Abstract
Genome sequencing is revealing a vast mutational landscape in leukemia, offering new opportunities for treatment with targeted therapy. Here we identify two patients with acute myeloid leukemia and B-cell acute lymphoblastic leukemia whose tumors harbor point mutations in the ALK kinase. The mutations reside in the extracellular domain of ALK and are potently transforming in cytokine-independent cellular assays and primary mouse bone marrow colony formation studies. Strikingly, both mutations conferred sensitivity to ALK kinase inhibitors, including the FDA approved drug crizotinib. On the basis of our results, we propose that tumors harboring ALK mutations may be therapeutically tractable for personalized treatment of certain aggressive leukemias with ALK inhibitors.
INTRODUCTION
Anaplastic Lymphoma Kinase (ALK) is a receptor tyrosine kinase in the insulin receptor subfamily with homology to leukocyte tyrosine kinase (LTK), insulin-like growth factor-1 receptor kinase (IGF1R) and insulin receptor kinase (INSR) (1). ALK consists of a large ligand-binding extracellular domain, transmembrane region, and cytoplasmic domain comprised largely of the tyrosine kinase domain. The extracellular domain consists of two MAM (meprin, A5 protein and receptor protein tyrosine phosphatase mu) domains, a LDLa (low-density lipoprotein) and a glycine rich region (2). Although its normal physiological role is not entirely clear, ALK is proposed to play a role in the development of the nervous system based on its high level of expression in embryonic neural tissue (2).
ALK was originally identified as part of a gene fusion in patients with anaplastic large cell lymphoma (3). This fusion is a result of an in-frame fusion of the cytoplasmic domain of ALK to the N-terminus of nucleolar phosphoprotein (NPM) (3). In anaplastic large cell lymphoma STAT3 is a key mediator, which is required for the neoplastic transformation and prevents cell death(4,5). ALK rearrangements have also been identified in non-small-cell lung carcinomas (NSCLC), inflammatory myofibroblastic tumors, and other solid tumors (1). In NSCLC, the most common ALK fusion partner is echinoderm microtubule-associated protein-like 4 (EML4), which was found in 6.7% of cases (6). ALK overexpression has been observed in multiple tumor types (2). In neuroblastoma, activating point mutations are found in the ALK kinase domain (7).
Multiple ALK inhibitors are being developed clinically. Crizotinib, (PF-2341066, Xalkori, Pfizer) an ATP-competitive MET, ALK and ROS1 inhibitor (8), is the most clinically advanced and is now FDA-approved for front-line treatment in ALK-positive NSCLC. In a phase 3 clinical trial the response rate for patients with ALK positive NSCLC was 65% (9). ALK is therefore a promising therapeutic target in a variety of tumor types.
We recently sequenced primary samples from leukemia patients and found that aside from a few relatively frequent mutations, there are large numbers of mutations that occur at low frequency. A similar mutational landscape of cancer is emerging from large datasets produced from other efforts(10). Understanding which of these mutations are oncogenic drivers that can be therapeutically targeted remains a major challenge. We report sequencing from two leukemia patients with somatic mutations in the extracellular domain of ALK that were of unknown significance. Here, we show that these mutations are oncogenic and cells transformed by these mutant versions of ALK are sensitive to crizotinib and other ALK inhibitors.
MATERIALS AND METHODS
Sequencing of leukemia patient samples
Primary blood and bone marrow specimens were obtained after written informed consent from patients with hematologic malignancies according to a protocol approved by the OHSU institutional review board. Deep sequencing was performed on 1862 kinase and kinase associated genes as described previously (11). 185 patient samples were sequenced, including 96 Acute Myeloid Leukemia, 51 Acute Lymphoblastic Leukemia, and 38 Myeloproliferative Neoplasms. The ALK A348D mutation was verified by Sanger sequencing using the following M13F and M13R tagged primers (ALK-e4-L gtaaaacgacggccagtCCACAGAGCTACTGCTGGTC and ALK-e4-R caggaaacagctatgaccACCAAAAGCCAAATCACCTG) and then sequenced using M13F (gtaaaacgacggccagt) and M13R primers (caggaaacagctatgacc) by Eurofins MWG Operon. The ALK F856S mutation was verified by Sanger sequencing by Genewiz Inc.
Cloning
A gateway compatible entry clone containing the ALK cDNA was obtained from Genecopoeia (ALK pDONR, GC-T1863). The ALK A348D and F856S mutations were made by site directed mutagenesis using the Quikchange II XL Kit (Agilent Technologies, Inc.) and the following primers: ALK_A348D_F cactgcacactggacgtctcggtgcac, ALK_A348D_R gtgcaccgagacgtccagtgtgcagtg, ALK_F856S_F ggccaagacagacacgagccacccagagagactg, ALK_F856S_R cagtctctctgggtggctcgtgtctgtcttggcc.
Mutated cDNAs were then transferred into a Gateway-compatible MSCV-IRES-GFP vector using the LR Clonase enzyme (Life Technologies). Constructs were verified by Sanger sequencing.
Cell Culture and Virus Production
293T17 cells (ATCC) were cultured in DMEM (Invitrogen) supplemented with 10% FBS (Atlanta Biologicals), L-glutamine, penicillin/streptomycin (Invitrogen), and amphotericin B (HyClone). To produce murine retrovirus, 293T17 cells were co-transfected with pEcopac helper packaging plasmid and MSCV-IRES-GFP as an empty vector or containing WT or mutant versions of ALK. Viral supernatants were harvested 48 hours post-transfection.
Ba/F3 cells were grown in RPMI (Invitrogen) supplemented with 10% FBS (Atlanta Biologicals), L-glutamine, penicillin/streptomycin (Invitrogen), amphotericin B (HyClone), and 15% WEHI conditioned medium (a source of IL3). Ba/F3 mutant lines were generated by spinoculation with viral supernatants and polybrene at 2500 rpms for 90 minutes. GFP positive infected cells were then sorted using a FACS Aria flow cytometer.
Immunoblot Analysis
Immunoblot analysis was performed on the Ba/F3 cell lines as described previously(11). The primary antibodies used were rabbit anti-ALK (Cell Signaling Technology #3333) and Rabbit anti-GAPDH (Santa Cruz Biotechnology # #25778).
IL3-Independence Assays
Ba/F3 cells expressing either WT ALK or the ALK mutations were generated by retroviral infection. Ba/F3 lines expressing WT ALK or ALK mutants were washed three times in RPMI medium with 10% FBS (to remove all traces of IL3 containing WEHI conditioned medium). Cells were plated at 5 × 105 cells per mL and counted on a Guava personal flow cytometer every other day and divided as necessary.
Colony Assays
Bone marrow was isolated from BALB/c mice, cells were stimulated overnight with IL3, IL6 and stem cell factor, and then spinoculated with ALK expressing retrovirus, polybrene and Hepes buffer on two successive days. Cells were plated in methylcellulose (Stem Cell Technologies, M3234) and then counted on day 7 post-plating. Animal work was conducted in accordance with OHSU IACUC protocol number IS00002726.
RESULTS AND DISCUSSION
Identification of ALK point mutations in leukemia patient samples
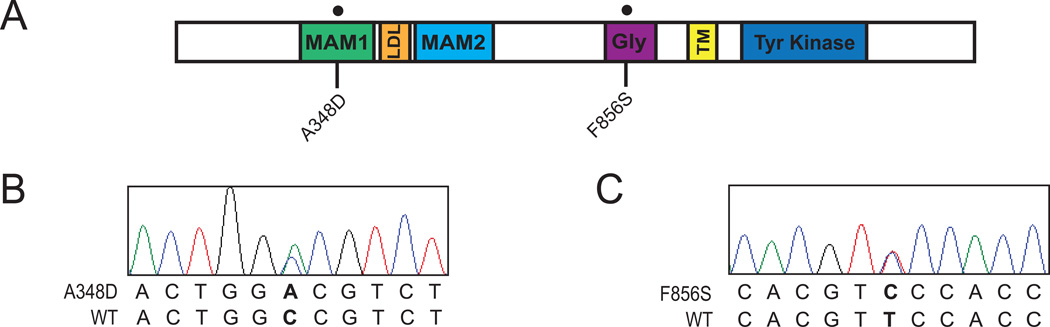
Deep sequencing of 1862 kinase and kinase associated genes in 185 leukemia samples revealed oncogenic point mutations in ALK in specimens from two patients diagnosed with hematologic malignancies. The first specimen from a pediatric patient with B-cell acute lymphoblastic leukemia (B-ALL) exhibited an ALK A348D mutation that resides within the MAM1 domain of ALK (Figure 1A). The other specimen was obtained from an adult patient with acute myeloid leukemia (AML) and exhibited an ALK F856S point mutation within the glycine-rich domain (Figure 1A). The presence of both of these mutations was confirmed by Sanger sequencing, which showed that both mutations were heterozygous (Figures 1B and 1C). Interestingly the B-ALL sample also harbored the NRAS mutations, G12C and G12S. Oncogenic mutations of known significance were not found in the AML sample, but it should be noted that the capture library is focused on kinase-associated genes, and there may therefore be other mutations present that were not assessed in this platform.
Figure 1. Identification of ALK mutations in leukemia patient samples.
(A) Schematic of the location of the ALK mutations identified by deep sequencing. Including the location of the following domains: MAM1, LDL-A (LDL), MAM2, glycine Rich (Gly), transmembrane (TM), and Tyrosine Kinase. (B,C) Sanger sequencing confirms that tumor cells from a pediatric B-ALL leukemia sample harbored the ALK A348D mutation in the MAM1 domain, and tumor cells from a patient with Adult AML exhibited the ALK F856S mutation in the glycine-rich domain.
Leukemia-associated ALK point mutations exhibit oncogenic capacity
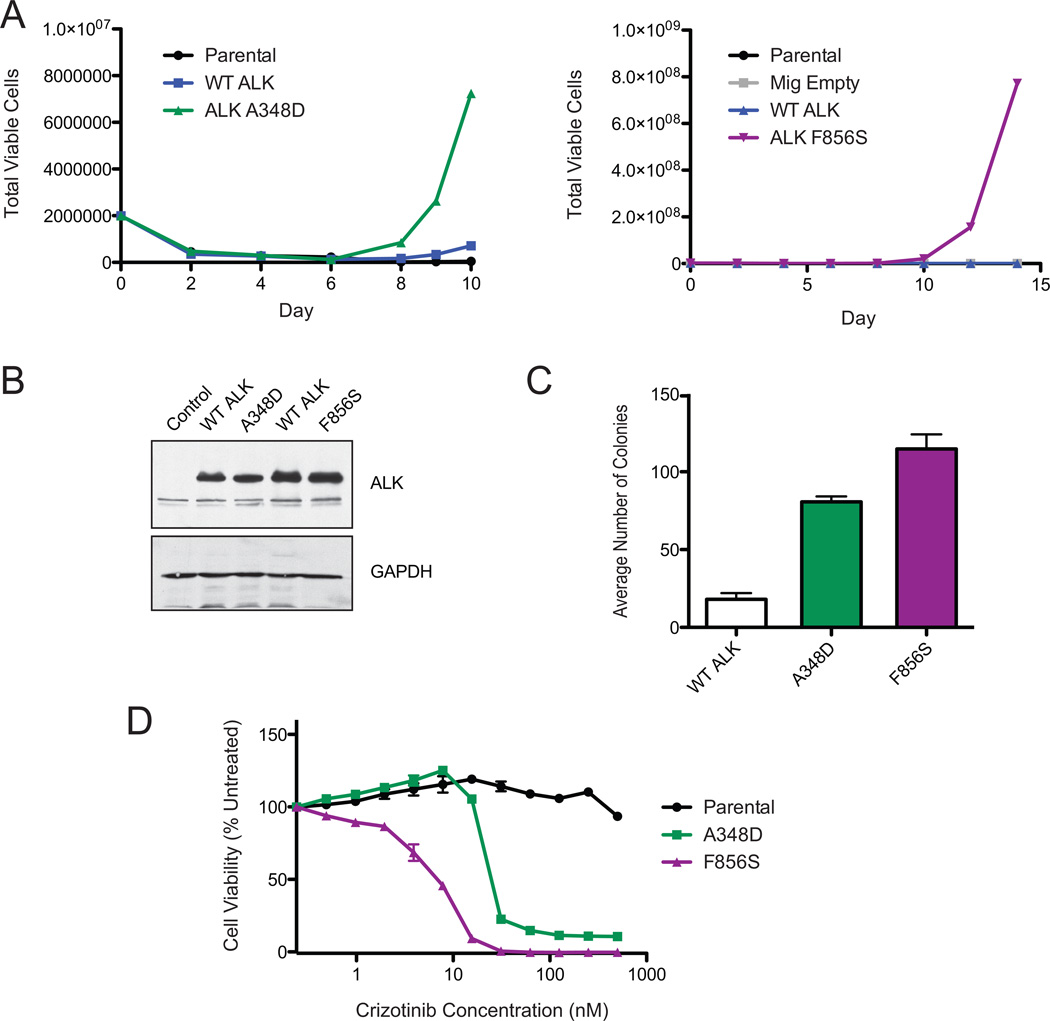
To determine whether the ALK mutations identified in AML and B-ALL patient specimens carried oncogenic capacity, we employed a cytokine-independence transformation assay. The murine pro-B cell Ba/F3 cell line normally requires the cytokine IL3 for growth. In the presence of certain transforming oncogenes, Ba/F3 cells can grow indefinitely in the absence of IL3. Both ALK A348D and ALK F856S mutant expressing Ba/F3 cell lines proliferated in the absence of IL3, while overexpression of WT ALK did not confer IL3-independent growth (Figures 2A). The mutant forms of ALK were expressed at similar levels to WT ALK (Figure 2B). To confirm that these mutations carried transformative capacity by an independent assay, we infected murine bone marrow with ALK mutants and tested their ability to form colonies in methylcellulose. The ALK A348D and F856S mutations were able to induce mouse bone marrow colony formation in the absence of exogenous cytokines, providing additional evidence that these mutations have oncogenic capacity (Figure 2C).
Figure 2. ALK point mutations found in leukemia samples are oncogenic, and sensitive to Crizotinib.
(A) The ALK A348D and F856S mutations transforms the murine Ba/F3 pro-B cell line to cytokine independent growth. Ba/F3 cells expressing WT ALK, ALK A348D, F856S, Ba/F3 cells harboring an empty vector (Mig Empty), or parental Ba/F3s were grown in the absence of the cytokine IL3. Total viable cells are plotted over time. (B) ALK mutations are expressed at the same level as WT ALK. Immunoblot analysis of Ba/F3 cells expressing ALK mutations along with their respective WT ALK controls from each Ba/F3 IL3 withdrawal experiment. GAPDH serves as a loading control. (C) ALK point mutations induce colony formation in mouse bone marrow. Mouse bone marrow cells were infected with retrovirus expressing WT ALK, A348D or F856S and then plated in colony formation medium in the absence of cytokines. The average number of colonies formed from three replicates are shown. Error bars represent the standard error of the mean. (D) ALK point mutant expressing Ba/F3 cells are sensitive to Crizotinib. IL3-independent Ba/F3 cells expressing the ALK A348D or F856S mutations were treated with Crizotinib in triplicate. Cell viability was determined using a tetrazolamine based viability assay. Viability is represented as a percentage of the untreated control. The mean of three replicates are shown, along with the standard error.
We next wanted to determine if ALK was expressed in these samples. Although material was not available for expression analysis from the AML sample, we were able to analyze ALK expression in the B-ALL sample with the ALK A348D mutation by Affymetrix exon arrays. We utilized a set of 25 samples, which had expression measurements using the Affymetrix Exon array. To determine expression we used a metric known as the probeset detected above background measure (PSDABG) which tests whether a given probeset displays significantly higher intensity than background regions with similar GC content(12,13). We computed the PSDABG measure for the 40 probesets belonging to the high confidence 'core' annotation group defined by Affymetrix. We found that 25/40 (62.5%) of the probesets exhibited a PSDABG value < .05 with 2 of them surviving a Bonferroni correction (Supplemental Figure 1). As each probeset was designed to target an exon, this suggests that the majority of the exons of ALK are expressed above background for this sample.
ALK inhibitors are being used therapeutically for patients with ALK rearrangements in lung cancer(9,14). We tested the most clinically advanced ALK inhibitor, crizotinib, against the ALK mutant Ba/F3 cell lines. The growth of Ba/F3 cells expressing both mutants was highly sensitive to crizotinib, with IC50s of 33 nM for ALK A348D cells and 6 nM for the ALK F856S cells (Figure 2D).
ALK point mutations are sensitive to multiple ALK inhibitors
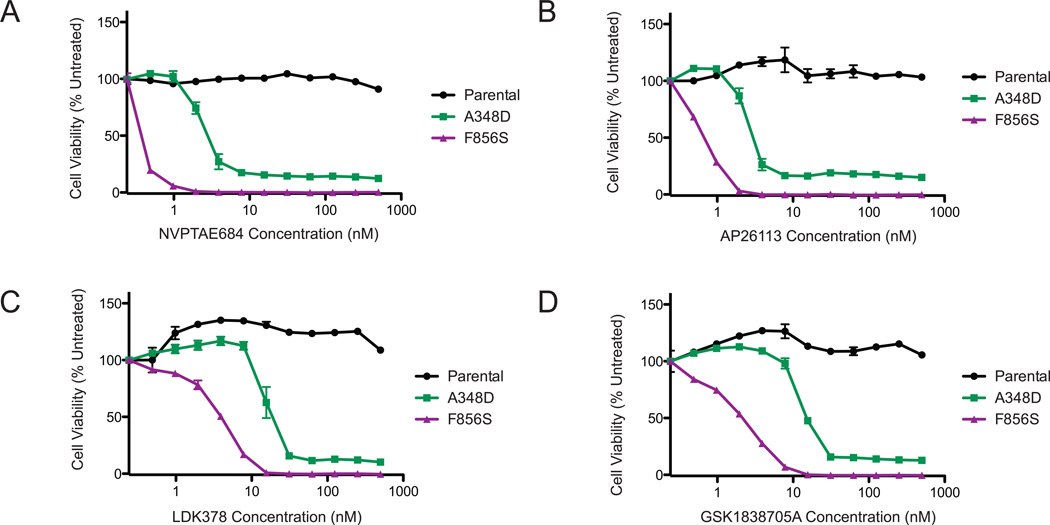
To further test the sensitivity of the ALK A348D and F856S mutations to ALK inhibitors, we used four additional ALK inhibitors. The NVP-TAE684 compound was developed as a potent and specific inhibitor of NPM-ALK fusion and blocks the proliferation of lung cancer cell lines harboring ALK gene fusions (15). NVP-TAE684 inhibited the cell survival and proliferation of Ba/F3 ALK A348D and ALK F856S cells with IC50s of 2.5 nM and ~0.5 nM respectively. The second-generation ALK inhibitor AP26113 was developed to overcome crizotinib resistance (14) mediated by the emergence of ALK L1196M mutation (16,17) and is in Phase 1/2 development. Treatment of ALK mutant-transformed Ba/F3 cells with AP26113 potently inhibited cell growth with an IC50 of 3 nM for the A348D mutant and 0.6 nM for the F856S mutant.
LDK378 is highly selective for ALK with 30–40 fold sensitivity for ALK over IGF1R and INSR, and showing an IC50 for growth of NPM-ALK-transformed Ba/F3 cells at 26 nM as well as an IC50 of 0.2 nM in cell free kinase assays (18). This compound inhibited ALK A348D cell growth with an IC50 of 22 nm and F856S cell growth with an IC50 of 4 nM. Finally, the GSK1838705A compound is potent against IGF1R, INSR and ALK (with an IC50 of 0.5 nM)(19). It was also highly potent in inhibiting growth of ALK-transformed cell lines with IC50s of 18 nM and 2nM for the A348D and F856S cells respectively. In aggregate, these data show that the ALK point mutations found in leukemia patient samples are highly sensitive to a variety of inhibitors directed against ALK.
The identification of oncogenic ALK point mutations in acute leukemia (AML and B-ALL) is unexpected given that ALK fusions are exceedingly rare in leukemia. RANB2-ALK fusions have been detected in an adult patient with acute myelomonocytic leukemia (20), and three cases of childhood myeloid leukemia (21). Here we demonstrate that these ALK point mutations are potently oncogenic. It is interesting that both mutations reside in the extracellular domain of ALK. In neuroblastoma, ALK point mutations are found primarily in the kinase domain (2), while they can occur in both the kinase domain and the extracellular domain in patients with lung cancer, although due to the lack of expression of ALK in the lung, the significance of these mutations to cancer progression is not yet clear (22). These extracellular domain mutations have been identified in both the MAM domains as well as the glycine rich domain, similarly to the mutations described here (22). The extracellular mutations identified in lung cancer were able to increase tumor burden in xenograft models (22). These extracellular domain mutations had variable activation of downstream STAT3, AKT and ERK signaling pathways (22). Although the function of the MAM and glycine rich domains is still under investigation, it is clear that these regions are important for ALK’s role in the proper development of the Drosophila gut (23). Interestingly, the neuroblastoma cell line, NB1, had an activating deletion in the first MAM domain leading to increased STAT3 signaling, possibly due to receptor mis-localization (24). Future structural studies of the extracellular regions of ALK would provide a framework for analysis of the structural impact of individual point mutations. Although, oncogenic, the ALK mutations likely require other cooperating mutation in the progression to leukemia, as evidenced by the presence of known oncogenic NRAS mutations in the B-ALL sample.
The ALK A348D and F856S extracellular mutations were highly sensitive to the approved ALK inhibitor, crizotinib, and a variety of other ALK inhibitors in clinical development. As we move towards individualized cancer therapy based on mutational profiles of patient tumors, ALK point mutations, although present in a small percentage of samples, represent an exciting therapeutic target. Characterization of additional ALK mutations and demonstration of clinical efficacy will be required to fully implement ALK directed therapy for leukemia patients.
Supplementary Material
Figure 3. The ALK A348D and F856S mutations confer sensitivity to ALK inhibitors.
IL3-independent Ba/F3 cells expressing the ALK A348D or F856S mutations were treated with NVPTAE684 (A), AP2631 (B), LDK378 (C) or GSK1838705A (D). Parental BaF3 cells in IL3 containing medium were used as a control. All drugs were used at the following concentrations: 500, 250, 125, 62.5, 31.25, 15.6, 7.8, 3.9, 1.95, 0.98, 0.49 and 0 nM. Cell viability was determined using a tetrazolamine based viability assay. Viability is represented as a percentage of the untreated control. The mean of three replicates is plotted, along with the standard error.
Table 1.
Summary of IC50 values for inhibition of Ba/F3 cell growth by ALK inhibitors calculated from data presented in Figures 2 and 3.
| IC50 (nM) | ||
|---|---|---|
| A348D | F856S | |
| Crizotinib | 33 | 6 |
| NVP-TAE684 | 2.5 | 0.5 |
| AP26113 | 3 | 0.6 |
| LDK378 | 22 | 4 |
| GSK1838705 | 18 | 2 |
ACKNOWLEDGEMENTS
We would like to than Dorian La Tocha for assistance with sorting of GFP+ Ba/F3 cells. This work was supported by Howard Hughes Medical Institute Funding to BJD. J.M. is supported by a NCI K99 CA190605-01, a Leukemia & Lymphoma Society Fellow Award and a Medical Research Foundation Early Clinical Investigator Award. B.H.C is supported by the St. Baldrick’s Foundation. J.W.T. is supported by grants from the V Foundation for Cancer Research, the Gabrielle’s Angel Foundation for Cancer Research, and the National Cancer Institute (4 R00CA151457-03).
Footnotes
Conflict of Interest Statements: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Barreca A, Lasorsa E, Riera L, Machiorlatti R, Piva R, Ponzoni M, et al. Anaplastic lymphoma kinase in human cancer. Journal of molecular endocrinology. 2011;47(1):R11–R23. doi: 10.1530/JME-11-0004. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. The Biochemical journal. 2009;420(3):345–361. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263(5151):1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 4.Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nature medicine. 2005;11(6):623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 5.Zamo A, Chiarle R, Piva R, Howes J, Fan Y, Chilosi M, et al. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene. 2002;21(7):1038–1047. doi: 10.1038/sj.onc.1205152. [DOI] [PubMed] [Google Scholar]
- 6.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 7.George RE, Sanda T, Hanna M, Frohling S, Luther W, 2nd, Zhang J, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455(7215):975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Molecular cancer therapeutics. 2007;6(12 Pt 1):3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. The New England journal of medicine. 2013;368(25):2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. The New England journal of medicine. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. The New England journal of medicine. 2013;368(19):1781–1790. doi: 10.1056/NEJMoa1214514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26(19):2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark TA, Schweitzer AC, Chen TX, Staples MK, Lu G, Wang H, et al. Discovery of tissue-specific exons using comprehensive human exon microarrays. Genome biology. 2007;8(4):R64. doi: 10.1186/gb-2007-8-4-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. The lancet oncology. 2011;12(11):1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galkin AV, Melnick JS, Kim S, Hood TL, Li N, Li L, et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(1):270–275. doi: 10.1073/pnas.0609412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. The New England journal of medicine. 2010;363(18):1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 17.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(18):7535–7540. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsilje TH, Pei W, Chen B, Lu W, Uno T, Jin Y, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. Journal of medicinal chemistry. 2013;56(14):5675–5690. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 19.Sabbatini P, Korenchuk S, Rowand JL, Groy A, Liu Q, Leperi D, et al. GSK1838705A inhibits the insulin-like growth factor-1 receptor and anaplastic lymphoma kinase and shows antitumor activity in experimental models of human cancers. Molecular cancer therapeutics. 2009;8(10):2811–2820. doi: 10.1158/1535-7163.MCT-09-0423. [DOI] [PubMed] [Google Scholar]
- 20.Lim JH, Jang S, Park CJ, Cho YU, Lee JH, Lee KH, et al. RANBP2-ALK fusion combined with monosomy 7 in acute myelomonocytic leukemia. Cancer genetics. 2014;207(1–2):40–45. doi: 10.1016/j.cancergen.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Rottgers S, Gombert M, Teigler-Schlegel A, Busch K, Gamerdinger U, Slany R, et al. ALK fusion genes in children with atypical myeloproliferative leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24(6):1197–1200. doi: 10.1038/leu.2010.18. [DOI] [PubMed] [Google Scholar]
- 22.Wang YW, Tu PH, Lin KT, Lin SC, Ko JY, Jou YS. Identification of oncogenic point mutations and hyperphosphorylation of anaplastic lymphoma kinase in lung cancer. Neoplasia. 2011;13(8):704–715. doi: 10.1593/neo.11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loren CE, Englund C, Grabbe C, Hallberg B, Hunter T, Palmer RH. A crucial role for the Anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO reports. 2003;4(8):781–786. doi: 10.1038/sj.embor.embor897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okubo J, Takita J, Chen Y, Oki K, Nishimura R, Kato M, et al. Aberrant activation of ALK kinase by a novel truncated form ALK protein in neuroblastoma. Oncogene. 2012;31(44):4667–4676. doi: 10.1038/onc.2011.616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.