Abstract
Our objective for this study was to discuss the usability of mean platelet volume, which is associated with numerous vascular pathologies, in the early diagnosis of acute mesenteric ischemia. Acute mesenteric ischemia is an uncommon, life-threatening clinical condition mostly seen in the elderly. Early diagnosis of acute mesenteric ischemia and correction of blood circulation before necrosis occurs are important factors affecting prognosis. A total of 95 patients who underwent emergency surgery for acute mesenteric ischemia and 90 healthy volunteers as control group were included in this study. Age, gender, hemoglobin values, white blood cell counts, mean platelet volume, and platelet counts are recorded for evaluation. The mean platelet volume values were significantly higher in patients with acute mesenteric ischemia than in the controls (9.4 ± 1.1 fL and 7.4 ± 1.4 fL, respectively; P < 0.001). Receiver-operating characteristic analysis demonstrated a cutoff value of mean platelet volume as 8.1 fL (area under the curve, 0.862), a sensitivity of 83.2%, and a specificity of 80%. As a result, in the patients who are admitted to the hospital with acute nonspecific abdominal pain and suspected of having acute mesenteric ischemia, high mean platelet volume values in routine hemograms support the diagnosis of acute mesenteric ischemia.
Key words: Acute mesenteric ischemia, Mean platelet volume, Diagnosis
Acute mesenteric ischemia (AMI) is an infrequent, life-threatening clinical condition mostly seen in the elderly. Despite advances in the diagnosis of and therapy for AMI, mortality rates remain high and show dissimilarity (30%–97%) in the literature. Atypical symptoms, cases frequently being elderly patients with cardiovascular diseases, and diagnostic difficulties all cause delays in surgical intervention and ultimately high mortality rates.1,2 AMI, as a result of mesenteric vascular insufficiency, causes damage in other vital organs besides the intestines. AMI may progress due to obstruction of mesenteric vessels as a result of embolism or thrombosis, or for nonobstructive reasons, such as hypotension or cardiac insufficiency. AMI is frequently seen in patients with serious concomitant diseases.3–5 Early diagnosis of AMI and correction of blood circulation before necrosis occurs are important factors affecting prognosis.6 In every patient, platelets show heterogeneity in size and density. Mean platelet volume (MPV) is a blood parameter used for measuring platelet size and can be determined in routine blood tests. It is cost-effective and yields results in a short amount of time.7 Larger platelets are more active metabolically and enzymatically,8 and their prothrombotic potential is greater than that of smaller ones.9 Elevated MPV is associated with other markers of platelet activity, including increased platelet aggregation, increased thromboxane synthesis, increased β-thromboglobulin release, and increased expression of adhesion molecules.10 This suggests a common mechanism by which these factors may increase the risk of cardiovascular disease.7 In patients with a myocardial infarction or cerebrovascular disease,7–12 platelet volume has been found to be increased, and MPV is independently associated with peripheral artery disease.13 However, data in mesenteric vascular disease are lacking. Our objective in this study is to discuss the usability of MPV, which is associated with numerous vascular pathologies, in the early diagnosis of AMI.
Patients and Methods
Study design and patients
The study is designed as a retrospective study involving two groups: AMI and control.
The AMI group consists of 95 patients (39 women and 56 men; mean age, 68.4 ± 14.4 years) who had undergone emergency surgery for AMI in the General Surgery Department, Dicle University Medicine Faculty, between January 2006 and December 2011. The control group consists of 90 healthy volunteers (42 women and 48 men; mean age, 67.1 ± 8.7 years) who had been admitted to the general surgery policlinic for routine checkup examinations, with no active symptoms. Patients' files were analyzed retrospectively. Age, gender, hemoglobin (Hb) values (g/dL), white blood cell (WBC) counts (×109/L), MPV (fL), and platelet (PLT) counts (×109/L) were recorded for evaluation.
Laboratory data
Peripheral blood sample was drawn from the antecubital vein using a 21-gauge needle with a syringe and minimal stasis. The blood sample was collected into tubes containing ethylene diamine tetraacetic acid for Hb, WBC count, PLT count, and MPV analysis. Using an automated system (the Cell-Dyn 3700 Hematology Analyzer, Abbott Laboratory, Abbott Park, Illinois), Hb (g/dL), MPV (fL), WBC count (×109/L), and PLT count (×109/L) were measured. The reference ranges in our hematology laboratory are as follows: Hb, 11.2 to 16.2 g/dL; WBC count: 4 × 109/L to 10 × 109/L; PLT, 150 × 109/L to 450 × 109/L; and MPV, 6.8 to 10.8 fL.
Statistical analysis
In the evaluation of the study results, the SPSS (Chicago, Illinois) Windows 15.0 program was used for statistical analysis. The quantitative data were indicated as mean ± SD. Kolmogorov-Smirnov test was used for the compatibility of normally distributed data. In comparisons of the groups, Student's t-test was used in analysis of parametric data, whereas χ2 test was used in the analysis of categoric data. P values <0.05 were accepted to be significant for all variables. Receiver-operating characteristic (ROC) analyses were used to determine the cutoff values and the sensitivity/specificity of MPV.
Results
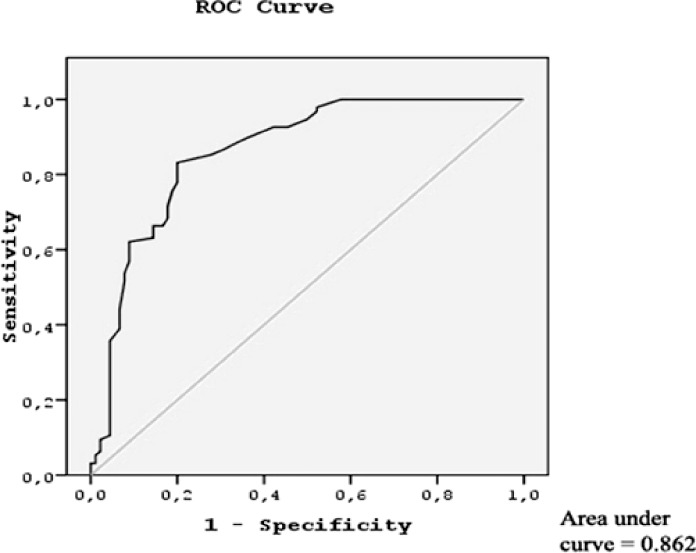
Baseline demographic and laboratory parameters are given in Table 1. Age, gender, PLT count, and Hb values of patients were similar in both groups. The MPV values were significantly higher in patients with AMI than in the controls (9.4 ± 1.1 fL and 7.4 ± 1.4 fL, respectively; P < 0.001). In addition, the WBC counts of AMI patients (probably because they had sepsis) were found to be significantly higher than those for the control group of patients (P < 0.001). ROC analysis demonstrated an MPV cutoff value of 8.1 fL (area under the curve, 0.862), sensitivity of 83.2%, and specificity of 80% (Fig. 1).
Table 1.
Baseline demographic and laboratory characteristics of the study population
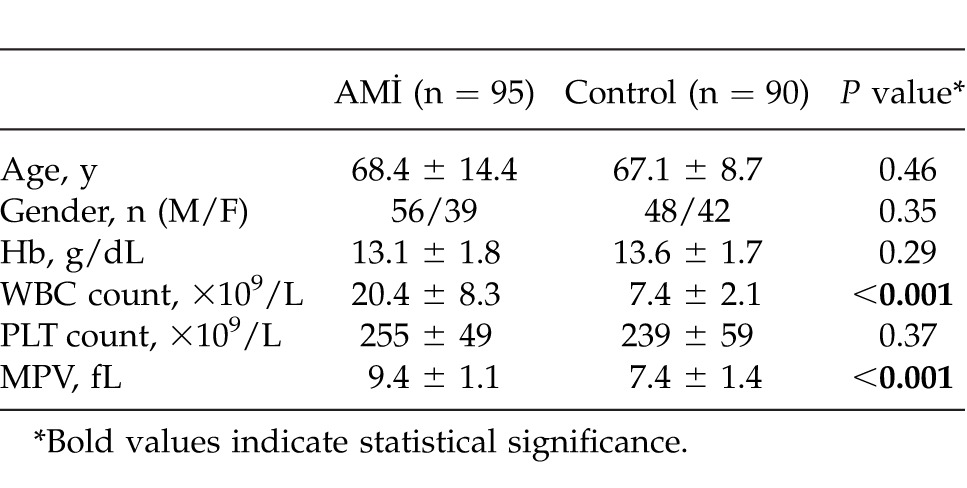
Fig. 1.
Receiver-operating characteristic curves for predictors of acute mesenteric ischemia. MPV values (area under the curve, 0.862). Straight line, reference line.
Discussion
AMI is a clinical condition that needs to be diagnosed rapidly because of its high mortality rates when left untreated. Because of the insignificant and nonspecific clinical findings and limitations in diagnostic tests, diagnosis is the most important step in the course of AMI.14 Serum laboratory tests are usually unhelpful in the diagnosis of AMI. Leukocytosis is common in AMI, but it is a nonspecific marker for inflammation and infection.15 Approximately 50% of patients have metabolic acidosis, a late finding to show intestinal infarct, and 25% have hyperamylasemia.16 Prerenal azotemia, and increased levels of phosphate, lactate, and alkaline phosphatase may also accompany intestinal necrosis.5,17 The number of studies focusing on the search of a specific biochemical, serologic parameter in the early diagnosis of AMI has increased recently.18 Platelets are very important in the process of thrombosis and hemostasis. They adhere to each other when activated. Soluble adhesion molecules released from activated platelets mediate this interaction. In detail, following endothelial damage, the subendothelial tissue is exposed directly to the circulating blood components. This activates the circulating platelets, and they adhere to the subendothelial tissue. In the next step, platelets aggregate and release their vasoactive substances; afterward, further aggregation with fibrin production occurs, and this finally results in thrombotic occlusion. However, venous thrombi that occur in low flow or during stasis contain greater proportions of erythrocytes and fibrin.19 In every individual, platelets show heterogeneity in size, functional activity, density, and metabolism. Larger platelets are more active enzymatically and metabolically than smaller ones. They also contain more prothrombotic material, with increased thromboxane A2 and B2 per unit volume and glycoprotein IIb-IIIa receptor expression.9,20 Recently, high levels of MPV have been shown to be associated with many cardiovascular diseases. The PLT count can predict the risk of major adverse cardiovascular events.21 A meta-analysis study conducted by Chu et al7 demonstrated that high MPVs play a role in the etiopathogenesis of cardiovascular diseases and raised the mortality rate for postmyocardial infarction patients. Gulcan et al22 showed that patients with deep vein thrombosis have higher MPV levels than the control group. In our study, patients with AMI seemed to have significantly higher MPV values compared with the control group upon initial admission to the hospital. ROC analyses were performed to determine the best MPV cutoff values for predicting AMI. The cutoff value of 8.1 fL for MPV was found to be highly sensitive and specific for predicting AMI. However, most AMI patients have concomitant cardiovascular diseases, so it remains untold whether high levels of MPV are due to a predisposing cardiovascular condition or AMI.
Recently, Altıntoprak et al23 reported a study investigating the relationship between MPV values and AMI prognosis in 30 patients. They concluded that MPV values were higher in nonsurvivors than in survivors and might be beneficial in predicting patients with poor prognosis and in the planning of reoperations. This was the first study investigating the relationship between MPV and the prognosis of AMI. To the best of our knowledge, our study is the first study suggesting that MPV is useful in the diagnosis of AMI patients. Although Altıntoprak et al23 suggested that high MPV might be an indicator of poor prognosis, we found that it may also be used as a marker for the diagnosis of disease in AMI patients. In analyzing both studies together, it can be seen that the results support each other.
Conclusion
The present study showed that high MPV values in routine hemograms may support the diagnosis of AMI in patients who are admitted to the hospital with acute nonspecific abdominal pain and are suspected of having AMI.
Acknowledgments
We are grateful to Dicle University Scientific Research Projects (DUBAP) for its sponsorship of the English editing of this manuscript.
References
- 1.Merle C, Lepouse C, De Garine A, Frayssinet N, Leymarie F, Leon A, et al. Surgery for mesenteric infarction: prognostic factors associated with early death within 72 hours. J Cardiothorac Vasc Anesth. 2004;18(6):734–741. doi: 10.1053/j.jvca.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Huang HH, Chang YC, Yen DH, Kao WF, Chen JD, Wang LM, et al. Clinical factors and outcomes in patients with acute mesenteric ischemia in the emergency department. J Chin Med Assoc. 2005;68(7):299–306. doi: 10.1016/S1726-4901(09)70165-0. [DOI] [PubMed] [Google Scholar]
- 3.Scheider TA, Longo WE, Ure T, Verrnava AM., III Mesenteric ischemia: acute arterial syndromes. Dis Colon Rectum. 1994;37(11):1163–1174. doi: 10.1007/BF02049824. [DOI] [PubMed] [Google Scholar]
- 4.Yasuhara H. Acute mesenteric ischemia: the challenge of gastroenterology. Surg Today. 2005;35(3):185–195. doi: 10.1007/s00595-004-2924-0. [DOI] [PubMed] [Google Scholar]
- 5.McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307–318. doi: 10.1016/s0039-6109(05)70550-8. [DOI] [PubMed] [Google Scholar]
- 6.Mansour MA. Management of acute mesenteric ischemia. Arch Surg. 1999;134(3):328–331. doi: 10.1001/archsurg.134.3.328. [DOI] [PubMed] [Google Scholar]
- 7.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karpatkin S. Heterogeneity of human platelets, II: functional evidence suggestive of young and old platelets. J Clin Invest. 1969;48(6):1083–1087. doi: 10.1172/JCI106064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamath S, Blann AD, Lip GY. Platelet activation: assessment and quantification. Eur Heart J. 2001;22(17):1561–1571. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]
- 10.Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7(2):157–161. [PubMed] [Google Scholar]
- 11.Van der Loo B, Martin JF. A role for changes in platelet production in the cause of acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1999;19(3):672–679. doi: 10.1161/01.atv.19.3.672. [DOI] [PubMed] [Google Scholar]
- 12.Bath P, Algert C, Chapman N, Neal B. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35(3):622–626. doi: 10.1161/01.STR.0000116105.26237.EC. [DOI] [PubMed] [Google Scholar]
- 13.Berger JS, Eraso LH, Xie D, Sha D, Mohler ER., III Mean platelet volume and prevalence of peripheral artery disease, the National Health and Nutrition Examination Survey, 1999–2004. Atherosclerosis. 2010;213(2):586–591. doi: 10.1016/j.atherosclerosis.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall JC, Vincent JL, Fink MP, Cook DJ, Rubenfeld G, Foster D, et al. Measures, markers, and mediators: toward a staging system for clinical sepsis: a report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25–26, 2000. Crit Care Med. 2003;31(5):1560–1567. doi: 10.1097/01.CCM.0000065186.67848.3A. [DOI] [PubMed] [Google Scholar]
- 15.Eldrup-Jorgensen J, Hawkins RE, Bredenberg CE. Abdominal vascular catastrophes. Surg Clin North Am. 1997;77(6):1305–1320. doi: 10.1016/s0039-6109(05)70619-8. [DOI] [PubMed] [Google Scholar]
- 16.Tsai CJ, Kuo YC, Chen PC, Wu CS. The spectrum of acute intestinal vascular failure: a collective review of 43 cases in Taiwan. Br J Clin Pract. 1990;44(12):603–608. [PubMed] [Google Scholar]
- 17.Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335(8):540–546. doi: 10.1056/NEJM199608223350802. [DOI] [PubMed] [Google Scholar]
- 18.Gönüllü D, Yankol Y, Işiman F. Akyildiz, Iğdem A, Yücel O et al. pH value and potassium level of diagnostic peritoneal lavage fluid in the early diagnosis of acute mesenteric ischemia secondary to arterial occlusion in rats. Ulus Travma Acil Cerrahi Derg. 2007;13(4):261–267. [PubMed] [Google Scholar]
- 19.Chiang TM, Woo-Rasberry V, Cole F. Role of platelet endothelial form of nitric oxide synthase in collagen-platelet interaction: regulation by phosphorylation. Biochim Biophys Acta. 2002;1592(2):169–174. doi: 10.1016/s0167-4889(02)00311-7. [DOI] [PubMed] [Google Scholar]
- 20.Giles H, Smith RE, Martin JF. Platelet glycoprotein IIb–IIIa and size are increased in acute myocardial infarction. Eur J Clin Invest. 1994;24(1):69–72. doi: 10.1111/j.1365-2362.1994.tb02062.x. [DOI] [PubMed] [Google Scholar]
- 21.Thaulow E, Erikssen J, Sandvi L, Stormorken H, Cohn PF. Blood platelet count and function are related total and cardiovascular death in apparently healthy men. Circulation. 1991;84(2):613–617. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]
- 22.Gulcan M, Varol E, Etli M, Aksoy F, Kayan M. Mean platelet volume is increased in patients with deep vein thrombosis. Clin Appl Thromb Hemost. 2012;18(4):427–430. doi: 10.1177/1076029611427437. [DOI] [PubMed] [Google Scholar]
- 23.Altintoprak F, Arslan Y, Yalkin O, Uzunoglu Y, Ozkan OV. Mean platelet volume as a potential prognostic marker in patients with acute mesentericischemia-retrospective study. World J Emerg Surg. 2013;8(1):49. doi: 10.1186/1749-7922-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]