactions to CK, vimentin, desmin, CD34, WT-1, and NSE (Figs. 5–8). The patient did not take fluorescence in situ hybridization (FISH) test or polymerase chain reaction (PCR) owing to the restriction of laboratory equipment.
Fig. 5.
Positive result of CK, H&E (×100).
Fig. 6 Positive result of vimentin, H&E (×100).
Fig. 7 Positive result of NSE, H&E (×100).
Fig. 8 Positive result of WT-1, H&E (×100).
The postoperative course was uneventful, and the patient was discharged home 10 days after surgery.
She received no adjuvant radiotherapy or chemotherapy. A 6-month postoperative follow-up was performed, and monthly CT scans failed to find any recurrence.
Discussion
DSRCT is an extremely rare, highly aggressive, malignant mesenchymal tumor of unidentified histogenesis. It was first described by Gerald and Rosai in 1989 as a newly featured clinicopathologic entity.1 DSRCT has a predilection for young males in their second and third decades of life, affecting mostly adolescent males with a male to female ratio of 4:1.3 The tumor typically develops in the abdominal or pelvic cavity and is associated with multiple peritoneal implants.4 Very rarely, DSRCT can arise from the head and neck, base of skull, paratesticular region, ovary, brain, or thoracic viscera.10 Extraperitoneal organ involvement is inconstant and secondary; including lung,11 liver,12 kidney,13 stomach,14 and pancreas.15 Liver and lungs are the 2 most common sites of distant metastasis.2 DSRCT shares some similar characteristics with other small round cell tumors including lymphoma, rhabdomyosarcoma, neuroblastoma, primitive neuroectodermal tumor, small cell mesothelioma, EWS, and Wilms tumor. Therefore, DSRCT is recognized as a soft-tissue undifferentiated sarcoma.3,16
Clinical manifestations of DSRCT are nonspecific, including abdominal pain and/or abdominal distention, nausea, vomiting, weight loss, and ascites. Physical examination may reveal a palpable mass in the abdominal or pelvic cavity, possibly accompanied by tenderness, unclear border, hard texture, and low mobility. The lesions are usually multifocal, with sizes ranging from 3 to 5 mm up to ≥200 mm in diameter. Ultrasound, CT scan, and MRI may reveal multiple tumor nodules; however, the radiologic findings, including calcifications, hydronephrosis, urinary obstruction, bowel obstruction, nodular peritoneal thickening, and retroperitoneal lymphadenopathy, are always nonspecific.17 Because of the challenges of diagnosis through noninvasive imaging examination, biopsy is an appropriate recommendation. The confirmation of DSRCT is based on immunohistochemistry and cytogenetics. Gross examination of DSRCT may reveal grayish, nodular, or lobular surfaces with local necrosis.18 Typically, DSRCT, seen under microscope, is composed of large sheets of small, round, blue cells distributed within abundant desmoplastic stroma. Tumor cells are small to medium in size, with round or oval hyperchromatic nuclei, inconspicuous nucleoli, and increased nuclear/cytoplasm ratios.18 DSRCT simultaneously express markers of epithelial (CK and EMA), mesenchymal (vimentin), myogenic (desmin), and neural (CD56 and NSE) tissues.3,4 Almost all DSRCT are positive for WT-1, a polyclonal antibody against the amino terminus of the WT-1 protein.19 DSRCT is associated with a characteristic chromosomal translocation, t(11;22)(p13;q12), which fuses the N-terminus of the EWS gene to the C-terminus of the WT-1 gene. The presence of this translocation provides definitive diagnosis of DSRCT.5
The therapeutic protocol for treating DSRCT remains challenging owing to the rarity of the tumor and consequently the limited experience. Aggressive surgical debulking is the mainstay of the therapeutic strategy. Debulking surgery is defined as definitive removal of at least 90% of the tumor burden. It has been reported that identified gross-tumor resection had an overall 3-year survival rate of 58% as compared with 0% in nonresectable cases.9 There are few data on the role of radiation therapy. This approach is considered essentially to consolidate efficacy or to palliate symptoms. Its use for curative purposes is limited by peritoneal dissemination and radiosensitivity of intra-abdominal organs.20 Multi-agent chemotherapy leads to approximately 40% tumor response.10 The cytotoxic agents that seem to be effective in DSRCT are cyclophosphamide, ifosfamide, adriamycin, vincristine, etoposide, and topotecan.20 The benefit to the survival rate by treatment with high-dose chemotherapy remains uncertain. Treatment using hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin is thought by some to be effective at reducing the recurrence rate of DSRCT8; yet others have found no demonstrable effect on survival.7 Despite the combined therapeutic approach, including surgery, radiotherapy, and chemotherapy, DSRCT has a very poor prognosis, and its median survival ranges from 17 to 25 months, with fewer than 20% of patients achieving 5-year survival.9 In the present case, our patient underwent a complete gross-tumor resection with all 34 lymph nodes testing negative. No decision was made to use either chemotherapy or radiotherapy. The only treatment after surgery has been traditional Chinese herbs. However, no evidence could be confirmed that Chinese herbs benefitted the 6-month nonrecurrence of the tumor in our patient.
Conclusion
Desmoplastic small round cell tumor is a highly aggressive, malignant mesenchymal tumor with a very poor prognosis. The diagnosis of DSRCT relies on immunohistologic and cytogenetic findings. No consensus has been reached for the treatment protocol of DSRCT. However, debulking surgery with postoperative chemotherapy or radiotherapy might promise more optimistic results on survival.
References
- 1.Gerald WL, Rosai J. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol. 1989;9(2):177–183. doi: 10.3109/15513818909022347. [DOI] [PubMed] [Google Scholar]
- 2.Dufresne A, Cassier P, Couraud L, Marec-Bérard P, Meeus P, Alberti L, et al. Desmoplastic small round cell tumor: current management and recent findings. Sarcoma. 2012;2012:714986. doi: 10.1155/2012/714986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerald WL, Ladanyi M, de Alava E, Cuatrecasas M, Kushner BH, LaQuaglia MP, et al. Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol. 1998;16(9):3028–3036. doi: 10.1200/JCO.1998.16.9.3028. [DOI] [PubMed] [Google Scholar]
- 4.Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round cell tumor: report of 19 cases of a distinctive type of high grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15(6):499–513. [PubMed] [Google Scholar]
- 5.Rodriguez E, Sreekantaiah C, Gerald W, Reuter VE, Motzer RJ, Chaganti RS. A recurring translocation, t(11;22)(p13;q11.2), characterizes intra-abdominal desmoplastic small round-cell tumors. Cancer Genet Cytogenet. 1993;69(1):17–21. doi: 10.1016/0165-4608(93)90105-u. [DOI] [PubMed] [Google Scholar]
- 6.Talarico F, Iusco D, Negri L, Belinelli D. Combined resection and multi-agent adjuvant chemotherapy for intraabdominal desmoplastic small round cell tumour: case report and review of the literature. G Chir. 2007;28(10):367–370. [PubMed] [Google Scholar]
- 7.Lauridant-Philippin G, Ledem N, Lemoine F, Gouerant S, Guillemet C, Michel P, et al. Optimal treatment with systemic chemotherapy, complete surgical excision and hyperthermic intraperitoneal chemotherapy for a desmoplastic small round cell tumor in an adult male patient. Gastroenterol Clin Biol. 2010;34(4–5):321–324. doi: 10.1016/j.gcb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Jordan AH, Pappo A. Management of desmoplastic small round-cell tumors in children and young adults. J Pediatr Hematol Oncol. 2012;34(suppl 2):S73–S75. doi: 10.1097/MPH.0b013e31824e38ad. [DOI] [PubMed] [Google Scholar]
- 9.Lal DR, Su WT, Wolden SL, Loh KC, Modak S, La Quaglia MP. Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg. 2005;40(1):251–255. doi: 10.1016/j.jpedsurg.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Biswas G, Laskar S, Banavali SD, Gujral S, Kurkure PA, Muckaden M, et al. Desmoplastic small round cell tumor: extra abdominal and abdominal presentations and the results of treatment. Indian J Cancer. 2005;42(2):78–84. doi: 10.4103/0019-509x.16696. [DOI] [PubMed] [Google Scholar]
- 11.Muramatsu T, Shimamura M, Furuichi M, Nishii T, Takeshita S, Shiono M. Desmoplastic small round cell tumor of the lung. Ann Thorac Surg. 2010;90(6):e86–e87. doi: 10.1016/j.athoracsur.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Subbiah V, Murthy R, Anderson PM. [90Y]yttrium microspheres radioembolotherapy in desmoplastic small round cell tumor hepatic metastases. J Clin Oncol. 2011;29(11):e292–e294. doi: 10.1200/JCO.2010.32.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su MC, Jeng YM, Chu YC. Desmoplastic small round cell tumor of the kidney. Am J Surg Pathol. 2004;28(10):1379–1383. doi: 10.1097/01.pas.0000128676.64288.0f. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Zaid A, Azzam A, Alnajjar A, Al-Hussaini H, Amin T. Desmoplastic small round cell tumor of stomach. Case Rep Gastrointest Med 2013; 2013:907136. doi: 10.1155/2013/907136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bismar TA, Basturk O, Gerald WL, Schwarz K, Adsay NV. Desmoplastic small cell tumor in the pancreas. Am J Surg Pathol. 2004;28(6):808–812. doi: 10.1097/01.pas.0000126782.94781.dc. [DOI] [PubMed] [Google Scholar]
- 16.Amato RJ, Ellerhorst JA, Ayala AG. Intraabdominal desmoplastic small round cell tumor: report and discussion of five cases. Cancer. 1996;78(4):845–851. doi: 10.1002/(SICI)1097-0142(19960815)78:4<845::AID-CNCR22>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Chang F. Desmoplastic small round cell tumors: cytologic, histologic, and immunohistochemical features. Arch Pathol Lab Med. 2006;130(5):728–732. doi: 10.5858/2006-130-728-DSRCTC. [DOI] [PubMed] [Google Scholar]
- 18.Lae ME, Roche PC, Jin L, Lloyd RV, Nascimento AG. Desmoplastic small round cell tumor: a clinicopathologic, immunohistochemical, and molecular study of 32 tumors. Am J Surg Pathol. 2002;26(7):823–835. doi: 10.1097/00000478-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Hill DA, Pfeifer JD, Marley EF, Dehner LP, Humphrey PA, Zhu X, et al. WT1 staining reliably differentiates desmoplastic small round cell tumor from Ewing sarcoma/primitive neuroectodermal tumor: an immunohistochemical and molecular diagnostic study. Am J Clin Pathol. 2000;114(3):345–353. doi: 10.1093/ajcp/114.3.345. [DOI] [PubMed] [Google Scholar]
- 20.Stuart-Buttle CE, Smart CJ, Pritchard S, Martin D, Welch IM. Desmoplastic small round cell tumour: a review of literature and treatment options. Surg Oncol. 2008;17(2):107–112. doi: 10.1016/j.suronc.2007.11.005. [DOI] [PubMed] [Google Scholar]