Abstract
The social behavior in a cohort of adult animals who received ibotenic acid lesions of the amygdala (4 female, 3 male) or hippocampus (5 female, 3 male) as neonates, and sham-operated controls (4 female, 4 male) was evaluated in their home environments with the familiar opposite-sex monkey (pair-mate) with whom they were housed. Amygdala-lesioned animals spent less time with their familiar partners and engaged in higher frequencies of stress-related behaviors than control animals. Hippocampus-lesioned animals spent significantly more time socially engaging their pair-mates than both control and amygdala-lesioned animals. These results suggest that early damage to the amygdala or hippocampus subtly alter patterns of adult social behavior in a familiar context and stand in sharp contrast to extant studies of early damage to the amygdala or hippocampus and to the more dramatically altered patterns of behavior observed after damage to the adult amygdala.
Keywords: amygdala, hippocampus, social behavior, nonhuman primate, Macaca mulatta
Introduction
Lesion studies have investigated the functional role of the amygdala in social behavior and affective processing. (e.g., Brown & Schafer, 1888; Klüver & Bucy, 1939; Schreiner & Kling, 1956; Mirsky, 1960; Kling, 1968; Kling, Lancaster & Benitone, 1970; Kling & Cornell, 1971; Kling, 1974; Emery, Capitanio, Mason, Machado, Mendoza, & Amaral, 2001; Machado, Emery, et al., 2008). In general, compared to intact animals, animals that receive bilateral damage to the amygdala in adulthood are uninhibited. They approach both conspecifics and objects regardless of their novelty or threat potential (Mirsky, 1960; Emery et al., 2001; Machado et al., 2008, Stefanacci, Clark, & Zola, 2003; Izquierdo, Suda, & Murray, 2005; Mason, Capitanio, Machado, and Mendoza, & Amaral et al., 2006). Most studies investigating the effects of damage to the hippocampus in adult monkeys focus on memory or learning, rather than social or affective behavior (Bachevalier, Beauregard, & Alvarado, 1999; Banta Lavenex, Amaral, & Lavenex, 2006). Those studies that do evaluate social behavior report minimal impact of adult hippocampus damage (Machado & Bachevalier, 2006).
While informative, studies of damage to medial temporal lobe structures that occurs during adulthood leave open questions about the importance of these structures for the development of normal social and affective behavior. One approach to address such questions is to eliminate the amygdala or hippocampus early in an animal’s development and then track his or her behavior over time. Early studies of this type that ablated the amygdala in infants and nursery reared them alone (i.e., they were housed without other monkeys) demonstrated that, compared to neurologically intact control animals, they were less reactive to novel stimuli (Thompson, Schwartzbaum, & Harlow, 1969) and more reactive during social interactions that occurred with non-operated animals (Thompson & Towfighi, 1976; Thompson, Bergland, & Towfighi, 1977). Similarly, early lesions of the hippocampus resulted in fairly subtle social behavior perturbations in infancy. Hippocampus-lesioned animals were less likely than controls to initiate social interactions with familiar partners and were more likely to be aggressed by them (Beauregard, Malkova, & Bachevalier, 1995; Bachevalier, Alvarado, & Malkova, 1999). Social behavior in these animals changed over time, however, and by adulthood they were much less socially engaged and demonstrated higher frequencies of motor stereotypies than control animals (Beauregard et al., 1995; Bachevalier, Alvarado et al., 1999).
A developmental approach was adopted in a longitudinal study that began in 2001 and has since chronicled the longitudinal effects of neonatal damage to the amygdala or hippocampus on the development of social and affective behavior in rhesus macaques (Bauman, Lavenex, Mason, Capitanio, & Amaral, 2004a; Bauman, Lavenex, Mason, Capitanio, & Amaral, 2004b; Bauman, Toscano, Mason, Lavenex, & Amaral, 2006; Bauman, Toscano, Babineau, Mason, & Amaral, 2008; Toscano, Bauman, Mason, & Amaral, 2009; Bliss-Moreau, Toscano, Bauman, Mason, & Amaral, 2010; Bliss-Moreau, Bauman, & Amaral, 2011; Bliss-Moreau, Toscano, Bauman, Mason, & Amaral, 2011; Bliss-Moreau, Moadab, Bauman & Amaral, 2013; Moadab, Bliss-Moreau, Bauman, & Amaral, under review). Notably, the patterning of the neonatal amygdala-lesioned and hippocampus-lesioned animals’ social behavior has changed over time. For example, early in development, amygdala-lesioned animals generated more frequent communicative signals (both fear/submission signals and affiliative signals) to both familiar and novel animals (Bauman et al., 2004a). Their heightened signaling subsided by 18 months of age such that amygdala-lesioned animals were less communicative than control animals in social interactions with familiar social partners but equally communicative in social interactions with novel social partners (Bliss-Moreau et al., 2013).
When the female animals from the present study were evaluated at four years of age while living in small social groups with other experimental females and an adult male, amygdala-lesioned animals, compared to their peers, were less socially interactive with the male, but equally interactive with peers (Moadab et al., under review). At this time point, hippocampus-lesioned animals were more social than control animals, engaging in more frequent and longer social interactions with their female peers (Moadab et al., under review). This stands in contrast to other published reports that have demonstrated that animals with neonatal damage to the hippocampus, compared to neurologically intact controls, became increasingly less social as they aged, such that they spent less time in social contact as adults (Beauregard et al., 1995; Bachevalier, Alvarado, et al., 1999). Continuing our longitudinal study, the present report investigated the social behavior of this cohort as adults (at approximately 8 years of age) in their day-to-day social environments in which they were housed with opposite sex partners.
Methods
Experimental procedures were developed in consultation with the California National Primate Research Center staff and approved by the University of California Davis Institutional Animal Care and Use Committee.
Animals and Living Conditions
Comprehensive rearing history and subject selection of the animals discussed in this paper has been described in previous publications (Bauman et al., 2004a, 2004b; Bliss-Moreau et al., 2010, 2011; Bliss-Moreau, Toscano et al., 2011; Bliss-Moreau et al., 2013; Moadab et al., under review). Twenty-four rhesus macaque monkeys received bilateral ibotenic acid lesions of either the amygdala (5 females, 3 males) or hippocampus (5 females, 3 males), or sham control operations (4 females, 4 males) at approximately two weeks of age.
Surgical Procedures
Surgical procedures have been detailed in previous publications (Bauman et al., 2004a, 2004b) and are briefly summarized here. On the morning of surgery, structural magnetic resonance imaging (MRI) was performed on each subject in order to determine the stereotaxic coordinates of the amygdala or hippocampus for ibotenic acid injections. Subjects were anesthetized with ketamine hydrochloride (15 mg/kg i.m.) and medatomidine (30µg/kg) prior to being placed in an MRI-compatible stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). Brain imaging occurred on a General Electric 1.5 T Gyroscan magnet (slice thickness = 1.0 mm, T1-weighted Inversion Recovery Pulse sequence, TR = 21, TE =7.9, NEX 3, FOV = 8cm, Matrix 256 × 256).
Following the MRI, subjects were intubated and anesthetized with a combination of isoflurane (1.0% - varied as needed to maintain an adequate level of anesthesia) and fentanyl (intravenous, 7–10 µg/kg/hour). Sham-operated controls were maintained under anesthesia for the average duration of the lesion surgeries and received a midline incision to expose the skull. Operated subjects received bilateral craniotomies over the amygdala or hippocampus and ibotenic acid (Biosearch Technologies Inc., 10 mg/ml in 0.1 M phosphate buffered saline) was injected simultaneously bilaterally at a rate of 0.2 µl/min into different locations of the amygdala or hippocampus using 10 µl Hamilton syringes (26 gauge beveled needles). Following surgery, infants were monitored closely by a veterinarian and were returned to their mothers once they were fully alert.
Lesion Analysis
T2-weighted images of coronal sections through the middle portion of the amygdala illustrating the extent of the damage have been documented in previous publications (Bauman et al., 2004a, 2004b; Bliss-Moreau, Bauman, & Amaral, 2011). Lesion extent was further characterized in a previous report using T1-weighted MRI images when animals were four years of age (Machado, Snyder, Cherry, Lavenex, & Amaral, 2008). From those structural MRI images, volumes of amygdalae and hippocampi were calculated for the 8 sham operated controls. Volumes of the structures were then calculated for the amygdala-lesioned and hippocampus-lesioned animals. The extent of atrophy was calculated by comparing the structural volumes in the lesioned animals to the structural volumes of the control animals. On average, amygdala-lesioned animals demonstrated a 72.56% (SD=4.57%) volumetric reduction of the amygdala and a 23.71% (SD=12.17%) volumetric reduction in the adjoining hippocampus. Similarly, hippocampus-lesioned animals demonstrated a 76.65% average (SD=7.46%) volumetric reduction of the hippocampus and a 15.17% average (SD=13.93) volumetric reduction of the amygdala.
Rearing History
After surgery, animals were returned to their mothers and housed in standard primate cages (61 cm W × 66 cm D × 81 cm H). Following recovery, subjects and their mothers were socialized in groups with other subjects and their mothers in large chain-link indoor enclosures (2.13m W × 3.35m D × 2.44m H). Each social group included six subject/mother pairs (two subjects from each experimental condition) and an adult male. These groups were held for three hours each day, five days per week. Subjects were separated from their mothers at 6 months of age and singly housed, and continued to socialize three hours per day with their groups without their mothers. Social groups remained in the same configuration (same peers and adult male) but with the addition of a novel adult female. At one year of age, subjects were permanently housed (24 hours per day) with their social groups in these large enclosures.
Animals moved with their current social groups to large outdoor enclosures (6.10m W × 4.27m D × 2.44m H) at approximately 3 years of age. After one year, they were relocated into standard indoor caging where they were pair housed with compatible same-sex social partners. At 4 years of age, females were moved into outdoor enclosures (4.9 m W × 4.3 m D × 2.4 m H) where they were housed with two other females (from differing lesion groups) and a novel adult male (see Moadab et al., under review for further details). Males were moved to smaller outdoor enclosures (2.5 m W × 4.8 m D × 2.1 m H) at this time, and housed with a compatible male from the project.
At approximately 6.5 years of age, animals were relocated indoors and housed with compatible same sex partners. Approximately one year later, animals were introduced to and housed with opposite-sex partners from the experiment or from the colony, based on the availability and compatibility of animals. Six experimental animals were paired with colony animals. There were 2 pairs of non-lesioned animals (1 of which was a pairing that included a colony animal), 5 amygdala-lesioned – non-lesioned pairs (2 of which were pairings that included a colony animal), 5 hippocampus-lesioned – non-lesioned pairs (3 of which were pairings that included a colony animal), and 2 amygdala-lesioned – hippocampus-lesioned pairs. The males in the study were vasectomized to prevent females from becoming pregnant. One female underwent tubal ligation at 4 years and 2.5 months of age for health reasons and therefore was paired with an intact male. The present observations occurred while animals were living indoors in these pairs. Testing in the current study occurred when animals were on average 7.62 years old (SD=0.44 year).
Animals were fed monkey chow twice daily (Lab Diet #5047, PMI Nutrition International INC, Brentwood, MO or Lab Diet #5045, PMI Nutrition International INC, Brentwood, MO), provided with fresh fruit and vegetables twice per week, and an assortment of enrichment such as pea-oat forage mixture on forage boards, and Kong toys. They were provided with access to water ad libitum. Housing rooms were maintained on a 12-hour light/dark cycle, between 17.78–28.89 degrees Celsius.
One amygdala-lesioned male animal died of causes unrelated to his lesion status at approximately one year of age (Bauman et al., 2004b). Another amygdala-lesioned male animal that underwent surgery at the same time as the present cohort replaced him. This subject did not participate in social group rearing from infancy. Instead, he was reared by his mother for the first year of life and pair housed with an age-matched female at 1 year. He was successfully introduced to his social group at 1 year and 3 months of age. A female amygdala-lesioned animal died at approximately 5 years of age of causes unrelated to her lesion; she was not replaced as a subject.
Behavioral Assessment Design and Procedures
Social Partners
All but two animals were housed with compatible opposite-sex partners prior to the start of data collection. Social partners (pair-mates) were selected by staff based on knowledge of the animals. Pairs were watched during early pairing to ensure compatibility and subsequently monitored by both laboratory staff and CNPRC staff. In two cases, animals were not compatible with their initial pair-mates and not successfully housed with an opposite sex partner until completion of the subsequent experiment at which time observations for this report were collected. One hippocampus-lesioned male was unable to be housed with a social partner due to high levels of social aggression. Data from that animal are not presented in this report.
Animals were allowed access to their pair-mates 5 days a week, 6 hours per day for an average of 4.64 months (SD=1.73) before data reported here were recorded. In one case, an amygdala-lesioned female was housed with her pair-mate only 11 days prior to the start of the study. However, this animal had participated in an additional experiment during which she met and interacted socially with her partner for two months prior to being housed with him.
Behavioral Sampling Procedure
All observations occurred in the cages in which the animals lived with their pair-mates (two side by side standard primate cages, 61 cm W × 66 cm D × 81 cm H, joined by a retractable door). Observations occurred in two conditions: Live and Video. In the Live condition, animals were observed in real time. The observer entered the room, sat down, and then began a series of observations. In the Video condition, animals were video-taped for the duration of the observation sample with no human present. The observer entered the room to start the video recording and then left the room for the duration of the recording. Behaviors were evaluated with the same ethogram as used for the Live condition at a later time point.
Social and affective behaviors were recorded while animals interacted with their social partner using the focal sampling technique (Altmann, 1974). Animals were observed twice a week for four weeks, resulting in 8 5-minute samples (4 Video and 4 Live). Observation order was pseudo-randomized. There were three trained observers with inter-observer reliability of greater than 90%. Two of the observers were blind to lesion condition. Observations were collected using The Observer 5.0 (Noldus, 1991) to record the frequency and duration of specific behaviors (See Table 1 for behavioral ethogram). Observers recorded behaviors that were initiated by the subject that was the focus of a given observation (i.e., the focal animal), as well as behaviors that were initiated by that animal’s social partner directed toward the focal animal. Observers recorded behaviors that had both frequency and duration (i.e., states), as well as behaviors that only are reported as frequencies (i.e., events; no duration).
Table 1.
Behavioral Ethogram
| Behavior | Description | |
|---|---|---|
| States | ||
| Social States | ||
| Extended Groom | Examination, picking, or licking of another animal’s fur or body. | |
| Proximity | Animal is within arm’s reach of another animal. | |
| Extended Mount | Any instance of mounting. | |
| Non-Social States | ||
| Nonsocial Activity | Animal remains out of all social states with head up, actively engaged in the environment. | |
| Nonsocial Inactivity | Animal remains out of all social states with head down, not engaged in environment, often staring off into space. | |
| Extended Pace | Repetitive, undirected walking or running with the same path repeated. | |
| Sleep States | ||
| Sleep Social | Animal is asleep within arm’s reach of the other animal. | |
| Sleep Solo | Animal is asleep but is not within arm’s reach of the other animal. | |
| Events | ||
| Total Communication | ||
| Affiliative | ||
| Approach | Intentional movement within arm’s reach of another animal. | |
| Accept Approach | Animal remains within arm’s reach after the other animal approaches. | |
| Coo * | Clear, soft sounds, moderate in pitch and intensity; usually sounds like “whoooooo..” | |
| Follow | Intentional follow of another animal. | |
| Groom | Examination, picking, or licking of another animal’s fur or body. | |
| Grunt | Deep, muffled, low-intensity vocalization. | |
| Lipsmack | Rapid lip movements with pursed or puckered lips, usually accompanied by smacking sounds. | |
| Huddle | Physical contact that involves one animal ventrally touching another animal. | |
| Joint Threat | Pair mate animals threaten other animals or observer together. | |
| Mount† | Mount that includes all of the following components: appropriate positioning of partner, hands on back, double foot clasp. | |
| Present Groom | Intentional presentation of neck, belly, or other part of body to another animal. | |
| Present Rump† | Rigid posture with rump and tail elevated and oriented toward another individual. | |
| Threat-Solicitation | Animal recruits the other animal in threatening the observer or another animal. | |
| Agonistic/”Aggression” | ||
| Aggression | Grabbing, slapping, and biting of another animal. | |
| Displacement | Physical movement in which an animal “takes the place” of another animal. | |
| Threat | Contains one or more of the following components: open mouth stare, head bobbing, ear flaps, bark vocalizations, or lunges. | |
| Submission/”Fear” | ||
| Avoid | Animal leaves the area when the other animal comes near or is about to approach. | |
| Grimace | Exaggerated movement of lips such that lips are pulled back with teeth showing. | |
| Flee | Rapid, intentional movement away from another animal. | |
| Freeze* | Stiff body posture without any movement for more than three seconds. | |
| Scream | High-pitched vocalization, with extreme high intensity; sounds like “eeeeeeeeee..” | |
| Exploration | ||
| Manual | Exploration of the cage or environment with the hands. | |
| Mirror Use | Animal plays with or uses mirror. | |
| Oral | Exploration of the cage or environment with the mouth. | |
| Toy-Use | Exploration of toy. | |
| Stress | ||
| Scratch | Scratches own body. | |
| Yawn | Yawn. | |
| Other Events | ||
| Self Sex† | Anogenital exploration of self. | |
| Stereotypies | ||
| Self-Directed | ||
| Rock | Repetitive swaying back and forth. | |
| Salute | Animal covers hand over eye or eye pokes. | |
| Self-Clasp | Unusual holding of body part or limb. | |
| Self-Bite | Biting at oneself. | |
| Self-Strum | Animal pulls at or strokes fur but is not self-grooming | |
| Whole Body | ||
| Backflip | Repetitive back flipping. | |
| Bounce | Repetitive hopping. | |
| Pace | Repetitive undirected movement with the same path repeated. | |
| Spin | Repetitive twirling. | |
| Swing | Repetitive swinging. | |
| Other | ||
| Heat Twist | Animal twists neck in a dramatic display. | |
| Other Stereotypy | Repetitive motor or abnormal behavior patterns not described by any of the above definitions. | |
Note: In order to be scored in a “state” behavior must occur for three seconds.
Behavior was not scored for any monkey during the study.
Behaviors were included in the Sexual Behavior composite category.
Data Analysis Strategy
Statistical analyses were completed using IBM SPSS Statistics version 22 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). Data from the Live and Video conditions were aggregated because preliminary analysis revealed that there were not significant differences between the two conditions. Condition effects are not presented here but are available upon request. Behaviors were grouped into broad categories as indicated in Table 1 based on the analysis strategy used in previous reports (Bliss-Moreau et al., 2013; Moadab et al., under review). Behavioral frequencies and durations were summed across each category and then averaged across the number of observations to create a mean per observation. In cases where data were not normally distributed, they were log10 (x+1) transformed for analyses. For the purposes of interpretation, raw data (means and variance indices) are presented; log transformed data are available upon request. Analysis of variance (ANOVA) was performed on each broad behavioral category with focal lesion group and sex as the between subjects factor, with post-hoc or follow-up t-tests where appropriate. P-values associated with LSD post hoc tests are indicated in the text where appropriate. In some cases, the omnibus test did not reach conventional levels of significance, but might be considered a “trend” (i.e., p was greater than 0.05 but less than 0.10), but visual inspection of each group’s marginal means suggested that there were significant differences between two of the three groups. In those cases, effects were further evaluated using t-tests so as not to miss important group variation that may have been masked in the omnibus tests. Cohen’s d effect size is reported for t-tests in order to evaluate the magnitude of the lesion effects. This series of steps was used for the sake of completeness, despite the fact that this approach differs from the traditional approaches that would probe between group differences when the omnibus effect was significant at p<0.05. We adopted this strategy because nonhuman primate studies of this sort are relatively rare, utilize small sample sizes, and are unlikely to be repeated using the exact same designs. In essence, the goal is to present all of the analyses that are relevant, thus creating a full scientific record and allowing the reader ample evidence from which to draw conclusions. In cases where data violated Levene’s test for equality of variance for t-tests, corrected degrees of freedom are presented using the Welch-Satterthwaite method.
Analyses were performed on a sample that included 7 amygdala-lesioned animals, 7 hippocampus-lesioned animals, and 8 control animals.
Results
Behavioral States
Social State Duration
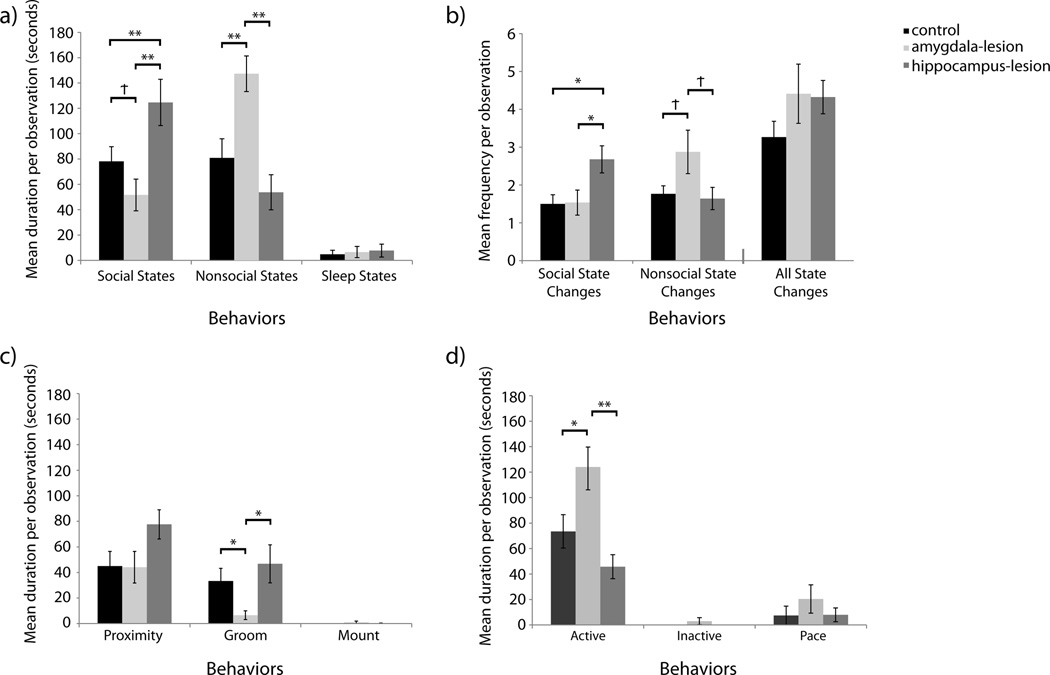
There was a significant effect of lesion condition on the duration of time that animals spent in social states—that is, sustained close social interactions that occur within arms’ reach of another animal (e.g., body-to-body contact, grooming, sitting next to each other)—which they initiated. Hippocampus-lesioned animals (H) spent more time in social interactions that they initiated with their familiar partners than control animals (C) and amygdala-lesioned animals (A); F(2,16)=4.471, p=0.029, ηp2=0.359 (H>C, p=0.0033; H>A, p=0.003; C>A, p=0.090) – Figure 1a. This effect was not driven by any unique social interaction type. Amygdala-lesioned animals did, however, tend to spend less time grooming their pair-mates than control or hippocampus-lesioned animals; F(2,16)=3.114, p=0.072, ηp2=0.280 (analyses on log transformed data). Despite the omnibus test not reaching conventional levels of significance, between-group differences were evaluated with t-tests because confidence intervals of the marginal means suggested that the amygdala-lesioned animals might differ significantly from the other two groups. Amygdala-lesioned animals differed significantly from the two other groups and the large effect sizes indicated that this difference was robust. Amygdala-lesioned animals spent less time grooming their pair-mates than both control animals (t(13)=2.430, p=0.030, d=1.269; analyses on log transformed data) and hippocampus-lesioned animals (t(12)=2.556, p=0.025, d=1.407; analyses on log transformed data) – Figure 1c. Lesion and sex together influenced the duration of time animals were groomed by their pair-mates.
Figure 1. Initiation of Social States.
[a] Duration of time spent in nonsocial states and states initiated with pair-mates. Raw means are presented in the figure although analyses for Sleep States were performed on log transformed data to account for non-normality data distributions. [b] Frequency of state changes. Raw means are presented in the figure although analyses for Nonsocial State Changes were performed on log transformed data to account for non-normality. [c] Duration of time spent in close social interactions initiated with pair-mates. Raw means are presented in the figure although analyses for Groom and Mount were performed on log transformed data to account for non-normality. [d] Duration of time spent alone. Raw means are presented in the figure although analyses for Active, Inactive, and Pace were performed on log transformed data to account for non-normality. Significant differences between lesion conditions as per independent sample t-tests are indicated using the following symbol key: Ϯ = p< .10, * = p< .05, ** = p< .01.
Variation in grooming behavior was not only observed in how much time focal animals spent grooming their partners but also in the duration that focal subjects were groomed by their partners. Hippocampus-lesioned females were groomed more often than the other lesion groups; F(2,16)=5.166, p=0.019, ηp2=0.392 (analyses on log transformed data). Follow up t-tests suggest that controls and hippocampus-lesioned females did not differ significantly in time being groomed by their pair-mate (t(3.389)=1.579, p=0.202, d=0.821; analyses on log transformed data) but that hippocampus-lesioned females did differ significantly from amygdala-lesioned females (t(7)=5.701, p=0.001, d=2.712; analyses on log transformed data – Figure 2a).
Figure 2. Duration of Time Spent in Close Social Interactions Initiated by Pair-Mates.
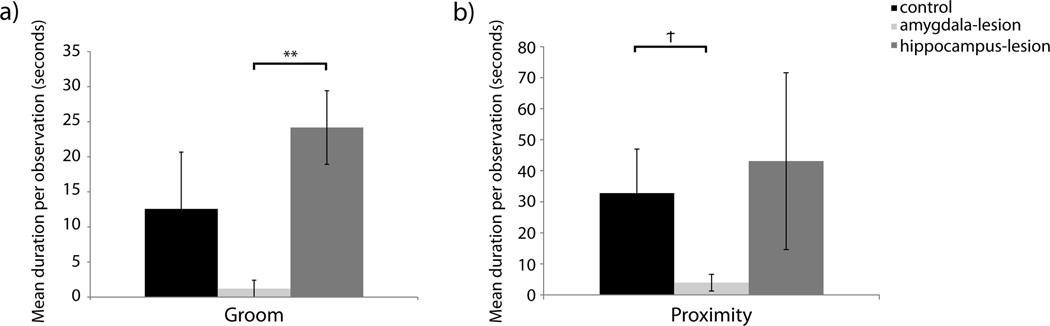
[a] Duration of time experimental females were groomed by their pair-mates. Raw means are presented in the figure although analyses for Groom were performed on log transformed data to account for non-normality. [b] Duration of time pair-mates sat near experimental males. Raw means are presented in the figure although analyses for Proximity were performed on log transformed data to account non-normality. Significant differences between lesion conditions as per independent sample t-tests are indicated using the following symbol key: Ϯ = p< .10, * = p< .05, ** = p< .01.
Lesion condition did not influence the duration of bouts of sitting near or mounting pair-mates; F(2,16)=2.472, p=0.116, ηp2=0.236 (analyses on log transformed data) and F(2,16)=1.380, p=0.280, ηp2=0.147 (analyses on log transformed data) respectively – Figure 1c. However, there was a tendency for pair-mates of amygdala-lesioned males to sit next to them for shorter durations as compared to pair-mates of control males; F(2,16)=3.022, p=0.077, ηp2=0.183 (analyses on log transformed data). Evaluation of the marginal means suggested a difference between amygdala-lesioned males and the other groups. When groups were compared individually using t-tests, the effect still did not reach conventional levels of significance although a large effect size suggests that the difference between amygdala-lesioned and control animals might be meaningful, t(5)=2.269, p=0.073, d=1.415; analyses on log transformed data) – Figure 2b.
Nonsocial State Duration
Nonsocial states are sustained periods of time spent outside of arms’ reach of another animal. Amygdala-lesioned animals spent more time in nonsocial states than control or hippocampus-lesioned animals; F(2,16)=9.734, p=0.002, ηp2=.549 (A>C, p=0.006; A>H, p=0.001) – Figure 1a. This effect was driven by the amount of time spent alert and active while being alone; F(2,16)=7.074, p=0.006, ηp2=0.469 (A>C, p=0.019; A>H, p=0.001) – Figure 1d. It is worth noting that the only animals who spent any time “inactive” (not alert) were two amygdala-lesioned males. One animal was inactive for an average of 1.12 seconds per observation and the other animal was inactive for an average of 19.52 seconds per observation. There were no lesion based difference in the duration of time spent pacing; F(2,16)=1.557, p=0.241, ηp2=0.163 (analyses on log transformed data) – Figure 1d.
Sleep State Duration
There were no group differences in time spent sleeping; F(2,16)=0.043, p=0.958, ηp2=0.005 (analyses on log transformed data) – Figure 1a.
Number of State Changes
As discussed above, the duration or length of some states differed by lesion group. The number of times animals entered a social or nonsocial state and the number of times that they changed states all together (giving some indication of restlessness, or lack of sustained engagement in either the environment or social interactions) were assessed. Lesion groups did not differ in number of times that they shifted between states (F(2,16)=1.750, p=0.205, ηp2=0.179), however, they did differ in how frequently they initiated close social states. Hippocampus-lesioned animals initiated close social states more frequently than both control and amygdala-lesioned animals; F(2,16)=5.590, p=0.014, ηp2=0.411 (H>C, p=0.014; H>A, p=0.020) – Figure 1b. Once engaged in close social interactions, amygdala-lesioned animals tended to leave them more frequently than controls or hippocampus-lesioned animals; F(2,16)=2.983, p=0.079, ηp2=0.272 (analyses on log transformed data). Evaluation of the marginal means suggested a difference between amygdala-lesioned animals and the other groups. While the direct comparison effects still did not reach conventional levels of significance, when amygdala-lesioned animals were compared directly to control animals (t(13)=1.833, p=0.090, d=0.960; analyses on log transformed data) or hippocampus-lesioned animals (t(12)=1.884, p=0.084, d=1.020; analyses on log transformed data), the large effect sizes suggest that the differences might be meaningful.
Communicative Signaling
Lesion groups did not differ in the frequencies with which they generated communicative signals directed towards their pair-mates; F(2,16)=0.040, p=0.961, ηp2=0.005 (analyses on log transformed data). Further investigation of specific types of communicative signaling indicated no lesion group differences in affiliative signaling (F(2,16)=0.040, p=0.961, ηp2=0.005; analyses on log transformed data), submissive signaling (F(2,16)=0.243, p=0.787, ηp2=0.029; analyses on log transformed data), or agonistic signaling (F(2,16)=1.016, p=0.384, ηp2=0.113; analyses on log transformed data) – Figure 3a.
Figure 3. Frequencies of Communicative Signals.
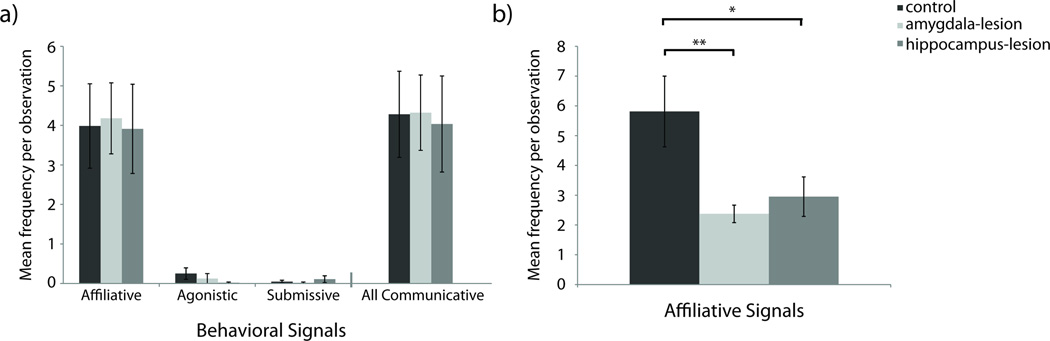
[a] Frequencies of communicative signals generated toward pair-mates. Raw means are presented in the figure although analyses for Affiliative, Agonistic, Submissive, and All Communicative Signals were performed on log transformed data to account for non-normality. [b] Frequencies of affiliative signals generated by pair-mates toward experimental females. Raw means are presented in the figure although analyses for Affiliative Signals were performed on log transformed data to account for non-normality. Significant differences between lesion conditions as per independent sample t-tests are indicated using the following symbol key: Ϯ = p< .10, * = p< .05, ** = p< .01.
Lesion and sex together influenced the number of affiliative signals that were directed towards subjects by their pair-mates. Pair-mates of control females had a tendency to produce higher frequencies of affiliative signals towards them than did the pair-mates of amygdala- or hippocampus-lesioned females; F(2,16)=3.315, p=0.062, ηp2=0.293 (analyses on log transformed data). Despite the omnibus test not reaching conventional levels of significance, between-group differences were evaluated with t-tests because the marginal means suggested that the control females might differ significantly from the females of the other two groups. When compared directly, pair-mates of control animals generated significantly more affiliative signals towards their partners than pair-mates of amygdala-lesioned females (t(6)=3.888, p=0.008, d=1.986; analyses on log transformed data) and pair-mates of hippocampus-lesioned females (t(7)=2.440, p=0.045, d=1.445; analyses on log transformed data – Figure 3b). The large effect sizes suggest robust effects.
Sexual Behavior
There were no lesion-group difference in frequencies of sexual behavior, F(2,16)=0.273, p=0.765, ηp2=0.033 (analyses on log transformed data raw means: Mamygdala-lesioned=0.982, SEamygdala-lesioned=0.406; Mhippocampus-lesioned=0.893, SEhippocampus-lesioned=0.326; Mcontrols=0.703, SEcontrols=0.310).
Nonsocial Behaviors
Exploratory Behaviors
Lesion condition did not influence the frequency of exploration, (F(2,16)=1.248, p=0.314, ηp2=0.135; analyses on log transformed data). However, there was a significant interaction of lesion and sex on the frequencies of exploration. Control females explored more frequently than amygdala- and hippocampus-lesioned females; F(2,16)=4.904, p=0.022, ηp2=0.380 (analyses on log transformed data). Follow up t-tests indicated that control females physically explored their environments significantly more than amygdala-lesioned females (t(6)=2.845, p=0.029, d=1.961; analyses on log transformed data) but did not differ from hippocampus-lesioned females (t(7)=1.845, p=0.108, d=1.376; analyses on log transformed data) – Figure 4a.
Figure 4. Frequencies of Exploration and Anxiety-Related Behaviors.
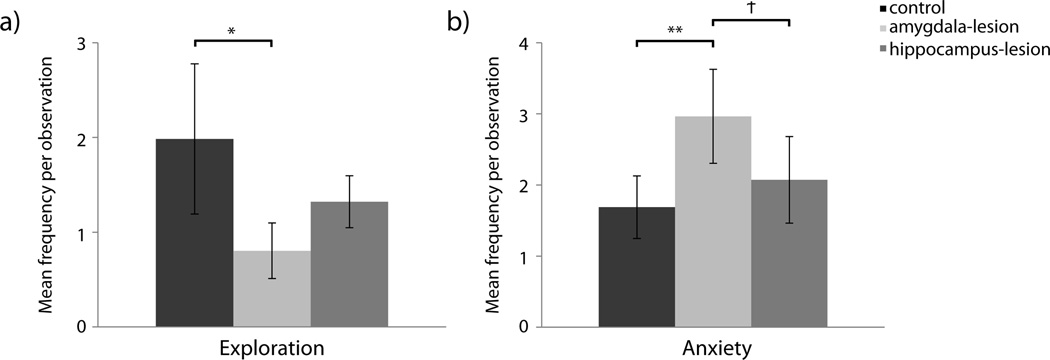
[a] Frequencies of exploratory behavior generated by experimental females. Raw means are presented in the figure although analyses for Exploration were performed on log transformed data to account for non-normality. [b] Frequencies of anxiety-related behaviors generated. Raw means are presented in the figure although analyses for anxiety-related behaviors were performed on log transformed data to account for non-normality. Significant differences between lesion conditions as per independent sample t-tests are indicated using the following symbol key: Ϯ = p< .10, * = p< .05, ** = p< .01.
Anxiety-Related Behaviors
Amygdala-lesioned animals demonstrated greater frequencies of anxiety-related behaviors such as yawning and scratching than control or hippocampus-lesioned animals; F(2,16)=5.700, p=0.014, ηp2=0.416 (A>C, p=0.009; A>H, p=0.084) (analyses on log transformed data) – Figure 4b.
Total Stereotypy Frequency
There was a tendency for control animals to produce fewer stereotypies than the amygdala-lesioned and hippocampus-lesioned animals; F(2,16)=2.723, p=0.096, ηp2=0.254 (analyses on log transformed data). When amygdala-lesioned animals are compared directly with controls, the effect still did not reach conventional levels of significance; t(13)=1.918, p=0.069, d=1.033 (analyses on log transformed data), although the large effect size suggests that the difference might be meaningful – Figure 5a. Controls and hippocampus-lesioned animals did not differ in the total frequency of stereotypies, t(13)=1.303, p=0.215, d=0.68 (analyses on log transformed data). Investigation of specific categories of stereotypies were evaluated and results are presented below.
Figure 5. Frequencies of Stereotypic Behavior.
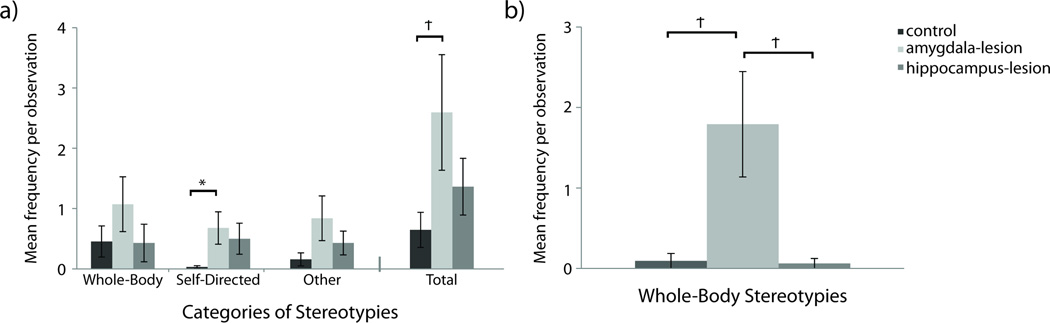
[a] Frequencies of Stereotypies. Raw means are presented in the figure although analyses for Whole-Body, Self-Directed, Other, and Total Stereotypies were performed on log transformed data to account for non-normality. [b] Frequencies of whole-body stereotypies generated by experimental males. Raw means are presented in the figure although analyses for Whole-Body Stereotypies were performed on log transformed data to account for non-normality. Significant differences between lesion conditions as per independent sample t-tests are indicated using the following symbol key: Ϯ = p< .10, * = p< .05, ** = p< .01.
Whole-body Stereotypies
Although lesion groups did not differ in their frequencies of exhibiting whole-body stereotypies, e.g. pacing, spinning in circles, and repetitive bouncing (F(2,16)=1.614, p=0.230, ηp2=0.168; analyses on log transformed data), there was an interaction of lesion and sex. Amygdala-lesioned males tended to engage in greater frequencies of whole-body stereotypies than control or hippocampus-lesioned males; F(2,16)=3.115, p=0.072, ηp2=0.280 (analyses on log transformed data). Between-group differences were evaluated with t-tests because the marginal means associated with the omnibus test suggested that the amygdala-lesioned males might differ significantly from the other two groups. While the direct comparison effects still did not reach conventional levels of significance, when amygdala-lesioned males where compared directly to control males (t(2.332)=3.038, p=0.077, d=2.089; analyses on log transformed data) or hippocampus-lesioned males (t(3)=2.487, p=0.089, d=2.150; analyses on log transformed data), the effect sizes were very large– Figure 5b.
Self-Directed Stereotypies
Amygdala-lesioned animals tended to engage in more self-directed stereotypies than control animals (e.g. rocking back and forth, self-biting, eye-poking); F(2,16)=2.767, p=0.093, ηp2=0.257 (analyses on log transformed data). Because evaluation of the marginal means suggested a difference between amygdala-lesioned animals and controls, control animals were compared directly to the amygdala-lesioned animals via t-test. Amygdala-lesioned animals differed significantly from controls; t(6.177)=2.579, p=0.041, d=1.066 (analyses on log transformed data) – Figure 5a.
Other Stereotypies
Lesion groups did not differ in the production of other stereotypies (e.g., head-twists, and other behaviors that are not included in whole-body or self-directed categories); F(2,16)=2.242, p=0.139, ηp2=0.219 (analyses on log transformed data) – Figure 5a.
Discussion
The results presented here demonstrate that social deficits in adult animals following early damage to the amygdala or hippocampus are subtle. Eight years after sustaining bilateral focal lesions of the amygdala or hippocampus, operated animals, as compared to controls, generated significantly different patterns of social behavior when evaluated in the context of their familiar social relationships.
Amygdala-lesioned animals were less social with familiar partners than control animals. They spent less time in social interactions, such as grooming, that occurred in close proximity. Compared to control animals, amygdala-lesioned animals also spent more time alone, out of reach of their pair-mates. When alone, they produced greater numbers of stereotypies than controls. They also displayed more stress-related behaviors than control animals. This reduced sociability and increased stereotypic behavior is consistent with previous observations conducted with these animals at earlier juvenile and adult developmental time points (Bliss-Moreau et al., 2013; Moadab et al., under review) but different from those observed in infancy (Bauman et al., 2004b). Thus, the social behavior and stereotypy patterns of these animals have changes as they have matured.
The evolution of behavioral patterns that we observed in this cohort of animals is consistent with previous studies of early amygdala damage. However, rather than becoming more “normal” (i.e., like controls), the social behavior of subjects from other studies became more perturbed over time. Early studies by Thompson and colleagues (Thompson, 1969; Thompson et al., 1969; Thompson & Towfighi, 1976; Thompson et al., 1977) demonstrated that animals with early amygdala damage had behavioral deficits that became more robust with age. Compared to control animals, adult animals who sustained amygdala damage as infants were significantly more submissive, hyperactive, and less social. When evaluated with familiar social partners (as in the present experiment), animals with early amygdala damage spent more time sitting passively alone, had higher frequencies of cage exploration, and more frequently moved from one behavior to the next (Thompson et al., 1977). The social trajectory of the animals in the Thompson studies stands in contrast to what was observed in the current study— social impairments have grown more subtle as our animals aged (Bauman et al., 2004b; Bliss-Moreau et al., 2013; Moadab et al., under review). As adults, the animals in the current study did not differ from controls in the frequencies of exploration or moving from one behavior to the next. In fact, the control females had higher frequencies of exploration than the amygdala-lesioned females. Amygdala-lesioned animals did not significantly differ from controls in total time spent socially, although they did spend significantly less time grooming. One primary difference between the study group in the present report and that of Thompson and colleagues is how the infants were socialized over development. It is possible, even probable, that differences in developmental trajectories between the two study groups are related to socialization over development.
The other extant study of animals with early amygdala damage further speaks to the idea that socialization influences the extent of behavioral alterations following early amygdala damage (Raper, Stephens, Sanchez, Bachevalier, & Wallen, 2014; Raper, Stephens, Henry, et al., 2014; Raper, Wilson, Sanchez, Machado, & Bachevalier, 2013; Raper, Bachevalier, Wallen, & Sanchez, 2013; Raper, Wallen, et al., 2013;). Following surgery, infants in that study were returned to large social groups with their mothers. These infants demonstrated only subtly impacted social behavior as infants with regards to mother-infant social dynamics (Raper, Stephens, Sanchez, Bachevalier, & Wallen, 2014). While male amygdala-lesioned infants behaved similarly to control animals with their mothers, female infants with amygdala damage spent less time in contact with their mothers. This pattern of results is different from what was observed with the present study group—that is both male and female amygdala-lesioned infants spent more time in contact with their mothers than control infants (Bauman et al., 2004a). Whether the social behavior of the infants in Raper, Stephens, Sanchez et al. (2014) changes over development has yet to be seen.
One interpretation of the present data is that close social interactions such as grooming were not rewarding for amygdala-lesioned animals to the extent that they were for control animals and therefore they were less motivated to engage in them. These amygdala-lesioned animals have blunted affective responsivity not only to negative or threatening stimuli (Bliss-Moreau et al., 2010; Bliss-Moreau et al., 2011; Bliss-Moreau, Bauman, & Amaral, 2011) but also to positive stimuli (Bliss-Moreau, Bauman, & Amaral, 2011). This leaves open the possibility that they may generate less pro-social behavior because such interactions are not inherently pleasant for amygdala-lesioned animals. These findings are also consistent with the idea that amygdala damage alters affective processing which in turn alters social processing. This is also consistent with findings from groups who have shown that animals with early amygdala lesions continue to demonstrate differences in affective processing across development (e.g., less reactive to novelty and threatening stimuli; Raper, Wilson, Sanchez, Machado, & Bachevalier, 2013; Raper, Bachevalier, Wallen, & Sanchez, 2013; Raper et al., 2013; Raper et al., 2014).
Consistent with the previous evaluations of these animals’ social behavior (Bliss-Moreau et al., 2013; Moadab et al., under review), animals with early hippocampus damage were more social with their pair-mates than control animals. Compared to control animals, hippocampus-lesioned animals spent more time in close social interactions and initiated these close social interactions more often. When this cohort was evaluated as juveniles, the propensity for hippocampus-lesioned animals to be more social was evident but did not reach conventional levels of significance. Their hyper-sociability has become more prominent as they have matured, resulting in significant differences between hippocampus-lesioned animals and control animals at this time point. These differences are notable because many extant studies evaluating the impact of early hippocampus damage in macaques have not explicitly evaluated social behavior (Bachevalier, Beauregard, & Alvarado, 1999; Munier, Nalwa, & Bachevalier, 2003; Lavenex, Banta Lavenex, & Amaral, 2007; Zeamer, Heuer, & Bachevalier, 2010; Heuer & Bachevalier, 2011). Previous studies that have evaluated social behavior following early hippocampus damage have generally found that animals with hippocampal damage were somewhat less social than controls in infancy, but were much less socially engaged as adults (Beauregard et al., 1995; Bachevalier, Alvarado et al., 1999).
Histological analyses that investigate neural reorganization following early hippocampus damage will hopefully shed light on the mechanisms subserving the heightened sociality that was observed in this cohort. It is well established that the hippocampus itself is a site of major plasticity in terms of neurogenesis as well as changes to existing neuronal structures (for a review Leuner & Gould, 2010) and damage to the hippocampus causes widespread changes to the brain including the structure and function of the prefrontal cortex and dopamine system among others (for a review, Tseng, Chambers, & Lipska, 2009). These findings are consistent with the remarkable behavioral plasticity that has been documented in the neonatal hippocampus-lesioned animals. Specifically, animals with neonatal hippocampal damage had intact spatial relational memory (Lavenex et al., 2007) while animals with adult damage did not (Banta Lavenex et al., 2006). Characterizing the neural plasticity is a critical future direction.
While the sample sizes in this experiment are small, especially if male and female animals are considered separately, the pattern of effects from this experiment is especially notable for two reasons. First, this experiment demonstrates that animals with early amygdala or hippocampus damage exhibit subtly altered social behavior outside of an experimental environment. Animals were observed in the cages and with the animals in which they lived. Often, animals with brain damage are evaluated in experimental environments such as novel test cages or test cages used specifically for monitoring social interactions (e.g., Thompson & Towfighi, 1976; Thompson et al., 1977; Beauregard et al., 1995; Bachevalier et al., 2001; Emery et al., 2001; Machado & Bachevalier, 2006; Machado, Emery, et al., 2008; Malkova, Mishkin, Suomi, & Bachevalier, 2010; Moadab et al., under review). Similarly, they are often evaluated with novel social interaction partners for short durations (Thompson & Towfighi, 1976; Thompson et al., 1977; Bachevalier, Malkova, & Mishkin, 2001; Emery et al., 2001; Machado & Bachevalier, 2006; Machado, Emery et al., 2008). Testing animals in novel environments and, or, with novel interaction partners sheds light on how animals develop social relationships in challenging contexts. In contrast, evaluating social behavior in familiar environments with familiar interaction partners provides information about animals’ “social baseline”. This provides information on how they interact with social partners in what should be a very low stress, unmanipulated environment. The data presented in this paper suggests that animals with neonatal amygdala damage and hippocampus damage demonstrate altered social behavior even after sustained exposure to a partner animal and environment.
The present findings contribute to a growing literature from multiple laboratories suggesting that impact of early damage to the amygdala or hippocampus on social and affective behavior is not the same as the impact of damage that occurs during adulthood and may change over time. One critical consideration in the evaluation of this literature is how the subjects were reared and socialized. Animals in the studies by Thompson and colleagues (Thompson et al., 1969; Thompson & Towfighi, 1978; Thompson et al., 1977) were isolate reared—that is, removed from their mothers on the day of birth and placed alone with a towel. The animals from the current study were reared with their mothers until 6 months of age and socialized in playgroups, housed individually and socialized in play groups between 6 and 12 months, and then finally, at 1 year of age, socially housed in various configurations across the rest of their lives (Bauman et al., 2004a; Bauman et al., 2004b; Bauman et al, 2006; Toscano et al., 2009; Bliss-Moreau et al., 2010; Bliss-Moreau, Bauman et al., 2011; Bliss-Moreau, Toscano et al., 2011; Bliss-Moreau et al., 2013; Moadab et al., under review). Early studies conducted by Bachevalier and colleagues (Beauregard et al., 1995; Bachevalier, Alvarado et al., 1999; Bachevalier, Beauregard, & Alvarado, 1999; Goursaud & Bachevalier, 2007; Zeamer et al., 2010; Heuer & Bachevalier, 2011; Raper, Wilson et al., 2013) utilized animals that were nursery reared with peers. Specifically, the neonates were removed from their mothers, housed individually, and socialized in small play-groups (i.e., with one or two other animals). Later studies by Bachevalier and colleagues (Raper, Bachevalier et al., 2013; Raper et al., 2013; Raper et al., 2014; Raper, Stephens, Sanchez et al., 2014; Stephens, Raper, Bachevalier, & Wallen, 2014; Goursaud, Wallen, & Bachevalier, 2014) had their subjects reared by their mothers in large social groups. It is likely that variation in these socialization procedures contributed to the variation observed in the animals’ social behavior.
The present findings, in concert with previous evidence collected from these animals over development, illustrates that neonatal damage to the amygdala or hippocampus impacts social behavior although the specific mechanisms are not yet clear. Animals with early damage to the amygdala or hippocampus demonstrate altered social behavior in adulthood when evaluated with familiar animals in a familiar location. Whether such effects hold with unfamiliar animals or unfamiliar environments (in which the threat processing capacity of the amygdala would be more relevant, Amaral, 2003) is unclear. Future histological and neuroimaging analyses will evaluate the extent to which neural systems have reorganized following early brain damage. Mechanisms subserving contextually dependent behavioral variation following early damage to the amygdala or hippocampus will be elucidated by evaluating neurobiological analyses in concert with a host of behavioral observations. The present study continues to emphasize the overall finding that early lesions lead to a lifetime course of behavioral alterations. The magnitude of behavioral alteration is dependent, in part, on the length of time that the brain has had to adapt to the lack of the lesioned tissue, the rearing practices during the early life of the animal and the environment and the test situation in which the animal finds itself. Identifying the neurobiological features of adaptation to early brain damage will have important implications towards enhancing recovery of function in human children who have suffered early brain damage.
Acknowledgments
This research was supported by funding from the National Institute of Mental Health (R37MH57502 to DGA), and by the base grant of the California National Primate Research Center (RR00169). EBM was supported by funding from the National Institute of Mental Health (F32MH087067 and K99MH10138) during data collection and the preparation of this manuscript. We thank the veterinary and husbandry staff of the California National Primate Research Center for excellent care of the animal subjects. We thank Dr. Pierre Lavenex, Jeffrey Bennett, and Pamela Tennant for assistance with surgical procedures.
References
- Amaral DG. The amygdala, social behavior, and danger detection. Annals of the New York Academy of Sciences. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Altmann J. Observational study of behavior: Sampling methods. Behavior. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M, Alvarado MC. Long-term effects of neonatal damage to the hippocampal formation and amygdaloid complex on object discrimination and object recognition in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 1999;113(6):1127–1151. doi: 10.1037//0735-7044.113.6.1127. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Málková L, Mishkin M. Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:545–559. doi: 10.1037//0735-7044.115.3.545. [DOI] [PubMed] [Google Scholar]
- Banta Lavenex P, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. Journal of Neuroscience. 2006;26:4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. Journal of Neuroscience. 2004a;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. Journal of Cognitive Neuroscience. 2004b;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in Rhesus Monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Babineau BA, Mason WA, Amaral DG. Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behavioral Neuroscience. 2008;122:1005–1015. doi: 10.1037/a0012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Malkova L, Bachevalier J. Sterotypies and loss of social affiliation after early hippocampectomy in primates. NeuroReport. 1995;6(18):2521–2526. doi: 10.1097/00001756-199512150-00018. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Developmental Psychobiology. 2010;52:487–503. doi: 10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behavioral Neuroscience. 2011;125:848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala lesions alter responsiveness to objects in juvenile macaques. Neuroscience. 2011;178:132–132. doi: 10.1016/j.neuroscience.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Moadab G, Bauman MD, Amaral DG. The impact of early amygdala damage on juvenile rhesus macaque social behavior. Journal of Cognitive Neuroscience. 2013;25:2124–2140. doi: 10.1162/jocn_a_00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Schafer EA. An Investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philosophical Transactions of the Royal Society of London. 1888;179:303–327. [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:515–544. [PubMed] [Google Scholar]
- Goursaud A, Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala and orbital frontal cortex. Behavioural Brain Research. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Goursaud A, Wallen K, Bachevalier J. Mother recognition and preference after neonatal amygdala lesions in rhesus macaques (Macaca mulatta) raised in a semi-naturalistic environment. Developmental Psychobiology. 2014;56(8):1723–1734. doi: 10.1002/dev.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Neonatal hippocampal lesions in rhesus macaques alter the monitoring, but not maintenance, of information in working memory. Behavioral Neuroscience. 2011;125:859–870. doi: 10.1037/a0025541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. The Journal of Neuroscience. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A. Effects of amygdalectomy & Testosterone on sexual behavior of male juvenile macaques. Journal of Comparative and Physiological Psychology. 1968;65:466–471. doi: 10.1037/h0025804. [DOI] [PubMed] [Google Scholar]
- Kling A. Differential effects of amygdalectomy in male and female nonhuman primates. Archives of Sexual Behavior. 1974;3:129–134. doi: 10.1007/BF01540996. [DOI] [PubMed] [Google Scholar]
- Kling A, Lancaster J, Benitone J. Amygdalectomy in the free-ranging vervet (Cercopithecus aethiops) Journal of Psychiatric Research. 1970;7:191–199. doi: 10.1016/0022-3956(70)90006-3. [DOI] [PubMed] [Google Scholar]
- Kling A, Cornell R. Amygdalectomy and social behavior in the caged stump-tailed macaque (Macaca speciosa) Folia Primatologica. 1971;14:190–208. doi: 10.1159/000155350. [DOI] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal loves in monkeys. Archives of Neurology & Psychiatry. 1939;42:979–1000. [Google Scholar]
- Lavenex P, Banta Lavenex P, Amaral DG. Spatial relational learning persists following neonatal lesions in macaque monkeys. Nature Neuroscience. 2007;10:234–239. doi: 10.1038/nn1820. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annual Review of Psychology. 2010;61:111–140. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in Rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in Rhesus monkeys (Macaca mulatta): Consistent pattern of behavior across different social contexts. Behavioral Neuroscience. 2008;22:251–266. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Snyder AZ, Cherry SR, Lavenex P, Amaral DG. Effects of neonatal amygdala or hippocampus lesions on resting brain metabolism in the macaque monkey: A microPET imaging study. Neuroimage. 2008;15:832–846. doi: 10.1016/j.neuroimage.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Mishkin M, Suomi SJ, Bachevalier J. Long-term effects of neonatal medial temporal ablations on socieoemotional behavior in monkeys (Macaca mulatta) Behavioral Neuroscience. 2010;124:742–760. doi: 10.1037/a0021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Mirsky AF. Studies of the effects of brain lesions on social behavior in Macaca mulatta: Methodological and theoretical considerations. Annals of the New York Academy of Sciences. 1960;85:785–794. doi: 10.1111/j.1749-6632.1960.tb50000.x. [DOI] [PubMed] [Google Scholar]
- Meunier M, Nalwa V, Bachevalier J. Reactions to familiar and novel objects in infant monkeys with neonatal temporal lesions. Hippocampus. 2003;13:489–493. doi: 10.1002/hipo.10143. [DOI] [PubMed] [Google Scholar]
- Moadab G, Bliss-Moreau E, Bauman MD, Amaral DG. Early amygdala or hippocampus damage impacts social behavior at the transition to sexual maturity. under review [Google Scholar]
- Noldus LPJJ. The Observer: a software system for collection and analysis of observational data. Behavior Research Methods, Instruments & Computers. 1991;23:415–429. [Google Scholar]
- Raper J, Bahevalier J, Wallen K, Sanchez M. Neonatal amygdala lesions alter basal cortisol levels in infant rhesus monkeys. Psychoneuroendocrinology. 2013;38(6):818–829. doi: 10.1016/j.psyneuen.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wilson M, Sanchez M, Machado CJ, Bachevalier J. Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. Psychoneuroendocrinology. 2013;38(7):1021–1035. doi: 10.1016/j.psyneuen.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wallen K, Sanchez MM, Stephens SB, Henry A, Villareal T, Bachevalier J. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. 2013 doi: 10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Stephens SB, Henry A, Villareal T, Bachevalier J, Wallen K, Sanchez MM. Neonatal amygdala lesions lead to increased activity of brain CRF systems and hypothalamic-pituitary-adrenal axis of juvenile rhesus monkeys. The Journal of Neuroscience. 2014;34(34):11452–11460. doi: 10.1523/JNEUROSCI.0269-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Stephens SB, Sanchez MM, Bachevalier J, Wallen K. Neonatal amygdala lesions alter mother-infant interactions in rhesus monkeys living in a species-typical social environment. Developmental Psychobiology. 2014;56(8):1711–1722. doi: 10.1002/dev.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner L, Kling A. Rhinencephalon and behavior. American Journal of Physiology. 1956;184:486–490. doi: 10.1152/ajplegacy.1956.184.3.486. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behavioral Neuroscience. 2003;117:1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- Stephens SB, Raper J, Bachevalier J, Wallen K. Neonatal amygdala lesions advance pubertal timing in female rhesus macaques. Psychoneuroendocrinology. 2014;51:307–317. doi: 10.1016/j.psyneuen.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CI. Time in test cage and behavior after amygdalectomy in infant rhesus monkeys. Physiology & Behavior. 1969;4:1027–1029. [Google Scholar]
- Thompson CI, Schwartzbaum JS, Harlow HF. Development of social fear after amygdalectomy in infant rhesus monkeys. Physiology & Behavior. 1969;4:249–254. [Google Scholar]
- Thompson CI, Towfighi JT. Social behavior of juvenile rhesus monkeys after amygdalectomy in infancy. Physiology & Behavior. 1976;17:831–836. doi: 10.1016/0031-9384(76)90049-4. [DOI] [PubMed] [Google Scholar]
- Thompson CI, Bergland RM, Towfighi JT. Social and nonsocial behaviors of adult rhesus monkeys after amygdalectomy in infancy or adulthood. Journal of Comparative and Physiological Psychology. 1977;91:533–548. doi: 10.1037/h0077352. [DOI] [PubMed] [Google Scholar]
- Toscano JE, Bauman MD, Mason WA, Amaral DG. Interest in infants by female rhesus monkeys with neonatal lesions of the amygdala or hippocampus. Neuroscience. 2009;162:881–891. doi: 10.1016/j.neuroscience.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behavioral Brain Research. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. The Journal of Neuroscience. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]