Abstract
Solitary fibrous tumor (SFT) is one of the mesenchymal tumors, which rarely arises in the abdominal space. We report a very rare case of abdominal SFT, mimicking another mesenchymal tumor. A 52-year-old Japanese man was referred to our hospital for further evaluation and treatment of gallbladder polyp. Contrast-enhanced computed tomography (CT) showed an enhanced nodule within the gallbladder, and incidentally, also showed a well-circumscribed mass adjacent to the small intestine. The mass was depicted as slightly high density in plain CT, and with contrast-enhancement, the mass was partially stained in early phase and the stained area spread heterogeneously in delayed phase. Magnetic resonance imaging showed that the abdominal mass was depicted as slightly high intensity on T2-weighted imaging and low intensity on T1-weighted imaging. With double-balloon endoscopy and capsule endoscopy, we did not find any tumor inside the small intestine. These visual findings lead us to diagnose it as gastrointestinal stromal tumor of the small intestine with extraluminal growth. We planned to resect both the gallbladder polyp and the intraperitoneal tumor at the same time for pathologic diagnosis and treatment. When the operation was performed, we found a milk-white lobulated tumor on the greater omentum and the tumor was entirely resected. Microscopically, the gallbladder polyp was diagnosed as tubular adenoma, and the omental tumor was diagnosed as SFT. It is important to bear in mind that omental SFTs sometimes mimic other mesenchymal tumors and should be included in the differential diagnosis of abdominal tumor not revealed by endoscopy.
Key words: Mesothelial tumor, Extrathoracic location
Solitary fibrous tumor (SFT) is one of the mesenchymal tumors, which was initially thought to usually arise in the pleura. In recent days, however, there have been several previously reported cases of extrapleural SFT; for example, orbit, thyroid, breast, and so on.1 Nevertheless, SFTs arising in the abdominal space are still rare; in particular, there have been few reports of omental SFT in the past literature. We herein report a case of SFT of the greater omentum, which mimicked another mesenchymal tumor.
Case Report
A 52-year-old Japanese man with no remarkable medical history received a routine medical check-up, and abdominal ultrasonography (AUS) revealed a gallbladder polyp that measured 4 mm. Five years later, in 2013, he underwent the second AUS. The AUS showed the gallbladder polyp had grown to 11 mm in size. He was referred to our hospital for further evaluation and treatment.
Contrast-enhanced computed tomography (CECT) showed a slightly enhanced nodule within the gallbladder (Fig. 1a, arrowhead), and incidentally, also showed a 16-mm well-circumscribed mass adjacent to the small intestine (Fig. 1a, arrows). The mass was depicted as slightly high density in plain CT (Fig. 1b). With contrast-enhancement, the mass was partially stained in early phase (Fig. 1c) and the stained area spread heterogeneously in delayed phase (Fig. 1d). Magnetic resonance imaging (MRI) showed that the abdominal mass was depicted as slightly high-intensity on T2-weighted imaging (Fig. 2a) and low-intensity on T1-weighted imaging (Fig. 2b). With double-balloon endoscopy and capsule endoscopy, we did not find any tumor inside the small intestine. The appearance and the enhancement pattern of the tumor, in both CT and MRI, led us to diagnose it as gastrointestinal stromal tumor (GIST) of the small intestine with extraluminal growth. We planned to resect both the gallbladder polyp and the intraperitoneal tumor at the same time for pathologic diagnosis and treatment.
Fig. 1.
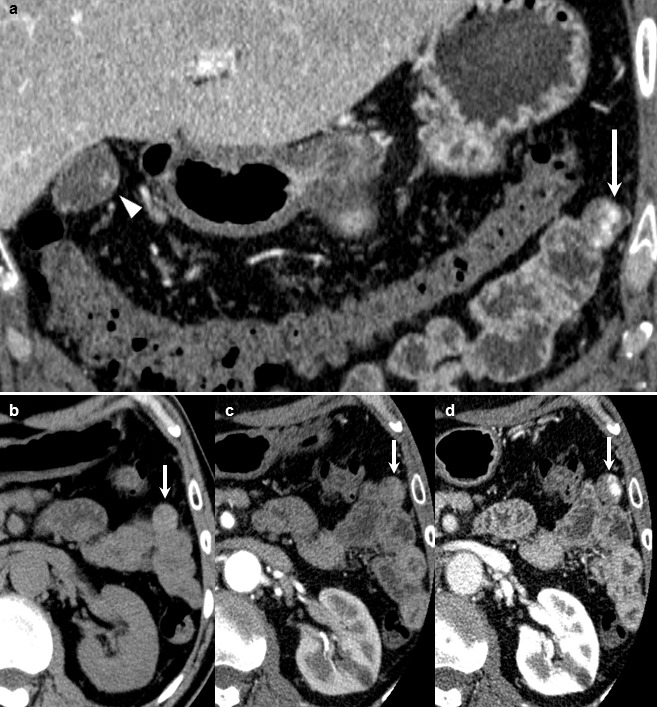
Preoperative CECT showed a slightly enhanced nodule within the gallbladder (a, arrowhead), and a well-circumscribed mass adjacent to the small intestine (a, arrow). The mass adjacent to the small intestine was depicted as slightly high density in plain CT (b, arrow). With contrast enhancement, the mass was partially stained in early phase (c, arrow) and the stained area spread heterogeneously in delayed phase (d, arrow).
Fig. 2.
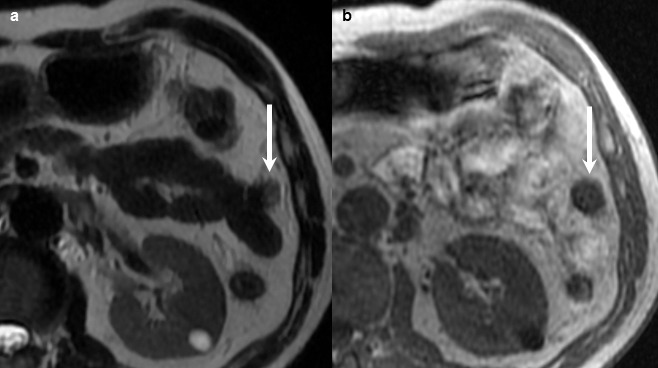
Preoperative MRI showed that the mass adjacent to the small intestine was depicted as slightly high intensity on T2-weighted imaging (a, arrow) and low intensity on T1-weighted imaging (b, arrow).
The operation was performed. First, we resected the gallbladder under laparoscopic view. Then, we observed the abdominal space and found a milk-white lobulated tumor on the greater omentum (Fig. 3a). To the best of our laparoscopic detection, there were no other intraperitoneal tumors. The tumor was entirely resected by open mini-laparotomy (Fig. 3b) and the operation was finished. This smoothly lobulated tumor had an elastic consistency.
Fig. 3.
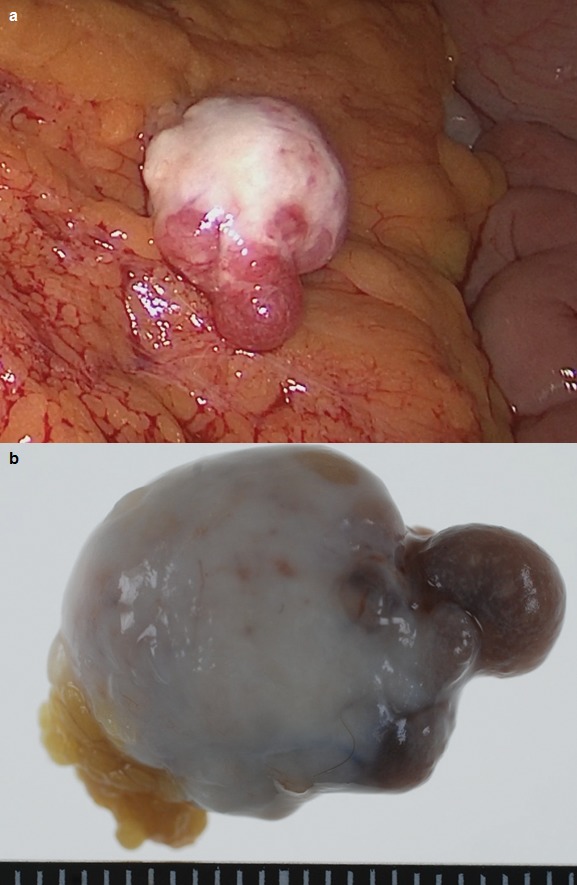
Under laparoscopic view, a milk-white lobulated tumor on the greater omentum was detected (a). The tumor was entirely resected (b).
Microscopically, the gallbladder polyp was diagnosed as tubular adenoma and the omental tumor turned out to be “solitary fibrous tumor.” The tumor was characterized by a cellular proliferation of spindle-shaped cells with no remarkable atypia in a marked collagenous matrix (Fig. 4a). Immunohistochemical staining revealed the tumor was diffusely positive for CD34 (Fig. 4b) and STAT6 (Fig. 4c) and negative for C-kit, S-100, desmin, and 1A4, which were the final clinchers of its diagnosis.
Fig. 4.
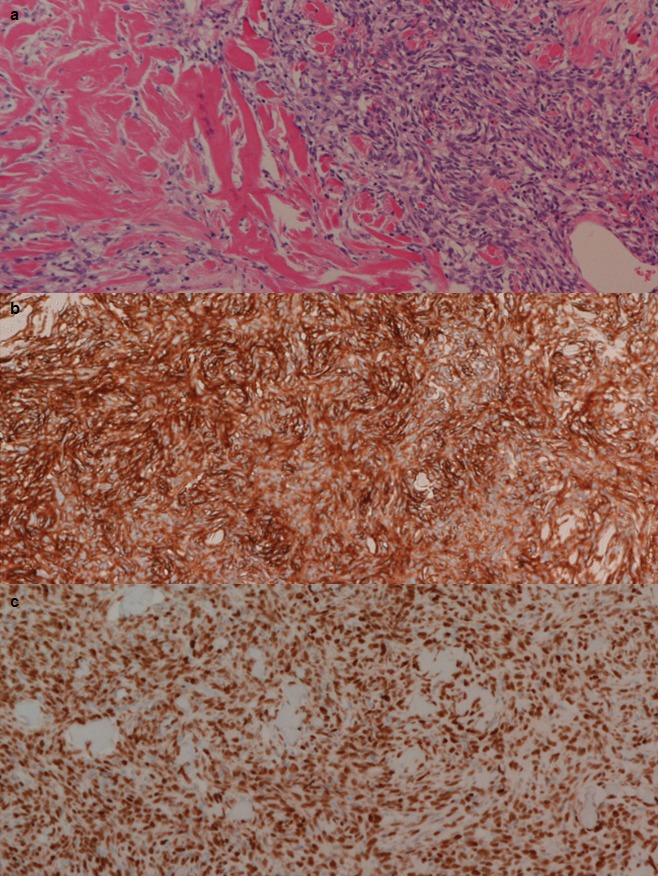
Microscopically, the omental tumor was characterized by a cellular proliferation of spindle-shaped cells with no remarkable atypia in a marked collagenous matrix (a, H–E stain ×100). Immunohistochemical test showed the tumor was positive for CD34 (b, ×100) and STAT6 (c, ×100).
The retrospective interpretation of the preoperative CT revealed that the feeding artery to the omental tumor might be the left gastroepiploic artery (Fig. 5).
Fig. 5.
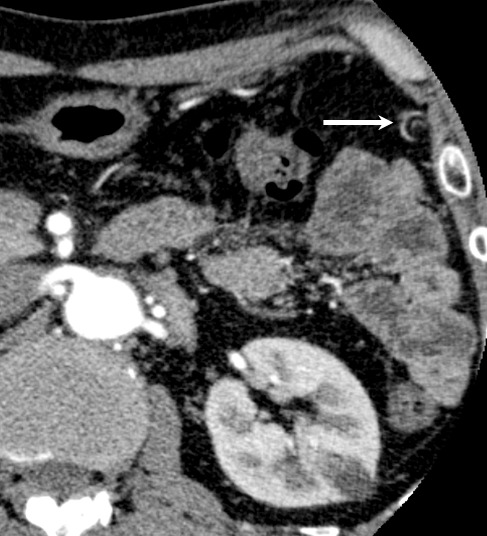
Preoperative CT revealed that the feeding artery to the omental tumor might be the left gastroepiploic artery (arrow).
After the operation, he has visited us regularly and, following surveillances, disclosed no evident disease recurrence 11 months post-operation.
Discussion
SFTs were first originally described in 1931 as tumors arising from the pleural space2 and have been well known to occur most often in the pleura. As mentioned above, however, there have been several cases of extrapleural SFTs. According to the revised World Health Organization classification (2002),3 SFT is anatomically ubiquitous and occurs often in somatic soft tissue. Currently, it is estimated that about 30–40% of reported SFTs in the English literature are extrapleural.4 However, SFTs in the intraperitoneal space are still rare: there have been only 6 reported cases of SFTs arising from greater/lesser omentum.5
Histologic diagnostic features of benign SFTs are as follows: (1) circumscription; (2) alternating hypercellular foci and hypocellular sclerotic foci; (3) bland-looking short spindly or ovoid cells with scanty and poorly defined cytoplasm; few mitotic figures (< 4/10 high power field); (4) haphazard, storiform, or fascicular arrangement of spindly cells; and (5) intimate intertwining of thin or thick collagen fibrosis with spindly cells.1 Histologic malignant features are as follows: (1) high cellularity with crowding and overlapping of nuclei; (2) high mitotic activity (> 4/10 high power field); and (3) pleomorphism judged as mild, moderate, or marked, based on nuclear size, irregularity, and nucleolar prominence.6 Immunochemically speaking, CD34 is a very useful positive biomarker for SFT. A few studies reported a 100% positivity rate.7,8 Chan1 said it is likely that solitary fibrous tumor represents the neoplastic counterpart of the ubiquitous CD34-positive dendric intestinal cell. Furthermore, what is attracting a great deal of recent attention is STAT6-positivity of SFTs. Robinson et al9 sequenced the exome of an SFT from a patient with recurrent tumors and detected a fusion between the NAB2 and STAT6 genes. Chmielecki et al10 reported whole-exome sequencing of 17 SFTs, and analysis of 53 tumors showed the NAB2-STAT6 fusion was present in 29 (55%) of tumors. Nowadays, expression of STAT6 is put to clinical use for distinguishing from some other histologic mimickers. Also in the current case, indeed, the positivity of CD34 and STAT6 was the most important clincher of the diagnosis. As differential diagnosis for this spindle cell tumor, GIST, neurogenic tumors, myogenic tumors or vascular tumors were thought to be diagnostically unlikely because of the negativity of C-kit (well-known positive marker for GIST),11 S-100 (neurogenic marker), desmin (myogenic marker), and 1A4 (leiomyogenic marker). The tumor's microscopic appearance with no atypia implied less possibility of malignancy, such as fibrosarcoma.
SFTs are generally benign; approximately 78 to 88% of SFTs are graded as benign on clinical diagnosis12 and would not affect the patients' prognoses a lot unless they grow to an extremely large size. The rest, 12 to 22% of SFTs, are deemed as malignant tumors, behaving in invasive and metastatic fashion. Limited to extrathoracic SFTs, Vallat-Decouvelaere et al reported that their recurrence rate was 6% (5 of 78 patients).13 Considering the possibility of malignancy, it would be recommended to surgically resect the whole tumor with negative margins when facing this rare entity. Gold et al4 reported SFTs greater than 10 cm had a statistically significant worse outcome for metastasis and also showed a trend toward an increased local recurrence disease survival. The current case might have relatively low risk of recurrence because of its smaller size. However, a previous article reported that it might be difficult to accurately evaluate biologic malignancy of SFTs only according to their morphology.13 Also in the current case, a sufficient span of follow-up certainly would be needed.
When making a decision of whether to excise tumors, CT and magnetic resonance imaging (MRI) are useful diagnostic modalities for their ability to evaluate the relationship between the tumor and its neighboring structures. However, these modalities sometimes displayed misleading findings as in our case, and detection of a tumor's feeding artery might be helpful for its diagnosis. Moreover, it is difficult to distinguish SFTs from other mesenchymal tumors only by these modalities, because appearance of SFTs vary a lot in accordance with the proportion of their fibrous component, the grade of their cellularity, or the existence of hemorrhage.14 We should keep in mind these limitations.
In summary, we came across a rare case of omental SFT mimicking GIST of the small intestine. We have to know omental SFT could mimic some other mesenchymal tumor. When facing abdominal tumor not revealed by endoscopy, SFT should be included in its differential diagnosis. The detection of a tumor's feeding artery might be helpful for the diagnosis.
References
- 1.Chan JK. Solitary fibrous tumor – everywhere, and a diagnosis in vogue. Histopathol. 1997;31(6):568–576. doi: 10.1046/j.1365-2559.1997.2400897.x. [DOI] [PubMed] [Google Scholar]
- 2.Klemperer P, Rabin CB. Primary neoplasms of the pleura: a report of five cases. Arch Pathol. 1931;11(3):385–412. [Google Scholar]
- 3.Fletcher CDM, Unni K, Mertens F. World Health Organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon, France: IARC Press; 2002. [Google Scholar]
- 4.Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94(4):1057–1068. [PubMed] [Google Scholar]
- 5.Zong L, Chen P, Wang GY, Zhu QS. Giant solitary fibrous tumor arising from greater omentum. World J Gastroenterol. 2012;18(44):6515–6520. doi: 10.3748/wjg.v18.i44.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13(8):640–658. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Westra WH, Gerald WL, Rosai J. Solitary fibrous tumour, consistent CD34 immunoreactivity and occurrence in the orbit. Am J Surg Pathol. 1994;18(10):992–998. doi: 10.1097/00000478-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Renshaw AA, Pinkus GS, Corson JM. CD34 and AE1/AE3, diagnostic discriminants in the distinction of solitary fibrous tumour of the pleura from sarcomatoid mesothelioma. Appl Immunohistochem. 1994;2(2):94–102. [Google Scholar]
- 9.Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45(2):180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chmielecki J, Crago AM, Rosenberg M, O'Connor R, Walker SR, Ambrogio L, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45(2):131–132. doi: 10.1038/ng.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirota S, Isozaki K, Moriyama Y, Hasimoto K, Nishida T, Ishiguro S, et al. Gain-of-function of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 12.Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control. 2006;13(4):264–269. doi: 10.1177/107327480601300403. [DOI] [PubMed] [Google Scholar]
- 13.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22(12):1501–1511. doi: 10.1097/00000478-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Ali SZ, Hoon V, Hoda S, Heelan R, Zakowski MF. Solitary fibrous tumor. A cytologic-histologic study with clinical, radiologic, and immunohistochemical correlations. Cancer. 1997;81(2):116–121. doi: 10.1002/(sici)1097-0142(19970425)81:2<116::aid-cncr5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]


