Abstract
We aimed to present our clinical experience with FG treatment. Fournier's gangrene (FG) is a rare but serious disease characterized by progressive necrosis in the genitourinary and perineal region. The retrospective study included 43 patients. Patients were divided into 2 groups as survivors and nonsurvivors. Included in the analysis were data pertaining to demographics, predisposing factors, comorbidities, results of bacteriologic analyses, number of debridements, duration of treatment, FG Severity Index (FGSI) score, fecal diversion methods (trephine ostomy or Flexi-Seal Fecal Management System-FMS), and dressing methods (wet or negative aspiration system). In the nonsurvivor group, urea, WBC, and age were significantly higher, whereas albumin, hematocrit, platelet count, and length of hospital stay (LOHS) were significantly lower compared to the survivor group. Mean FGSI was lower in survivors in comparison with nonsurvivors (5.00 ± 1.86 and 10.00 ± 1.27, respectively; P < 0.001). We conclude that FGSI is an important predictor in the prognosis of FG. Vacuum-assisted closure (VAC) should be performed in compliant patients in order to enhance patient comfort by reducing pain and the number of dressings. Fecal diversion should be performed as needed, preferably by using FMS. The trephine ostomy should be the method of choice in cases where an ostomy is necessary.
Key words: Fournier's gangrene, Trephine ostomy, Vacuum-assisted closure, Mortality
Fournier's gangrene (FG) is a rare but serious disease characterized by progressive necrosis in the genitourinary and perineal region.1 FG was named after Jean Alfred Fournier, who presented the first report of FG in 1883. In the report, the etiology of FG was considered to be idiopathic, although FG is now believed to result from a polymicrobial infection, which includes both aerobic and anaerobic pathogens.2,3 The infection mostly affects the gastrointestinal and genitourinary regions as well as cutaneous and soft tissues.4 The progression of the infection is extremely rapid and characterized by the formation of fascial planes and progressing into the abdominal wall, pelvic region, and retroperitoneal area. The overall incidence of FG is 1.6 cases per 100,000 males per year.5 FG is mostly seen in men aged 40 to 50, although it has also been reported in women.6 In spite of advancements in treatment methods and intensive care systems, FG is associated with high mortality, with rates ranging between 7.5%5 and 45%.7 A number of factors, including diabetes mellitus (DM), advanced age, end-stage liver disease, vasculopathy, malignancy, chronic alcoholism, obesity, paraplegia, and renal failure are believed to predispose individuals to the development of FG, although 30% to 50% of FG cases are not associated with any known comorbidities or other risk factors.8
The most common symptom of FG is pain in the perianus or perineum, and FG is primarily diagnosed via clinical examination. Common clinical signs of FG include inflammation, necrosis of soft tissues, crepitus, and tenderness. Early detection of FG is possible using radiologic methods such as computed tomography (CT).9 The essential treatment of FG includes prompt and aggressive surgical debridement of the tissues involved, followed by wide-spectrum antibiotic treatment.10,11
FG is a rapidly progressing disease, requiring repeated debridements and thus longer hospitalization periods. For these reasons, FG is associated with high treatment costs. To the best of our knowledge, no previous large patient series are available for FG, and there is no detailed documentation regarding the use of new methods in the treatment of FG. In this retrospective study, we aimed to present our clinical experience with FG treatment.
Materials and Methods
The retrospective study included 43 patients who were treated surgically for FG in the Department of General Surgery, Medical School, Dicle University, Diyarbakır, Turkey, between 2005 and 2014. FG was diagnosed via patient history and the findings of the physical examination at the time of presentation. Medical records and surgical notes were evaluated, and relevant physical and surgical findings were recorded. Included in the analysis were data pertaining to demographics (age, gender), time from onset of symptoms to presentation, predisposing factors, comorbidities, results of bacteriologic analyses, number of debridements, duration of treatment, fecal diversion methods (Trephine ostomy or Flexi-Seal Fecal Management System) and dressing methods (wet or negative aspiration system).
Routine blood test, blood gas analysis, and other laboratory tests were performed in all patients. In addition, a CT scan was performed in the patients with suspected abdominal and retroperitoneal infections. The Fournier's Gangrene Severity Index (FGSI) score was assessed for each patient in evaluating disease severity of the basis of body temperature, heart rate, respiratory rate, serum sodium concentration, serum potassium concentration, serum creatinine, hematocrit, white blood cell count, and blood bicarbonate concentration. The patients were divided into 2 groups as (I) survivors and (II) nonsurvivors.
Following the physical examination, aggressive fluid resuscitation was performed in all patients, followed by the administration of wide-spectrum antibiotic therapy (third-generation cephalosporin + metronidazole). Hemodynamic support was provided as needed. Patients with cardiopulmonary insufficiency required prolonged mechanical ventilation, invasive monitoring, and inotropic support as a result of sepsis. Following the debridement, the necrotic tissues were completely excised and sent for microbiologic and histologic examination along with the samples obtained from the hypodermic and fascial tissues. Hydrogen peroxide and povidone/iodine solution were used to soak the dressings that were used to pack the areas of debridement. Following the primary surgery, the wound area was carefully evaluated and sufficient nutritional support was supplied in order to facilitate the recovery process. Debridement was repeated within the first or second day after the first debridement, and additional tissues were debrided in cases where the infection seemed highly progressive and the diameter of the necrosis in the wound area was critically large. For the purpose of this analysis, we defined debridement as the procedures performed in the operating room under general and spinal anesthesia, and did not include procedures performed outside the operating room under local anesthesia. Daily wound dressing changes were conducted by conventional methods, and vacuum-assisted closure (VAC) was used when applicable after eliminating the necrosis. VAC was applied at 75 to 125 mmHg and the dressings were changed every 72 hours.
Wound repair procedures were promptly initiated when re-approximation of healthy tissues was possible. Split-thickness skin grafts or rotational cutaneous flaps were applied as needed for repair of large wounds.
Colostomy or Flexi-Seal Fecal Management System (FFMS) was applied in patients who required fecal diversion. FFMS is comprised of several components including a soft silicone catheter with a retention balloon that is inserted into the patient's rectum and a syringe used for rectal irrigation and a collection bag.
Colostomy was applied for fecal diversion when the infection originated from the anorectal area and proceeded to the sphincter. Additional indications for colostomy included including the detection of a rectal perforation, a large wound in the rectum, or ongoing systemic sepsis. In all patients, colostomy was performed according to the Trephine technique.
For each continuous variable, mean and standard deviations were calculated and median values were defined for discrete variables. Proportions were compared between groups using the chi-square test. Both visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk's test) were used evaluate normal distribution of the variables. Normally distributed variables were compared using the Student's t-test; variables with distributions that did not conform to the normal distribution were compared using the Mann-Whitney U test. A P value of <0.05 was considered significant. All the data were analyzed using SPSS 12.0 for Windows (Chicago, Illinois).
Results
A total of 43 patients were diagnosed and treated at the University Hospital of Dicle by the Department of General Surgery during the years 2005–2014. Thirty-four (79.1%) of the 43 patients were male and the mean age was 51.98 (range, 26–90) years. The most common comorbidity was diabetes mellitus, which was seen in 18 (41.9%) patients. Demographic data and comorbidities are shown in Table 1. Of the 43 patients studied, 6 died and 37 survived, resulting in an overall mortality rate of 14.0%.
Table 1.
Demographics and comorbidities

The most common complaint at presentation was perianal/scrotal pain and swelling (72.9%), followed by tachycardia (58.13%), purulent discharge from the perineum (55.81%), crepitus (51.16%), and fever (41.86%). The most common clinical presentation was necrosis in the perineal and genital regions.
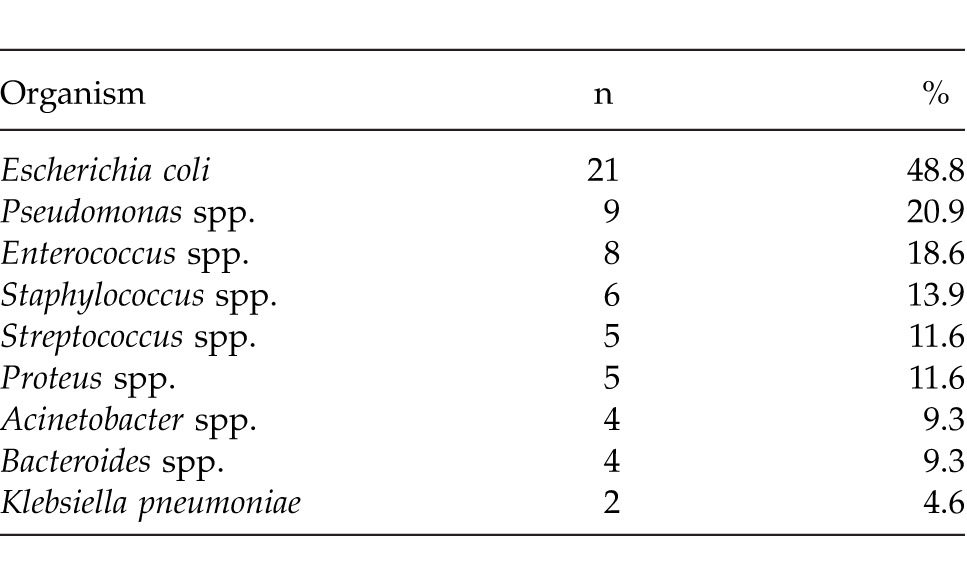
Table 2 presents the results of bacteriologic studies at presentation. The most common bacterial organisms found in the wounds were Escherichia coli (48.8%), Pseudomonas spp. (20.9%), Enterococcus spp. (18.6%), Staphylococcus spp. (13.9%), Streptococcus spp. (11.6%), Proteus spp. (11.6%), Acinetobacter spp. (9.3%), Bacteroides spp. (9.3%), and Klebsiella pneumoniae (4.6%).
Table 2.
Bacterial organisms found in the wound cultures of the patients with Fournier's gangrene
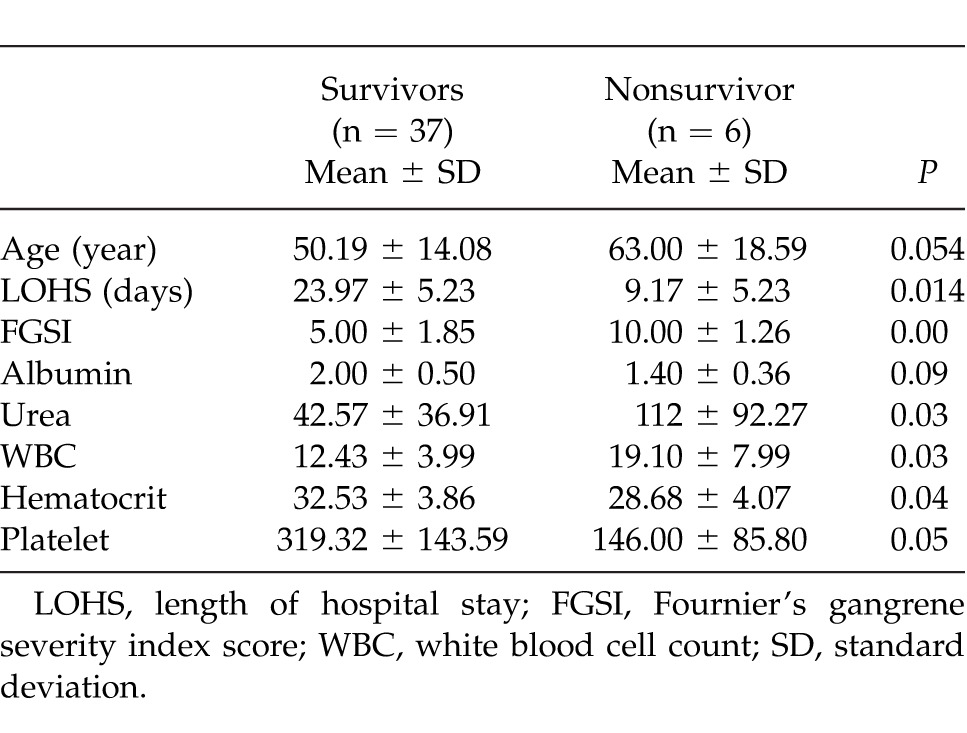
In the nonsurvivor group, urea, WBC, and age were significantly higher, whereas albumin, hematocrit, platelet count, and length of hospital stay (LOHS) were significantly lower in the nonsurvivor group compared to the survivor group (Table 3).
Table 3.
Mortality according to patient characteristics
The mean duration of symptoms at presentation was less than 48 hours in 16 (37.2%) patients and more than 48 hours in 27 (62.8%) patients. Delayed admission occurred in all nonsurvivors (100%) and 21 (56.8%) patients in the survivor group.
Mean LOHS of 43 patients was 22.58 ± 13.39 days (range, 7–79 days). All the patients spent more than 24 hours in the intensive care unit, and their mean length of stay in the unit was 5.5 days (range, 5–45 days). Mean length of hospital stay (LOHS) was 23.97 ± 5.23 days in the survivor group and 9.17 ± 5.23 days in the nonsurvivor group, a difference that was statistically significant (P = 0.014). Mean age was 50.19 ± 14.08 years in the survivor group and 63.00 ± 18.59 years in the nonsurvivor group, a difference which approached statistical significance (P = 0.054; Table 3).
While 16 patients (37.2%) underwent a single debridement, 27 (62.8%) patients required repeated debridements. However, there was no significant difference in the number of debridements performed between survivors and nonsurvivors (P = 0.504).
Radiologic imaging was performed in 8 patients (18.6%) in order to visually evaluate surgical progress. Imaging was performed by CT (Fig. 1). A pelvic abscess was detected in 1 patient, which was drained. A total of 12 patients underwent Trephine colostomy and 5 patients underwent Flexi-Seal for the treatment of infection originating from the perirectum or involving the anal sphincter. Tissue reconstruction was performed when the patient was clinically stabilized and the wound site was cleaned (Figs. 2, 3).
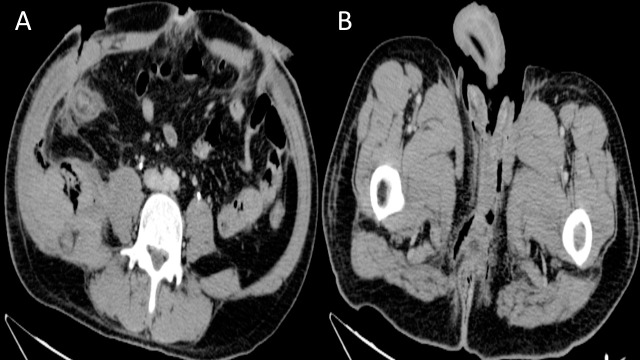
Fig. 1.
Abdominal computed tomography of a patient with Fournier's gangrene. (A) Axial CT image showing bilateral abdominal wall defect, air densities on the lateral fascia (on the right), and mild thickening of the plantar fascia. (B) Axial CT image showing air densities and mild thickening of the plantar fascia at the perinial level.
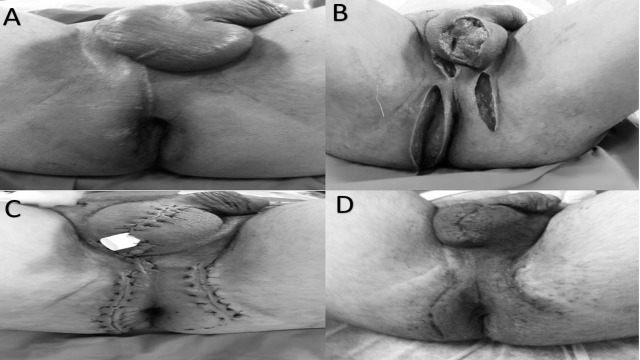
Fig. 2.
(A) The patient with Fournier's gangrene before treatment. (B) The wound was cleaner following a second debridement and VAC. (C) Patient after wound primary closure in the operating room. (D) Surgical site at first postoperative month.
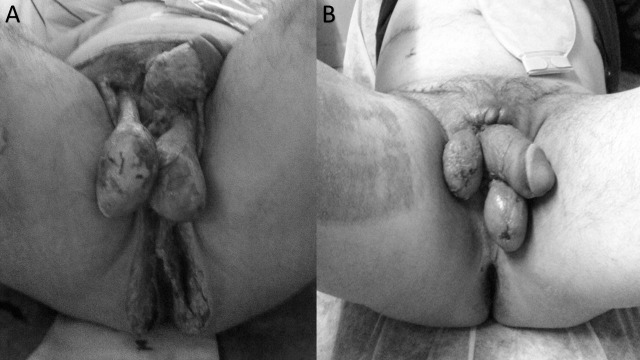
Fig. 3.
(A) The patient with Fournier's gangrene after debridement. (B) Patient after wound closure and skin graft.
Mean FGSI was 5.79 ± 2.45 (range, 0–12), with a mean FGSI of 5.00 ± 1.86 in the survivors and 10.00 ± 1.27 in the nonsurvivors (P < 0.001, t test).
Discussion
FG was first defined by Fournier in 1883 as an idiopathic condition.3 However, the majority of FG patients present with an infection of the perianal tissue or urinary tract and a history of local trauma. The infection primarily affects the genitourinary region and is typically characterized by acute rapidly progressing and potentially life-threatening infection.12 FG is associated with high mortality (3–67%) although significant advances have been made in the understanding of the etiology, diagnosis, and treatment of FG.13 In the present case series, the overall mortality rate was 14%.
Males older than 40 to 50 years of age constitute the largest proportion of FG cases.6 When the overall incidence of FG is examined, male patients outnumber female patients by a ratio of 10:1.14 However, in the present case series the ratio of males to females (4:1) was low relative to previous reports. Our patients had a mean age of 52 years, with age ranging from 26 to 90 years.
A number of systemic diseases including DM, vasculopathy, malignancy, chronic alcoholism cirrhosis, renal failure, and the use of steroids are known risk factors for the development of FG.5,14,15 In the present case series, DM was the most common risk factor, affecting 40.5% of the survivors and 66.7% of nonsurvivors. Although some studies have reported no association between DM status and clinical outcomes in FG,16–18 we observed increased mortality among patients with DM.
FG is currently thought to originate from a polymicrobial infection.2,19 Although the aerobes and anaerobes are culturable in the vast majority of the FG patients, the isolation of the anaerobes is less common.20 Blood cultures are generally negative. Among the bacterial organisms, Enterobacteriaceae, particularly E. coli, are the most frequently cultured species, followed by streptococcal species. In addition, other organisms such as Staphylococci, P. aeruginosa, Peptostreptococci, Bacteroides spp., and Clostridia are frequently isolated. These organisms are normally found in the gastrointestinal tract and in the perineal site.16,21 In the present study, E. coli, P. aeruginosa, Enterococcus spp., and Bacteroides were cultured from the wound sites of the patients.
A number of clinical symptoms are commonly used in the diagnosis of FG, including genital discomfort and pruritus in the prodromal period, followed by scrotal edema, genital erythema, scrotal pain, partial necrosis, induration, crepitations, feculent odor, and fever.22 This pattern of symptoms may not be present in every patient, making it difficult to differentiate FG from other soft-tissue diseases.23 According to the report by Ersay et al, FG cases primarily present with a perianal/scrotal pain followed by tachycardia, purulent discharge from the perineum, crepitus, and fever.13 On the other hand, Ferreira et al reported that scrotal swelling, fever, and pain were the most common symptoms.24 In our study, the majority of patients presented with perianal or scrotal swelling and pain, tachycardia, crepitation, purulent discharge with feculent odor, and fever.
A prognostic index known as FGSI was developed by Laor et al to assess the severity and prognosis of FG patients.16 This index was subsequently validated by Yeniyol and Tuncel.22,25 With this index, it is possible to compare disease severity between patient and to assess the probability of survival using the variables that are recorded at the time of presentation. In the present series, the mean FGSI was 5.79 ± 2.45 and the mean FGSI among the survivors was almost half the mean FGSI of nonsurvivors. Ersay et al reported that nonsurvivors had significantly lower hematocrit and albumin levels and higher levels of blood urea and lactic dehydrogenase.13 Moorthy et al found that a low hematocrit, high blood urea and creatinine, and a low serum albumin were associated with higher mortality.26 In contrast, Clayton et al reported that only blood urea nitrogen >50 mg/dL was a statistically significant predictor of mortality among the many parameters evaluated.27 Laor et al reported that hematocrit, serum calcium, albumin, and cholesterol levels were higher and blood urea nitrogen and alkaline phosphatase levels were lower in the survivors relative to the time of admission.16 In the present study, nonsurvivors had significantly lower serum hematocrit, platelet counts, and serum albumin and higher blood urea and WBC. Some authors, including Clayton et al27 and Benizri,28 have suggested that there is a strong correlation between mortality and patient age. We also found a significant correlation between mortality and age (Table 3).
The treatment of FG requires prompt and aggressive surgical debridement of the necrotic tissues, followed by wide-spectrum antibiotic treatment. This treatment is highly similar to the procedure undertaken for patients with severe sepsis. Since the general condition of the patient with FG is poor, it is essential to ensure fluid resuscitation and hemodynamic stability. In addition, blood transfusion may be required and albumin and vasopressors may be needed in the patients presenting with septic shock. Wide-spectrum antibiotic therapy (penicillin, metronidazole, and third-generation cephalosporin with gentamicin) should be administered prior to surgery and should be revised depending on the results of culture analyses. During surgical debridement, all necrotic areas should be removed and debridements should be repeated if necrosis continues.10,11,13 In the present case series, debridement was repeated in 27 (62.8%) patients who presented with progressive necrosis.
Proper dressing with simultaneous exploration is the key to wound therapy success in FG since it facilitates the detection of changes in the wound site. A well-known conventional method of dressing is known as wet-to-dry dressing. Despite drawbacks such frequent dressing changes, this method is beneficial and widely used because it is and cost-effective and keeps the wound site clean. A newly developed method known as negative pressure wound therapy (NPWT) has also been suggested as an effective method in FG wound therapy. The application of NPWT following elimination of necrosis facilitates wound healing processes.29–31 Ozturk et al32 have reported that conventional methods and VAC treatment methods yielded equivalent effects in terms of wound healing and were associated with similar costs. In the present study, we applied the conventional wound healing method in 35 patients and the VAC method in 8 patients. There was no correlation between VAS use and mortality or LOHS. However, as Ozturk et al32 reported previously, the use of VAC led to fewer dressing changes, less pain, fewer skipped meals, and greater mobility while reducing hands-on treatment time for the clinician.
Benefits associated with fecal diversion include a reduction in the number microbes in the perineum, remarkable improvement in the wound healing process, and local control of the infection.33 Fecal diversion is performed using the Flexi-Seal Fecal Management System (FMS) or diverting colostomy. FMS is an alternative to diverting colostomy in the management of fecal stream in FG patients. When compared to colostomy, FMS is cost effective and improves patient comfort for short-term fecal diversion.31,34 Moreover, in addition to preventing fecal contamination in the wound site, FMS avoids the complications associated with colostomy.34 Nevertheless, the use of FMS is not advised in cases presenting with rectal neoplasm and penetrating rectal injuries or fistulas. Diverting colostomy is indicated when the anal sphincters and the rectum are involved, incontinence is permanent, or the patient is noncompliant.35 Yan-Dong et al reported that mortality was remarkably reduced by the applications of diverting colostomy in patients with FG.36 However, the diverting colostomy necessitates repeated debridements.35 In the present study, we also found that the use of colostomy was not associated with the number of debridements. However we suggest that colostomy enabled better clinical outcomes among the most severely ill patients.
Moreover, early oral intake may be possible with the use of colostomy and this may facilitate wound healing by enabling nutritional support and reducing the risk of contamination in the wound site. In the present study, we found that the use of colostomy improved outcomes among the most severely ill patients, reducing morbidity and mortality and well as LOHS in this high risk patient group. In addition, the use of colostomy did not affect mortality and LOHS, which is an indicator that we were highly selective in the use of colostomy and only applied this technique to patients with clear indications. In our clinical algorithm, we consider ostomy only as a final option for the treatment of FG. FMS is our first choice for fecal diversion. In cases where an ostomy is indicated, the Trephine ostomy is out method of choice as it is clearly the safest and simplest method. This method also provides minimal invasion and can be applied for fast fecal diversion in patients who have no indications for laparotomy and in high risk patients presenting.37
Conclusion
Overall mortality among our FG patients was 14%. We conclude that early diagnosis, prompt surgical debridement, and wide-spectrum antibiotic treatment are vital steps to be taken in the essential treatment of FG. We also suggest that FGSI is an important predictor in the prognosis of FG. VAC should be performed in compliant patients in order to enhance patient comfort by reducing pain and the number of dressings. Fecal diversion should be performed as needed, preferably by using FMS. The trephine ostomy should be the method of choice in cases where an ostomy is necessary.
Acknowledgments
The authors declare that they have no conflicts of interest.
References
- 1.Smith GL, Bunker CB, Dinneen MD. Fournier's gangrene. Br J Urol. 1998;81(3):347–355. doi: 10.1046/j.1464-410x.1998.00532.x. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez Alonso A, Pérez García MD. Núñez López A, Ojea Calvo A, Alonso Rodrigo A, Rodríguez Iglesias B et al. Fournier's gangrene: anatomo-clinical features in adults and children. Therapy update. Actas Urol Esp. 2000;24(4):294–306. doi: 10.1016/s0210-4806(00)72452-1. [DOI] [PubMed] [Google Scholar]
- 3.Fournier A. Overwhelming gangrene [in French] Semaine Med. 1883;3:345–348. [Google Scholar]
- 4.Eke N. Fournier's gangrene: a review of 1726 cases. Br J Surg 2000n; 87(6):718–728. doi: 10.1046/j.1365-2168.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen MD, Krieger JN, Rivara FP, Broghammer JA, Klein MB, Mack CD, et al. Fournier's Gangrene: population based epidemiology and outcomes. J Urol. 2009;181(5):2120–2126. doi: 10.1016/j.juro.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Arena G, Pietrantuono G, Buccino E, Pacifico G, Musto P. Fournier's gangrene complicating hematologic malignancies: a case report and review of literature. Mediterr J Hematol Infect Dis. 2013;5(1):e2013067. doi: 10.4084/MJHID.2013.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hejase MJ, Simonin JE, Bihrle R, Coogan CL. Genital Fournier's gangrene: experience with 38 patients. Urology. 1996;47(5):734–739. doi: 10.1016/s0090-4295(96)80017-3. [DOI] [PubMed] [Google Scholar]
- 8.Vick R, Carson CC., III Fournier's disease. Urol Clin North Am. 1999;26(4):841–849. doi: 10.1016/s0094-0143(05)70224-x. [DOI] [PubMed] [Google Scholar]
- 9.Sherman J, Solliday M, Paraiso E, Becker J, Mydlo JH. Early CT findings of Fournier's gangrene in a healthy male. Clin Imaging. 1998;22(6):425–427. doi: 10.1016/s0899-7071(98)00073-4. [DOI] [PubMed] [Google Scholar]
- 10.Yanar H, Taviloglu K, Ertekin C, Guloglu R, Zorba U, Cabioglu N, et al. Fournier's gangrene: risk factors and strategies for management. World J Surg. 2006;30(9):1750–1754. doi: 10.1007/s00268-005-0777-3. [DOI] [PubMed] [Google Scholar]
- 11.Fajdic J, Gotovac N, Hrgovic Z. Fournier gangrene: our approach and patients. Urol Int. 2011;87(2):186–191. doi: 10.1159/000327510. [DOI] [PubMed] [Google Scholar]
- 12.Shyam DC, Rapsang AG. Fournier's gangrene. Surgeon. 2013;11(4):222–232. doi: 10.1016/j.surge.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Ersay A, Yilmaz G, Akgun Y, Celik Y. Factors affecting mortality of Fournier's gangrene: review of 70 patients. ANZ J Surg. 2007;77(1–2):43–48. doi: 10.1111/j.1445-2197.2006.03975.x. [DOI] [PubMed] [Google Scholar]
- 14.Koukouras D, Kallidonis P, Panagopoulos C, Al-Aown A, Athanasopoulos A, Rigopoulos C, et al. Fournier's gangrene, a urologic and surgical emergency: presentation of a multi-institutional experience with 45 cases. Urol Int. 2011;86(2):167–172. doi: 10.1159/000321691. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Rodríguez R, Ponce de León J, Caparrós J, Villavicencio H. Fournier's gangrene: a monographic urology center experience with twenty patients. Urol Int. 2009;83(3):323–328. doi: 10.1159/000241676. [DOI] [PubMed] [Google Scholar]
- 16.Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI. Outcome prediction in patients with Fournier's gangrene. J Urol. 1995;154(1):89–92. [PubMed] [Google Scholar]
- 17.Corcoran AT, Smaldone MC, Gibbons EP, Walsh TJ, Davies BJ. Validation of the Fournier's gangrene severity index in a large contemporary series. J Urol. 2008;180(3):944–948. doi: 10.1016/j.juro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Paty R, Smith AD. Gangrene and Fournier's gangrene. Urol Clin North Am. 1992;19(1):149–162. [PubMed] [Google Scholar]
- 19.Morpurgo E, Galandiuk S. Fourniers gangrene. Surg Clin North Am. 2002;82(6):1213–1224. doi: 10.1016/s0039-6109(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 20.Baskin LS, Carroll PR, Cattolica EV, McAninch JW. Necrotising soft tissue infections of the perineum and genitalia. Bacteriology, treatment and risk assessment. Br J Urol. 1990;65(5):524–529. doi: 10.1111/j.1464-410x.1990.tb14801.x. [DOI] [PubMed] [Google Scholar]
- 21.Tahmaz L, Erdemir F, Kibar Y, Cosar A, Yalcýn O. Fournier's gangrene: report of thirty-three cases and a review of the literature. Int J Urol. 2006;13(7):960–967. doi: 10.1111/j.1442-2042.2006.01448.x. [DOI] [PubMed] [Google Scholar]
- 22.Yeniyol CO, Suelozgen T, Arslan M, Ayder AR. Fournier's gangrene: experience with 25 patients and use of Fournier's gangrene severity index score. Urology. 2004;64(2):218–222. doi: 10.1016/j.urology.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 23.Uluğ M, Gedik E, Girgin S, Celen MK, Ayaz C. The evaluation of microbiology and Fournier's gangrene severity index in 27 patients. Int J Infect Dis. 2009;13(6):e424–430. doi: 10.1016/j.ijid.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira PC, Reis JC, Amarante JM, Silva AC, Pinho CJ, Oliveira IC, et al. Fournier's gangrene: a review of 43 reconstructive cases. Plast Reconstr Surg. 2007;119(1):175–184. doi: 10.1097/01.prs.0000244925.80290.57. [DOI] [PubMed] [Google Scholar]
- 25.Tuncel A, Aydin O, Tekdogan U, Nalcacioglu V, Capar Y, Atan A. Fournier's gangrene: three years of experience with 20 patients and validity of the Fournier's Gangrene Severity Index Score. Eur Urol. 2006;50(4):838–843. doi: 10.1016/j.eururo.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Moorthy K, Rao PP, Supe AN. Necrotising perineal infection: a fatal outcome of ischiorectal fossa abscesses. J R Coll Surg Edinb. 2000;45(5):281–284. [PubMed] [Google Scholar]
- 27.Clayton MD, Fowler JE, Jr, Sharifi R, Pearl RK. Causes, presentation and survival of fifty-seven patients with necrotizing fasciitis of the male genitalia. Surg Gynecol Obstet. 1990;170(1):49–55. [PubMed] [Google Scholar]
- 28.Benizri E, Fabiani P, Migliori G, Chevallier D, Peyrottes A, Raucoules M, et al. Gangrene of the perineum. Urology. 1996;47(6):935–939. doi: 10.1016/S0090-4295(96)00058-1. [DOI] [PubMed] [Google Scholar]
- 29.Assenza M, Cozza V, Sacco E, Clementi I, Tarantino B, Passafiume F, et al. VAC (Vacuum Assisted Closure) treatment in Fournier's gangrene: personal experience and literature review. Clin Ter. 2011;162(1):e1–5. [PubMed] [Google Scholar]
- 30.Czymek R, Schmidt A, Eckmann C, Bouchard R, Wulff B, Laubert T, et al. Fournier's gangrene: vacuum-assisted closure versus conventional dressings. Am J Surg. 2009;197(2):168–176. doi: 10.1016/j.amjsurg.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 31.Ozkan OF, Koksal N, Altinli E, Celik A, Uzun MA, Cıkman O, et al. Fournier's gangrene current approaches. Int Wound J. doi: 10.1111/iwj.12357. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum assisted closure therapy in the management of Fournier's gangrene. Am J Surg. 2009;197(5):660–665. doi: 10.1016/j.amjsurg.2008.04.018. discussion 665. [DOI] [PubMed] [Google Scholar]
- 33.Bronder CS, Cowey A, Hill J. Delayed stoma formation in Fournier's gangrene. Colorectal Dis. 2004;6(6):518–520. doi: 10.1111/j.1463-1318.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- 34.Padmanabhan A, Stern M, Wishin J, Mangino M, Richey K, DeSane M, et al. Clinical evaluation of a flexible fecal incontinence management system. Am J Crit Care. 2007;16(4):384–393. [PubMed] [Google Scholar]
- 35.Mallikarjuna MN, Vijayakumar A, Patil VS, Shivswamy BS. Fournier's gangrene: current practices. ISRN Surg. 2012;2012:942437. doi: 10.5402/2012/942437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YD, Zhu WF, Qiao JJ, Lin JJ. Enterostomy can decrease the mortality of patients with Fournier gangrene. World J Gastroenterol. 2014;20(24):7950–7954. doi: 10.3748/wjg.v20.i24.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson ID, Hill J, Vohra R, Schofield PF, Kiff ES. An improved means of faecal diversion: the trephine stoma. Br J Surg. 1992;79(10):1080–1081. doi: 10.1002/bjs.1800791031. [DOI] [PubMed] [Google Scholar]