Abstract
Regarding the complications of peptic ulcer, a perforation remains the most important fatal complication. The aim of our retrospective study was to determine relations between postoperative morbidity and comorbid disease or perioperative risk factors in perforated peptic ulcer. In total, 239 patients who underwent emergency surgery for perforated peptic ulcer in Ege University General Surgery Department, between June 1999 and May 2013 were included in this study. The clinical data concerning the patient characteristics, operative methods, and complications were collected retrospectively. One hundred seventy-five of the 239 patients were male (73.2%) and 64 were female (26.8%). Mean American Society of Anesthesiologists (ASA) score was 1 in the patients without morbidity, but mean ASA score was 3 in the morbidity and mortality groups. Primary suture and omentoplasty was the selected procedure in 228 of the patients. Eleven patients underwent resection. In total, 105 patients (43.9%) had comorbidities. Thirty-seven patients (67.3%) in the morbidity group had comorbid diseases. Thirteen (92.9%) patients in the mortality group had comorbid diseases. Perforation as a complication of peptic ulcer disease still remains among the frequent indications of urgent abdominal surgery. Among the analyzed parameters, age, ASA score, and having comorbid disease were found to have an effect on both mortality and morbidity. The controversial subject in the present study is regarding the duration of symptoms. The duration of symptoms had no effect on mortality nor morbidity in our study.
Key words: Peptic ulcer, Prognostic, Perforation
Each year, peptic ulcer disease (PUD) affects 4 million people around the world.1 PUD is associated with potentially life-threatening complications, including bleeding, perforation, penetration, and obstruction. Perforation is the second most frequent complication after bleeding.2 The main predisposing factors for peptic ulcer perforation are smoking, use of nonsteroidal anti-inflammatory drugs, chronic stress, Helicobacter pylori infection, and advanced age (>60 years).3,4 In last decades, with the introduction of proton pump inhibitors (PPIs) and increased knowledge of perforated peptic ulcer (PPU) etiology, the incidence of PPU has reportedly decreased in Western countries.5,6 But, mortality and morbidity for PPU remain high, despite improvements in anesthesiology and intensive care medicine. The mortality ranges were reported between 1.3% and 20%.7,8 Morbidity was also high (20%–50%) in patients treated surgically for peptic ulcer perforations (PUPs).9–11
The presence of gas under the diaphragm on plain abdominal erect X-ray is diagnostic in 75% of the cases.12 In addition, having significant symptoms of PUP makes diagnosis easier. Delayed diagnosis and treatment causes negative results for patients and increases costs. Successful results can be gained by early recognition and early treatment. Many surgical techniques have been recommended by authors since the first description of surgical treatment for PUP. With the introduction of H2 blockers, PPIs, and treatment to eradicate H. pylori, complicated surgical procedures have been decreasing, and surgeons use less-invasive and simpler surgical procedures. With recent advances in antiulcer therapy, many centers accept the simple closure of the perforation with an omental patch as a safe and simple surgical procedure for PPU.13
The prognostic factors reported in the literature include delay in treatment, localization of perforation, age, being female, having coexisting medical problems, having a failed primary surgery, and gastrectomy.11,12,14–16 Currently, the American Society of Anesthesiologists (ASA) score and the Boey score are the most frequently used prognostic scoring systems in patients with PPU.8,17,18 The aim of this study was to determine the factors affecting the mortality and morbidity of PUPs and describe the management of PUPs for better results.
Materials and Methods
In total, 239 patients, who underwent emergency surgery for PPU in Ege University General Surgery Department, between June 1999 and May 2013, were included in this study. Fifty-six patients with missing charts were excluded from the study. The clinical data concerning the patient characteristics, operative methods, and complications were collected retrospectively. In this study, we analyzed age, sex, ASA score, chronic alcohol consumption, smoking, use of nonsteroidal anti-inflammatory drugs (NSAIDs), the time passed from the onset of symptoms to the operation, history of previous PUD, diameter and localization of the ulcer, surgical technique applied, postoperative complications, and mortality rates as prognostic factors.
Statistical Analysis
Data analysis was performed using SPSS for Windows, Version 11.5 (SPSS Inc, Chicago, Illinois). Whether the distributions of continuous variables were normal or not was determined by Kolmogorov-Smirnov test. Continuous variables were shown as mean ± SD or median (minimum–maximum), where applicable.
The mean differences between groups were compared by Student's t-test; otherwise, Mann-Whitney U test was applied for comparisons of the median values. Nominal data were analyzed by Pearson's χ2 or Fisher's exact test, where applicable.
Determining the best predictor(s) which effect on both morbidity and mortality was evaluated by multiple logistic regression analysis after adjustment for all possible confounding factors. Any variable whose univariable test had a P value of <0.25 was accepted as a candidate for the multivariable model along with all variables of known clinical importance. Odds ratios (ORs) and 95% confidence intervals (CIs) for each independent variable were also calculated. A P value less than 0.05 was considered statistically significant.
Results
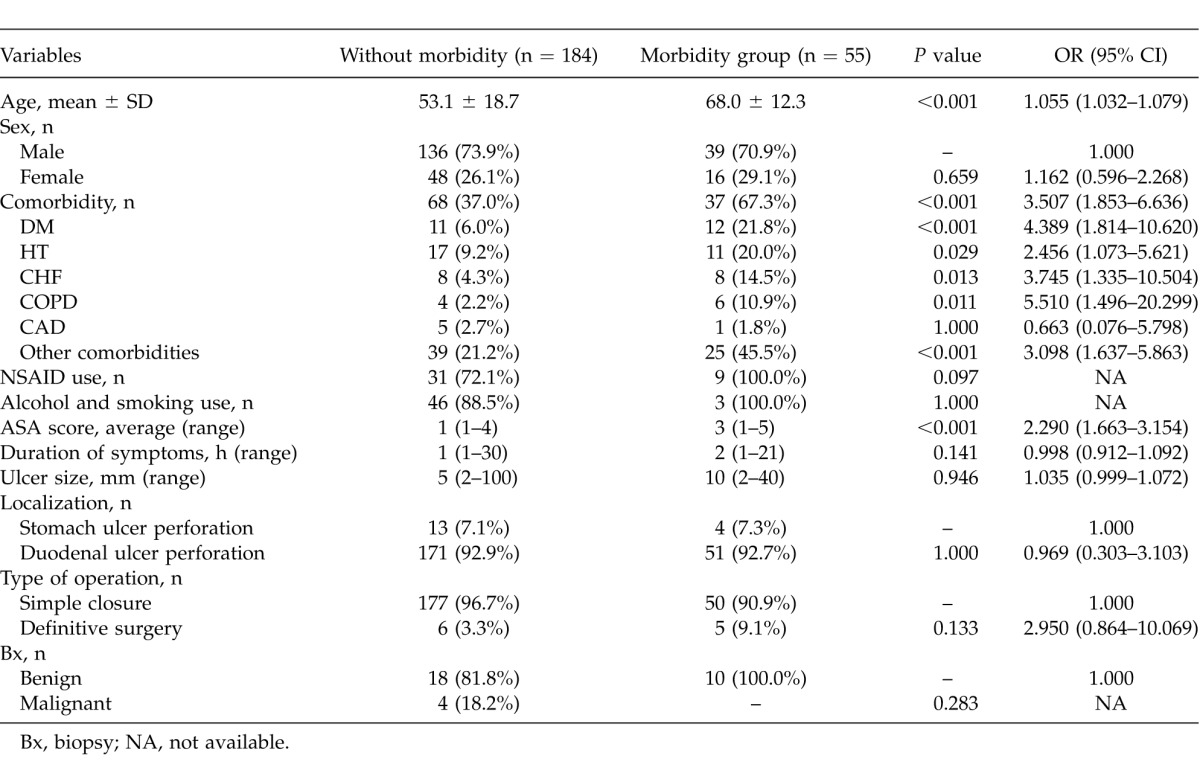
Postoperative complications (morbidity) affected 55 (23.0%) of 239 patients. Some patients experienced more than one complication. The most common morbidities were respiratory infection (33.3%), sepsis (18.0%), and wound infection (12.5%) (Table 1). The average age of the morbidity group was significantly higher than the group without morbidity (P < 0.001); that is, as the age of the patients increased, the morbidity risk increased (OR = 1.055; 95% CI, 1.032–1.079). The effect of the sex variable on morbidity was statistically insignificant (P = 0.659) (Table 2). Comorbidity was assessed for patients in terms of hypertension (HT), diabetes mellitus (DM), coronary artery disease (CAD), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), and others. The effect of comorbidity was statistically significant on postoperative morbidity (P < 0.05). Use of NSAIDs, smoking, alcohol consumption, duration of symptoms, localization and diameter of perforated ulcer, surgical procedure, and histologic diagnosis had no statistically significant effect on morbidity (P > 0.05). The ASA score was directly correlated with morbidity. As the ASA score increased, the risk of morbidity increased significantly (OR = 2.290; 95% CI, 1.663–3.154; P < 0.001) (Table 2).
Table 1.
Postoperative complications
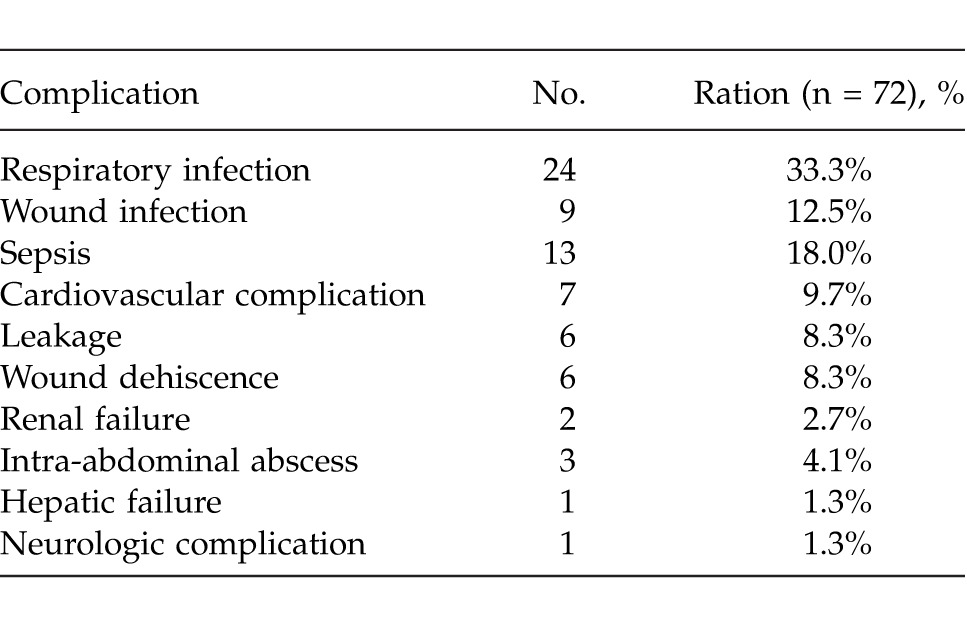
Table 2.
Demographic and clinical features regarding morbidity
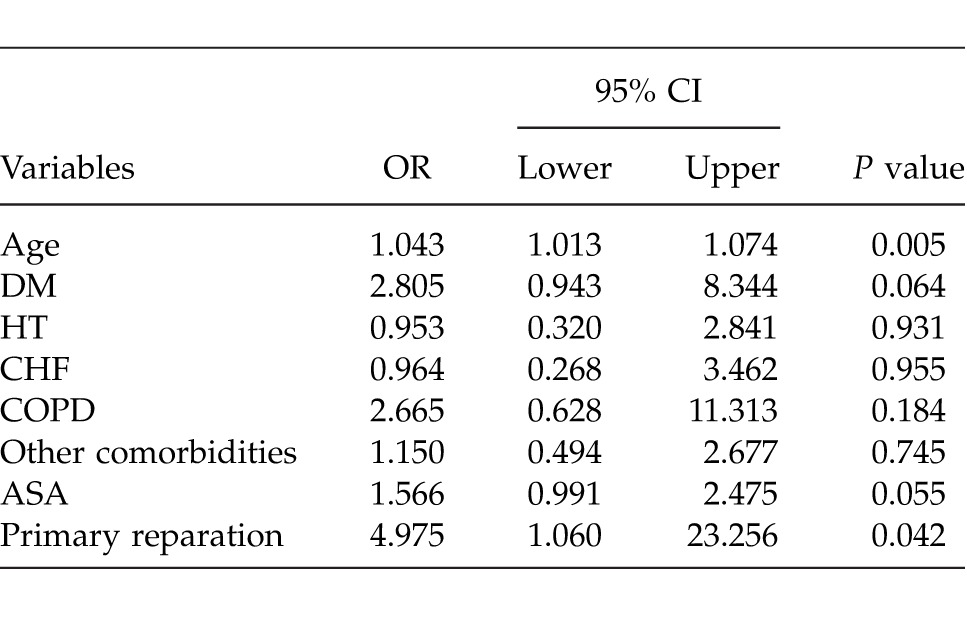
Determining the best predictor(s) which effect on morbidity was evaluated by multiple logistic regression analysis after adjustment for all possible confounding factors and the factors that were found to be effective on morbidity by single variable analysis. Any variable whose univariable test had a P value of <0.25 was accepted as a candidate for the multivariable model along with all variables of known clinical importance. According to these analyses, the factor having the most effect on morbidity was the age of the patient, and the factor having the second-most effect was primary closure of the perforation area (Table 3).
Table 3.
Results of multiple logistic regression analysis: discrimination of without morbidity and morbidity groups from each other
A total of 14 patients (5.8%) died at the hospital postoperatively. Respiratory failure and sepsis were the most common causes of mortality (Table 4). The mean age of the mortality group was 70.6 ± 13.5 years and was higher than the living group (mean 55.5 ± 18.4 years). The average age of the mortality group was significantly higher (P < 0.001). As the age of the patients increased, the risk of mortality significantly increased (OR = 1.059; 95% CI, 1.018–1.102). The sex variable had no statistically significant effect on mortality (P = 0.534). Among the patients who died postoperatively, 13 patients (92.9%) had comorbidities. Having at least a comorbid disease increased the risk of mortality (P < 0.001). Coronary artery disease and the other comorbidities had a statistically significant effect on mortality (P < 0.05). Use of NSAIDs, smoking, alcohol consumption, duration of symptoms, localization and diameter of perforated ulcer, surgical procedure, and histologic diagnosis had no statistically significant effect on mortality (Table 5). The average ASA score was 3 in the mortality group. With the rise of ASA score, the risk of mortality increased significantly (OR = 2.254; 95% CI, 1.311–3.875; P < 0.001).
Table 4.
Causes of postoperative mortality
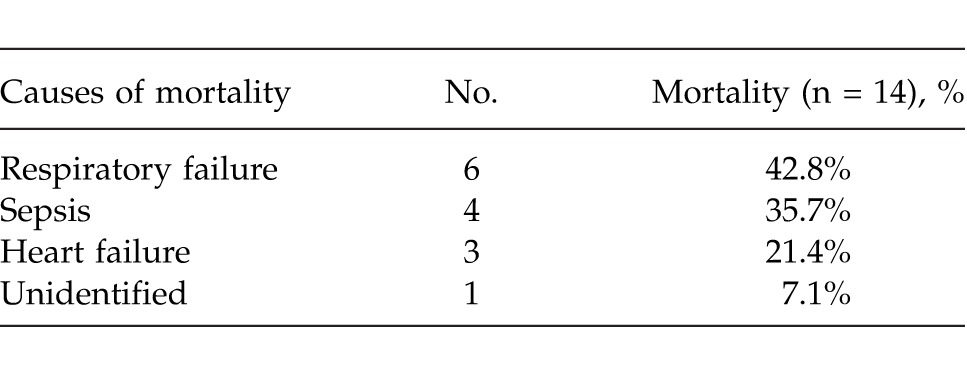
Table 5.
Demographic and clinical features regarding survival status
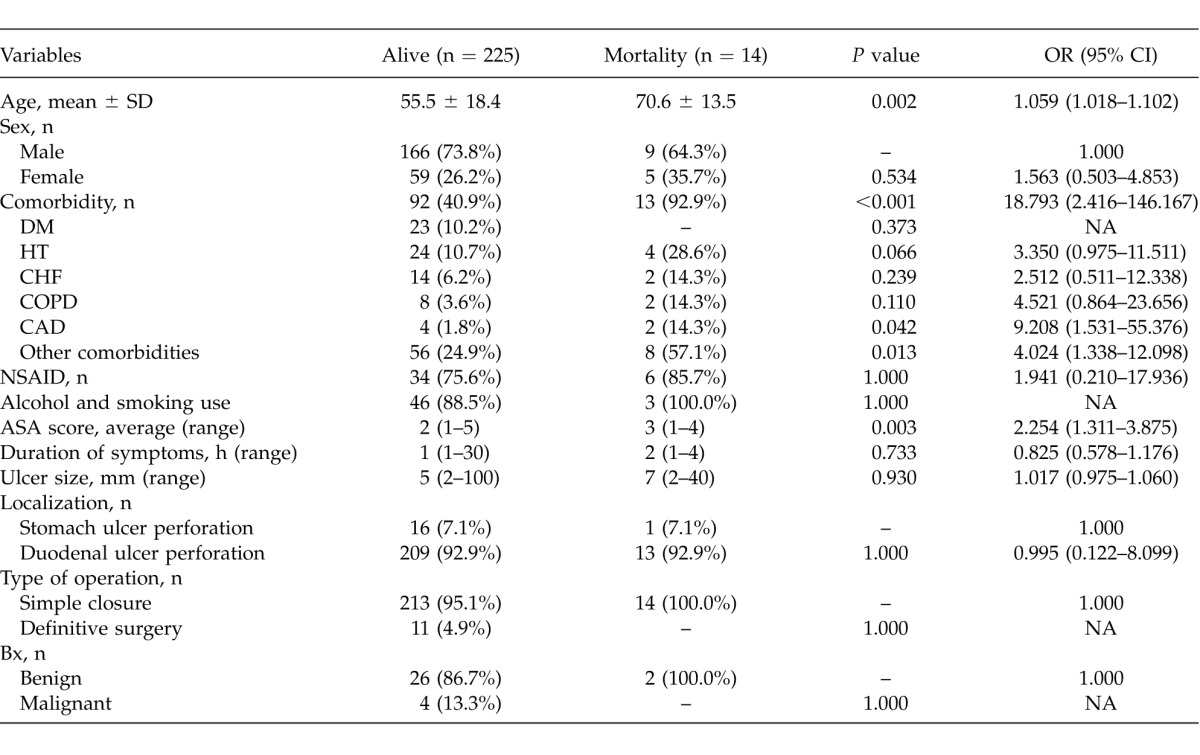
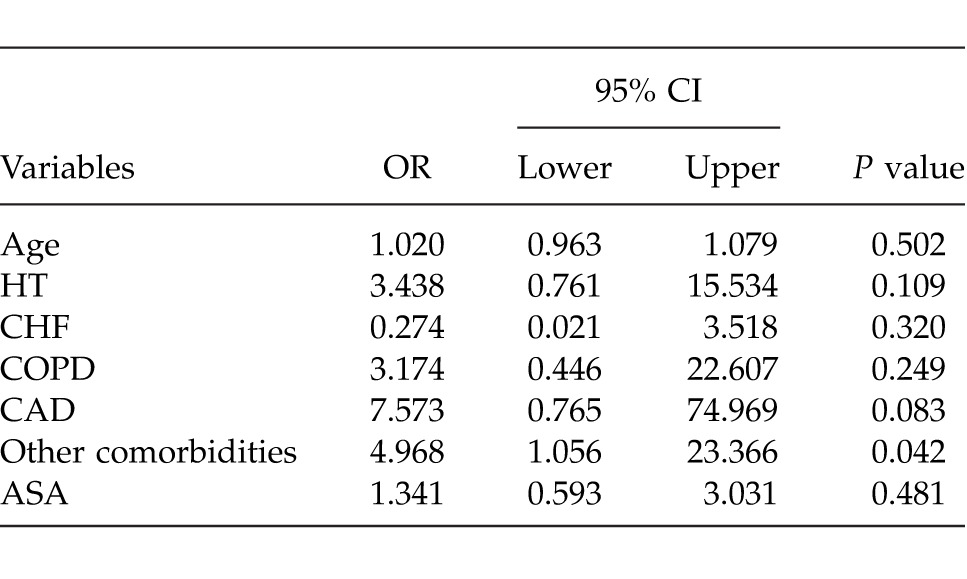
Determining the best predictor(s), which effect on mortality was evaluated by multiple logistic regression analyses after adjustment for all possible confounding factors. Any variable whose univariable test had a P value of <0.25 was accepted as a candidate for the multivariable model along with all variables of known clinical importance. According to multiple logistic regression analysis, the factor of other comorbid diseases had the most effect on mortality (P = 0.042) (Table 6).
Table 6.
Results of multiple logistic regression analysis: discrimination of alive and exitus groups from each other
Discussion
In the last few years, since the introduction of H2 blockers, PPIs, and treatment to eradicate H. Pylori, the number of patients with uncomplicated peptic ulcer has decreased.19,20 Despite the decrease in patients with PUD, the number of patients admitted with PUP has not declined.19,21–23
Arveen et al report that in their series, the male to female ratio was 10.3:1.0.20 Especially in Eastern countries, this ratio is similar in other studies.24,25 However, a certain increase in women patients has been reported in different reports.26–29 In the present study, there were 64 women (26.7%) who underwent surgery for PUP. We had a male to female ratio of 2.7:1.0 that can be favorably compared with Western studies.19 Postoperative morbidity was observed in 55 patients. Thirty-nine patients (70.9%) of the morbidity group were male and 16 patients (29.1%) were female. The morbidity ratio was similar to the sex ratio, and the sex variable was not found to influence morbidity in the present study. Despite our results, there are many studies suggesting that being female is a risk factor for PUP morbidities.11,20,30 A higher mortality rate among women has also been observed in other studies.20,26 However, we did not observe the sex variable to have any significant effect on mortality. This may relate to alcohol and smoking-related comorbidities because alcohol consumption and smoking are much more common among men in our country. This is the most likely explanation.
By multiple logistic regression analysis, older age is the most important risk factor for morbidity in the present study. Older age is also an important risk factor for mortality in univariate analysis. Previous studies in the literature support our results.20,30–33 More frequent presence of comorbid diseases in older patients may be the cause of higher morbidity and mortality. In the present study, 37 patients (67.3%) of the morbidity group had comorbid diseases, while the patients without morbidity had fewer (37.0%) comorbid diseases. DM (21.8%) and HT (20.0%) were the most common comorbidities in the morbidity group. The frequent comorbidities reported in the literature are pulmonary disease, hypertension, and diabetes mellitus.19,30,34 As reported in the literature,19,20,35 comorbidities are found to be important prognostic factors in our study. In our patients, comorbidities also had a significant effect on mortality, which is in agreement with other studies.19,32 With multiple logistic regression analysis, we found that the other comorbidities were the most important risk factor for mortality. Eight of the patients (57.1%) who died after surgery had other comorbidities. The other comorbidities for these 8 patients are multiple myeloma, brain metastasis with unknown primary, inoperable breast cancer, Parkinson disease, Alzheimer disease (in 3 patients), and morbid obesity.
Duration of symptoms is described as the time gap from onset of acute abdominal pain to presentation at the hospital. Median duration of symptoms was 2 days for morbidity and mortality groups. In our study, duration of symptoms did not have a significant effect on either mortality or morbidity, contrary to the literature.19,20,25,26,36,37 But, some other authors have stated doubts about this presumption.30,38 Alcohol consumption, smoking, and use of nonsteroidal anti-inflammatory drugs are strongly associated with PUD, but their effects on mortality and morbidity are in debate.11,12,39 In the present study, alcohol consumption, smoking, usage of nonsteroidal anti-inflammatory drugs had no significant effect on both mortality and morbidity. The effect of the site of the perforation is another controversial issue. Svanes et al state that gastric ulcer perforations are associated with higher mortality than duodenal ulcer perforations.15 However, some others did not find any correlation between the site of the perforation and the outcome of the perforation, as in our results.19,33 Kumar et al state that ulcer perforations greater than 5 mm are an independent risk factor for re-leak when a simple closure with omental patch alone is performed.40 In our department, we always perform feeding jejunostomy or pyloric exclusion with simple closure in large ulcer perforations. Probably as a result of this consolidation policy, we did not find any correlation between ulcer perforation size and either mortality or morbidity. With the multiple logistic regression analysis, simple closure of the perforation had a significant effect on morbidity in this study. This may be related to the low number of patients who had undergone definitive surgery or to the tendency to shorten the operating time for high-risk patients. The surgical option for PPU is still under discussion. Our first option is simple closure with omental patch, but the acceptable surgical option depends on the patient's clinical status. In this period, we had 4 gastric cancer perforations. We performed simple closure with omental patch and gastrostomy in these 4 patients. None of these patients had morbidity or postoperative mortality. But the number of patients with malignant tumors was not available for statistical analysis. An ASA score of grade 3 or more was identified as a significant risk factor associated with poor outcome. A higher ASA grade of patients who had undergone surgery for peptic ulcer perforation has been reported to be related to poor prognosis in several studies.20,26,36,37 In the present study, the ASA score was a significant risk factor for postoperative mortality and morbidity in univariate analysis as stated before in the literature.
In conclusion, advanced age, comorbidities, and higher ASA grade are the most important risk factors in patients with peptic ulcer perforation. These 3 risk factors are all related to each other. The controversial subjects are the preferred surgical option and the effect of the duration of symptoms. Simple closure with omental patch of the perforation had a significant effect on morbidity in our study. We believed this result was related to the low number of patients who had undergone definitive surgery or to the tendency to shorten the operating time for high-risk patients. Although the preferred surgical option depends on the patient's clinical status, our first option is always simple closure with omental patch if possible. In the present study, no correlation was found between the duration of symptoms and the outcome. Especially low prevalence of mortality may cause incidental significant or insignificant results in these types of studies with a high number of risk factors and a low number of patients. Therefore, the results should be discussed in accordance with clinical materiality.
Acknowledgments
The authors declare that they have no conflict of interest. The authors' contributions are as follows: Mutlu Unver, study conception and design, acquisition of data, analysis and interpretation of data, and drafting of manuscript; Özgür Fırat, study conception and design, acquisition of data, drafting of manuscript, and critical revision of manuscript; Ömer Vedat Ünalp, study conception and design, acquisition of data, and drafting of manuscript; Alper Uğuz, study conception and design and acquisition of data; Tufan Gümüş, acquisition of data; Taylan Özgür Sezer, study conception and design, acquisition of data, and drafting of manuscript; Şafak Öztürk, study conception and design, acquisition of data, and analysis and interpretation of data; Tayfun Yoldaş, study conception and design, acquisition of data, and drafting of manuscript; Sinan Ersin, critical revision and final approval; and Adem Güler, critical revision and final approval.
References
- 1.Zelickson MS, Bronder CM, Johnson BL, Camunas JA, Smith DE, Rawlinson D. et al. Helicobacter pylori is not the predominant etiology for peptic ulcers requiring operation. Am Surg. 2011;77(8):1054–1060. [PubMed] [Google Scholar]
- 2.Milosavljevic T, Kostić-Milosavljević M, Jovanović I, Krstić M. Complications of peptic ulcer disease. Dig Dis. 2011;29(5):491–493. doi: 10.1159/000331517. [DOI] [PubMed] [Google Scholar]
- 3.Gunshefski L, Flancbaum L, Brolin RE, Frankel A. Changing patterns in perforated peptic ulcer disease. Am Surg. 1990;56(4):270–274. [PubMed] [Google Scholar]
- 4.Wakayama T, Ishizaki Y, Mitsusada M, Takahashi S, Wada T, Fukushima Y, et al. Risk factors influencing the short-term results of gastroduodenal perforation. Surg Today. 1994;24(8):681–687. doi: 10.1007/BF01636772. [DOI] [PubMed] [Google Scholar]
- 5.Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993–2002: a population-based cohort study. Am J Gastroenterol. 2006;101(5):945–953. doi: 10.1111/j.1572-0241.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- 6.Svanes C. Trends in perforated peptic ulcer: incidence, etiology, treatment, and prognosis. World J Surg. 2000;24(3):277–283. doi: 10.1007/s002689910045. [DOI] [PubMed] [Google Scholar]
- 7.Hermansson M. Staël von Holstein C, Zilling T. Surgical approach and prognostic factors after peptic ulcer perforation. Eur J Surg. 1999;165(6):566–572. doi: 10.1080/110241599750006479. [DOI] [PubMed] [Google Scholar]
- 8.Boey J, Choi SK, Poon A, Alagaratnam TT. Risk stratification in perforated duodenal ulcers: a prospective validation of predictive factors. Ann Surg. 1987;205(1):22–26. doi: 10.1097/00000658-198701000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen S, Riis A, Nørgaard M, Sørensen HT, Thomsen RW. Short-term mortality after perforated or bleeding peptic ulcer among elderly patients: a population-based cohort study. BMC Geriatr. 2007;7:8. doi: 10.1186/1471-2318-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorsen K, Glomsaker TB, von Meer A, Søreide K, Søreide JA. Trends in diagnosis and surgical management of patients with perforated peptic ulcer. J Gastrointest Surg. 2011;15(8):1329–1335. doi: 10.1007/s11605-011-1482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae S, Shim KN, Kim N, Kang JM, Kim DS, Kim KM, et al. Incidence and short-term mortality from perforated peptic ulcer in Korea: a population-based study. J Epidemiol. 2012;22(6):508–516. doi: 10.2188/jea.JE20120056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalya PL, Mabula JB, Koy M, McHembe MD, Jaka HM, Kabangila R, et al. Clinical profile and outcome of surgical treatment of perforated peptic ulcers in Northwestern Tanzania: a tertiary hospital experience. World J Emerg Surg. 2011;6:31. doi: 10.1186/1749-7922-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez de la Peña C, Márquez R, Fakih F, Domínguez-Adame E, Medina J Simple closure or vagotomy and pyloroplasty for the treatment of a perforated duodenal ulcer: comparison of results. Dig Surg. 2000;17(3):225–228. doi: 10.1159/000018839. [DOI] [PubMed] [Google Scholar]
- 14.Rahman MM, Islam MS, Flora S, Akhter SF, Hossain S, Karim F. Mortality in perforated peptic ulcer patients after selective management of stratified poor risk cases. World J Surg. 2007;31(12):2341–2344. doi: 10.1007/s00268-007-9165-5. discussion 2345–2346. [DOI] [PubMed] [Google Scholar]
- 15.Svanes C, Lie RT, Svanes K, Lie SA, Søreide O. Adverse effects of delayed treatment for perforated peptic ulcer. Ann Surg. 1994;220(2):168–175. doi: 10.1097/00000658-199408000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng M, Li WH, Cheung MT. Early outcome after emergency gastrectomy for complicated peptic ulcer disease. Hong Kong Med J. 2012;18(4):291–298. [PubMed] [Google Scholar]
- 17.Lohsiriwat V, Prapasrivorakul S, Lohsiriwat D. Perforated peptic ulcer: clinical presentation, surgical outcomes, and the accuracy of the Boey scoring system in predicting postoperative morbidity and mortality. World J Surg. 2009;33(1):80–85. doi: 10.1007/s00268-008-9796-1. [DOI] [PubMed] [Google Scholar]
- 18.Arenal JJ, Bengoechea-Beeby M. Mortality associated with emergency abdominal surgery in the elderly. Can J Surg. 2003;46(2):111–116. [PMC free article] [PubMed] [Google Scholar]
- 19.Noguiera C, Silva AS, Santos JN, Silva AG, Ferreira J, Matos E, et al. Perforated peptic ulcer: main factors of morbidity and mortality. World J Surg. 2003;27(7):782–787. doi: 10.1007/s00268-003-6645-0. [DOI] [PubMed] [Google Scholar]
- 20.Arveen S, Jagdish S, Kadambari D. Perforated peptic ulcer in South India: an institutional perspective. World J Surg. 2009;33(8):1600–1604. doi: 10.1007/s00268-009-0056-9. [DOI] [PubMed] [Google Scholar]
- 21.Meisner S, Sørensen A, Wille-Jørgensen PA. Ulcer surgery during 1976–1978 and 1986–1988: significance of H2 blockaders in surgical training. Ugeskr Laeger. 1993;155(47):3828–3832. [PubMed] [Google Scholar]
- 22.Mäkelä J, Laitinen S, Kairaluoma MI. Complications of peptic ulcer disease before and after the introduction of H2-receptor antagonists. Hepatogastroenterology. 1992;39(2):144–148. [PubMed] [Google Scholar]
- 23.Walt R, Katschinski B, Logan R, Ashley J, Langman M. Rising frequency of ulcer perforation in elderly people in the United Kingdom. Lancet. 1986;1(8479):489–492. doi: 10.1016/s0140-6736(86)92940-5. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Kaushik R, Sharma R, Attri A. The management of large perforations of duodenal ulcers. BMC Surg. 2005;5:15. doi: 10.1186/1471-2482-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taj MH, Mohammad D, Qureshi SA. Outcome of omentopexy as primary repair in perforated duodenal ulcer. J Coll Physicians Surg Pak. 2007;17(12):731–735. [PubMed] [Google Scholar]
- 26.Kocer B, Surmeli S, Solak C, Unal B, Bozkurt B, Yildirim O, et al. Factors affecting mortality and morbidity in patients with peptic ulcer perforation. J Gastroenterol Hepatol. 2007;22(4):565–570. doi: 10.1111/j.1440-1746.2006.04500.x. [DOI] [PubMed] [Google Scholar]
- 27.Svanes C, Salvesen H, Stangeland L, Svanes K, Søreide O. Perforated peptic ulcer over 56 years: time trends in patients and disease characteristics. Gut. 1993;34(12):1666–1671. doi: 10.1136/gut.34.12.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blomgren LG. Perforated peptic ulcer: long-term results after simple closure in the elderly. World J Surg. 1997;21(4):412–414. doi: 10.1007/pl00012263. discussion 414–415. [DOI] [PubMed] [Google Scholar]
- 29.Bulut OB, Rasmussen C, Fischer A. Acute surgical treatment of complicated peptic ulcers with special reference to the elderly. World J Surg. 1996;20(5):574–577. doi: 10.1007/s002689900089. [DOI] [PubMed] [Google Scholar]
- 30.Kim JM, Jeong SH, Lee YJ, Park ST, Choi SK, Hong SC, et al. Analysis of risk factors for postoperative morbidity in perforated peptic ulcer. J Gastric Cancer. 2012;12(1):26–35. doi: 10.5230/jgc.2012.12.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano A, Bendix J, Adamsen S, Buck D, Mainz J, Bartels P, et al. 30-days mortality in patients with perforated peptic ulcer: a national audit. Risk Manag Healthc Policy. 2008;1:31–38. doi: 10.2147/RMHP.S4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imhof M, Epstein S, Ohmann C, Röher HD. Duration of survival after peptic ulcer perforation. World J Surg. 2008;32(3):408–412. doi: 10.1007/s00268-007-9370-2. [DOI] [PubMed] [Google Scholar]
- 33.Bas G, Eryilmaz R, Okan I, Sahin M. Risk factors of morbidity and mortality in patients with perforated peptic ulcer. Acta Chir Belg. 2008;108(4):424–427. doi: 10.1080/00015458.2008.11680254. [DOI] [PubMed] [Google Scholar]
- 34.Ko JW, Hong SJ, Ban JY, Kim JH. Risk factors associated with mortality in emergency surgery for perforated peptic ulcer. J Korean Surg Soc. 2004;67:373–378. [Google Scholar]
- 35.Hirsch IB, McGill JB. Role of insulin in management of surgical patients with diabetes mellitus. Diabetes Care. 1990;13(9):980–991. doi: 10.2337/diacare.13.9.980. [DOI] [PubMed] [Google Scholar]
- 36.Kujath P, Schwandner O, Bruch HP. Morbidity and mortality of perforated peptic gastroduodenal ulcer following emergency surgery. Langenbecks Arch Surg. 2002;387(7–8):298–302. doi: 10.1007/s00423-002-0331-9. [DOI] [PubMed] [Google Scholar]
- 37.Barut I, Tarhan OR, Cerci C, Karaguzel N, Akdeniz Y, Bulbul M. Prognostic factors of peptic ulcer perforation. Saudi Med J. 2005;26(8):1255–1259. [PubMed] [Google Scholar]
- 38.Agrez MV, Senthiselvan S, Henry DA, Mitchell A, Duggan JM. Perforated peptic ulcer in the Hunter region: a review of 174 cases. Aust N Z J Surg. 1992;62(5):338–343. doi: 10.1111/j.1445-2197.1992.tb07200.x. [DOI] [PubMed] [Google Scholar]
- 39.Torab FC, Amer M, Abu-Zidan FM, Branicki FJ. Perforated peptic ulcer: different ethnic, climatic and fasting risk factors for morbidity in Al-ain medical district, United Arab Emirates. Asian J Surg. 2009;32(2):95–101. doi: 10.1016/S1015-9584(09)60018-X. [DOI] [PubMed] [Google Scholar]
- 40.Kumar K, Pai D, Srinivasan K, Jagdish S, Ananthakrishnan N. Factors contributing to releak after surgical closure of perforated duodenal ulcer by Graham's Patch. Trop Gastroenterol. 2002;23(4):190–192. [PubMed] [Google Scholar]


