Abstract
Objective
To assess whether younger, but not older, women in China have higher in-hospital mortality following ST-Segment Elevation Myocardial Infarction (STEMI) compared with men, and whether this relationship varied over the last decade or across rural/urban areas.
Methods
We analysed a nationally representative sample of 11 986 patients with STEMI from 162 Chinese hospitals in 2001, 2006 and 2011, in the China PEACE-Retrospective AMI Study and compared in-hospital mortality between women and men with gender–age interactions in multivariable models.
Results
The overall in-hospital mortality rate was higher in women compared with men (17.2% vs 9.1%, p<0.0001; unadjusted OR 2.07, 95% CI 1.85 to 2.33). The unadjusted OR for mortality in women, compared with men, was 2.20 (95% CI 1.59 to 3.04), 2.21 (95% CI 1.74 to 2.79), 1.37 (95% CI 1.15 to 1.65) and 1.25 (95% CI 0.97 to 1.63) for ages <60, 60–69, 70–79 and ≥80 years, respectively. After adjustment for patient characteristics, hospital characteristics and year of study, the OR for mortality comparing women with men was 1.69 (95% CI 1.01 to 2.83), 1.64 (95% CI 1.24 to 2.19), 1.15 (95% CI 0.90 to 1.46) and 0.82 (95% CI 0.60 to 1.11) for ages <60, 60–69, 70–79 and ≥80 years, respectively. The gender–age interaction for mortality was statistically significant (p=0.009), even after adjustment for a wide range of confounders, and did not vary over time or across rural/urban areas.
Conclusions
Among a Chinese population with STEMI, gender differences in early mortality were age-dependent and greatest in the younger groups <70 years of age.
Trial registration number
Keywords: CORONARY ARTERY DISEASE
Introduction
Several studies from high-income areas, including the USA, Canada and Europe, have demonstrated gender differences in mortality following acute myocardial infarction (AMI) that vary by age,1–8 with a higher risk of death in younger women compared with their male counterparts. For example, in a US study of patients hospitalised with AMI between 1994 and 1998, women aged ≤50 years had a more than twofold greater in-hospital mortality compared with similarly aged men,2 but this difference was not present in older patients. A remaining question is whether this gender–age interaction in AMI mortality, in which gender differences are greater in younger patients, exists in diverse populations and healthcare systems, especially in low-income and middle-income countries.
In China, home to one-fifth of the world's women, there is a rising burden of cardiovascular disease.9 Moreover, as observed in the recently published China Patient-centered Evaluative Assessment of Cardiac Events (China PEACE)- Retrospective AMI Study of patients with ST-Segment Elevation Myocardial Infarction (STEMI), there was a fourfold increase in hospital admissions among both men and women over the last decade, with women persistently accounting for nearly 30% of all patients.10 In this growing population of women with AMI, it is critical to understand whether gender differences in survival among different age groups exist, especially as China prepares to embark on national efforts to improve the quality of AMI care.
Examining potential gender disparities in STEMI outcomes in China is important, as findings from Western countries may not be broadly applicable. Though prior studies from China have suggested higher rates of death in younger women after STEMI,11 these data are not contemporary and may not reflect the experience of average patients as they were based on clinical trial populations. Further investigation is needed to understand whether age–gender disparities exist among a nationally representative sample and whether differences in outcomes have changed over the last decade. Additionally, to inform future interventions, it is important to understand whether any differences observed can be explained by patient risk, hospital care management or the settings in which care is delivered.
Accordingly, we examined a nationally representative sample of patients with STEMI in the China PEACE-Retrospective AMI Study in 2001, 2006 and 2011. The objectives of this study were to (a) assess whether there is a significant gender–age interaction with in-hospital mortality among Chinese patients with STEMI; (b) identify factors that may explain this gender–age interaction and (c) determine whether this gender–age interaction has changed over time or varies across rural/urban areas. We hypothesised that younger, but not older, women with STEMI in China would have a higher risk of in-hospital mortality, compared with age-matched men. The findings of this study will assist in identifying vulnerable groups at risk for early mortality, identifying potential mediators of mortality differences, and stimulating quality improvement efforts to improve outcomes for younger women with STEMI.
Methods
Data source and study population
The design of the China PEACE-Retrospective AMI Study has been previously described.12 In brief, we created a nationally representative sample of hospitalisations for AMI during 2001, 2006 and 2011 using a 2-stage random sampling design. Since hospital volumes and clinical capacities differ between urban and rural areas, as well as among the 3 official economic–geographic regions of China, we identified hospitals in 5 strata: Eastern-rural, Central-rural, Western-rural, Eastern-urban and Central/Western-urban regions. We then used systematic random sampling to sample cases with AMI, which were identified using International Classification of Diseases versions 9 and 10, when available, or through principle discharge diagnosis terms from the selected hospitals.
We included patients with STEMI (n=13 815), which was confirmed by discharge diagnosis and electrocardiogram results. For the purposes of the present analysis, patients who were transferred in (n=559), transferred out (n=1148) or discharged within 24 h of admission (n=122) were excluded. The final study cohort consisted of 11 986 patients (n=3574 women, 29.8%).
The Chinese government, which provided financial support for the study, had no role in the design or conduct of the study; in the collection, management, analysis and interpretation of the data; or in the preparation or approval of the manuscript. The central ethics committee of the China National Center for Cardiovascular Diseases approved this study. All collaborating hospitals accepted the central ethics approval except for 5 hospitals that obtained local approval by internal ethics committees. The study is registered at http://www.clinicaltrials.gov (NCT01624883).
Medical record review and variables
Data were collected via standardised central medical chart abstraction using standardised data definitions. Variables abstracted from the medical records of each hospital included patient-level characteristics, hospital-level characteristics, year of discharge, length of stay, and in-hospital outcomes. In-hospital mortality was defined as a composite of death during hospitalisation or withdrawal from treatment due to a terminal status at discharge. Withdrawal from treatment is common in China due to many patients’/families’ reluctance to die in the hospital. The Chinese government uses in-hospital death or withdrawal from treatment as an indicator of quality for hospitals.13 14 Composite complications included re-infarction, cardiogenic shock, cardiac arrest, congestive heart failure, and ischaemic stroke (for definitions, see online supplementary appendix). Major bleeding included any intracranial bleeding; or absolute drop of haemoglobin of at least 5 g/dL; or bleeding resulting in hypovolemic shock or fatal bleeding (bleeding that directly results in death within 7 days).
Statistical analysis
Based on the distribution of in-hospital mortality among women and men, as well as the cut-off of age used in a previous landmark manuscript,2 we stratified the study sample into 4 pre-specified age groups (<60, 60–69, 70–79, and ≥80 years). Continuous variables were expressed as mean±SD and compared using unpaired t test. Using χ2 tests, we compared cardiovascular risk factors, medical history, clinical characteristics at admission, treatment during hospitalisation, in-hospital outcomes, hospital characteristics, and year of discharge between women and men in each age group. Crude ORs with 95% CIs for women versus men were reported.
We used multivariable logistic regression models to calculate adjusted OR and 95% CI for in-hospital mortality in the overall population and within each age group, and to determine whether there was a significant interaction between gender and age with respect to in-hospital mortality. Candidate variables for inclusion in the multivariable models included cardiovascular risk factors, medical history, clinical characteristics at admission, treatment during hospitalisation, length of stay, hospital characteristics, and year of discharge. The final models included only those variables that differed significantly between women and men (p<0.05). We also assessed 2 three-way interaction terms: (1) gender×age×year and (2) gender×age×rural/urban to assess whether age-dependent gender differences in in-hospital mortality changed either (a) over time or (b) across rural/urban areas. All comparisons were 2-tailed, with a p value<0.05 considered statistically significant. Statistical analyses were performed using SAS software (V.9.2, SAS Institute, Cary, North Carolina, USA) and R software (V.3.0.2).
Results
Baseline characteristics
Women presenting with STEMI were significantly older than men with STEMI (69.8±10.4 years vs 62.6±12.6 years, respectively, p<0.001). Younger women (<60 years) were more likely to have hypertension (OR=1.84; 95% CI 1.54 to 2.20), diabetes (OR=2.06; 95% CI 1.68 to 2.54) and were less likely to smoke (OR=0.09; 95% CI 0.07 to 0.12) compared with men. Women over the age of 60 years were also less likely to have had a prior AMI. Differences in these cardiovascular risk factors were attenuated in the older age groups (table 1).
Table 1.
Unadjusted OR for characteristics of patients and hospitals comparing women with men according to age
| All patients | Age group, year | |||||
|---|---|---|---|---|---|---|
| Women n=3574 |
Men n=8412 |
<60 n=3914 |
60–69 n=3213 |
70–79 n=3562 |
≥80 n=1297 |
|
| Per cent | OR* | |||||
| Cardiovascular risk factors | ||||||
| Hypertension | 56.4 | 45.5 | 1.84† | 1.43† | 1.37† | 1.13 |
| Diabetes | 25.6 | 16.3 | 2.06† | 1.98† | 1.68† | 1.27 |
| Current smoker | 10.1 | 44.5 | 0.09† | 0.16† | 0.26† | 0.26† |
| Medical history | ||||||
| MI | 9.2 | 10.8 | 0.87 | 0.72† | 0.80† | 0.50† |
| PCI | 1.2 | 1.8 | 0.64 | 0.58 | 0.85 | 0.16† |
| Stroke | 12.7 | 10.8 | 1.26 | 1.02 | 0.93 | 1.00 |
| Clinical characteristics at admission | ||||||
| Symptom onset to presentation (h) | ||||||
| ≤12 | 46.8 | 51.3 | 0.70† | 0.95 | 0.91 | 1.18 |
| >12 to ≤24 | 14.5 | 12.1 | 1.18 | 1.18 | 1.21 | 1.01 |
| >24 | 38.8 | 36.6 | 1.36† | 0.97 | 1.00 | 0.84 |
| Chest discomfort | 89.7 | 93.3 | 0.81 | 0.66† | 0.77† | 0.93 |
| SBP (mm Hg) | ||||||
| ≥140 | 39.2 | 32.1 | 1.57† | 1.22† | 1.27† | 1.29† |
| 90–139 | 54.0 | 62.4 | 0.66† | 0.81† | 0.78† | 0.69† |
| <90 | 6.8 | 5.6 | 0.91 | 1.09 | 1.06 | 1.62† |
| HR (bpm) | ||||||
| ≥100 | 19.7 | 13.2 | 1.68† | 1.47† | 1.44† | 1.29 |
| Anterior AMI | 23.9 | 23.6 | 0.95 | 0.94 | 1.06 | 1.22 |
| Cardiac arrest | 0.9 | 1.4 | 0.33† | 0.86 | 1.53 | 0.86 |
| Cardiogenic shock | 7.5 | 5.4 | 1.15 | 1.33 | 1.25 | 1.22 |
| eGFR (mL/min/1.73 m2) | ||||||
| ≥90 | 23.6 | 35.0 | 0.68† | 0.75† | 0.90 | 0.92 |
| ≥60–<90 | 31.2 | 32.5 | 1.19 | 0.81† | 0.83† | 0.76† |
| <60 | 28.6 | 16.9 | 2.08† | 1.61† | 1.26† | 1.23† |
| Unmeasured | 16.6 | 15.7 | 0.99 | 1.24† | 1.09 | 1.20 |
| LDL-C (mg/dL) | ||||||
| <100 | 27.7 | 34.9 | 0.77† | 0.55† | 0.66† | 0.73† |
| ≥100 to <130 | 23.8 | 24.1 | 1.00 | 1.04 | 1.04 | 1.15 |
| ≥130 | 19.6 | 16.2 | 1.08 | 1.37† | 1.62† | 1.52† |
| Unknown | 29.0 | 24.9 | 1.23† | 1.44† | 1.09 | 1.00 |
| Hospital characteristics | ||||||
| Economic–geographic region | ||||||
| Eastern | 62.4 | 57.6 | 1.24† | 1.35† | 1.16† | 0.96 |
| Central | 20.0 | 22.0 | 0.80† | 0.80† | 0.99 | 1.10 |
| Western | 17.6 | 20.5 | 0.92 | 0.79† | 0.80† | 0.95 |
| Rural/Urban | ||||||
| Rural | 40.3 | 37.4 | 1.29† | 0.98 | 0.98 | 1.09 |
| Urban | 59.7 | 62.6 | 0.78† | 1.02 | 1.02 | 0.92 |
| PCI-capable hospital | 58.7 | 61.0 | 0.88 | 0.93 | 0.99 | 0.93 |
| Hospital with CCU | 78.2 | 78.8 | 0.85 | 1.00 | 1.07 | 1.10 |
| Teaching hospital | 79.2 | 81.1 | 0.90 | 0.75† | 1.00 | 0.89 |
| Year of discharge | ||||||
| 2001 | 15.9 | 16.2 | 1.11 | 1.20† | 0.91 | 0.74 |
| 2006 | 29.1 | 30.2 | 0.94 | 0.93 | 0.95 | 0.88 |
| 2011 | 55.0 | 53.6 | 0.99 | 0.94 | 1.10 | 1.26† |
*The OR is for the comparison of women with men.
†The OR is significant between women and men.
CCU, coronary care unit; eGFR, estimated glomerular filtration rate; HR, heart rate; LDL-C, low-density lipoprotein cholesterol; MI: myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Clinical characteristics at presentation also differed between women and men with significant variations by age group. Younger women (<60 years) were more likely than similarly aged men to have symptoms lasting >24 h before hospital admission, while older women (aged 60–69 and 70–79 years) were less likely to have experienced chest discomfort compared with men. Younger women (<60 years) had higher systolic blood pressures and higher heart rates (≥100 bpm), but fewer cardiac arrests at presentation compared with similarly aged men and with older women. Additionally, younger women had worse renal function (glomerular filtration rate <60 mL/min) and higher low-density lipoprotein cholesterol levels than their male peers at presentation. Of note, women <60 years of age were more likely to live in rural than in urban areas (table 1).
In-hospital treatments also varied by gender and age. Younger women (<60 years) were less likely to receive clopidogrel within 24 h of admission (OR=0.80; 95% CI 0.67 to 0.95) or any statin therapy during hospitalisation (OR=0.79; 95% CI 0.65 to 0.96). Women aged 60–69 years were also less likely to receive aspirin, clopidogrel and β-blockers within 24 h of admission and statins during hospitalisation. With respect to reperfusion strategies, women aged <80 years were less likely to receive any reperfusion, especially those <60 years (OR=0.68; 95% CI 0.56 to 0.81), including less primary percutaneous coronary intervention (PCI) within 24 h (OR=0.54; 95% CI 0.40 to 0.73), compared with similarly aged men. All women were less likely to undergo cardiac catheterisation and those 60–69 and 70–79 years of age were less likely to undergo PCI or any revascularisation compared with their male peers (table 2).
Table 2.
Unadjusted OR for treatments, testing and in-hospital outcomes comparing women with men according to age
| All patients | Age group, year | |||||
|---|---|---|---|---|---|---|
| Women (n=3574) |
Men (n=8412) |
<60 (n=3914) |
60–69 (n=3213) |
70–79 (n=3562) |
≥80 (n=1297) |
|
| Per cent | OR* | |||||
| Acute medications | ||||||
| Aspirin≤24 h | 83.9 | 87.5 | 0.83 | 0.75† | 0.90 | 0.90 |
| Clopidogrel≤24 h | 51.5 | 56.6 | 0.80† | 0.81† | 0.86† | 1.01 |
| β-Blocker≤24 h | 43.8 | 48.5 | 0.87 | 0.84† | 1.06 | 0.98 |
| ACE-I/ARB‡ | 60.2 | 63.8 | 0.90 | 0.88 | 0.90 | 0.73† |
| Statin‡ | 73.0 | 75.2 | 0.79† | 0.85† | 1.02 | 1.02 |
| Reperfusion therapies ≤24 h | ||||||
| Primary PCI | 8.5 | 12.8 | 0.54† | 0.85 | 0.81 | 0.76 |
| Fibrinolytic therapy | 18.9 | 24.7 | 0.88 | 0.88 | 0.87 | 1.00 |
| Any reperfusion | 27.4 | 37.5 | 0.68† | 0.84† | 0.82† | 0.86 |
| Staged procedures | ||||||
| Cardiac catheterisation | 18.8 | 28.8 | 0.72† | 0.68† | 0.71† | 0.64† |
| PCI | 8.6 | 13.9 | 0.84 | 0.67† | 0.66† | 0.59 |
| CABG | 0.34 | 0.61 | 0.58 | 0.41 | 1.02 | – |
| Any revascularisation | 8.9 | 14.4 | 0.84 | 0.64† | 0.67† | 0.59 |
| Testing | ||||||
| Troponin | 48.9 | 49.9 | 0.85 | 0.90 | 0.96 | 1.06 |
| Echocardiogram | 46.1 | 52.6 | 0.97 | 0.67† | 0.90 | 0.93 |
| In-hospital outcomes | ||||||
| Death‡ | 12.5 | 6.7 | 2.29† | 2.21† | 1.20 | 1.38 |
| Death‡ + withdrawal from treatment | 17.2 | 9.1 | 2.20† | 2.21† | 1.37† | 1.25 |
| Death within 24h | 5.5 | 2.9 | 1.75 | 2.21† | 1.34 | 1.89† |
| Composite complications | 15.3 | 11.8 | 1.46† | 1.21 | 1.17 | 1.29 |
| Major bleeding | 0.8 | 1.0 | 0.22 | 0.74 | 1.07 | 0.44 |
| Length of stay (days) | ||||||
| <8 | 29.5 | 22.7 | 1.03 | 0.73† | 1.07 | 1.21† |
| 8–11 | 20.9 | 24.0 | 1.35† | 0.87 | 0.89 | 0.96 |
| 12–15 | 21.4 | 23.4 | 1.22† | 1.06 | 0.87 | 0.88 |
| ≥16 | 28.2 | 29.8 | 1.60† | 0.83 | 1.07 | 0.62† |
*The OR is for the comparison of women with men.
†The OR is significant between women and men.
‡During hospitalisation.
ACE-I/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; Composite complication: recurrent MI, cardiac shock, cardiac arrest, congestive heart failure and ischaemic stroke; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
Finally, women <60 years of age had higher rates of any composite complications than men, while there were no significant gender differences among other age groups. The rate of major bleeding was similar by gender in each age group (table 2).
Unadjusted in-hospital mortality
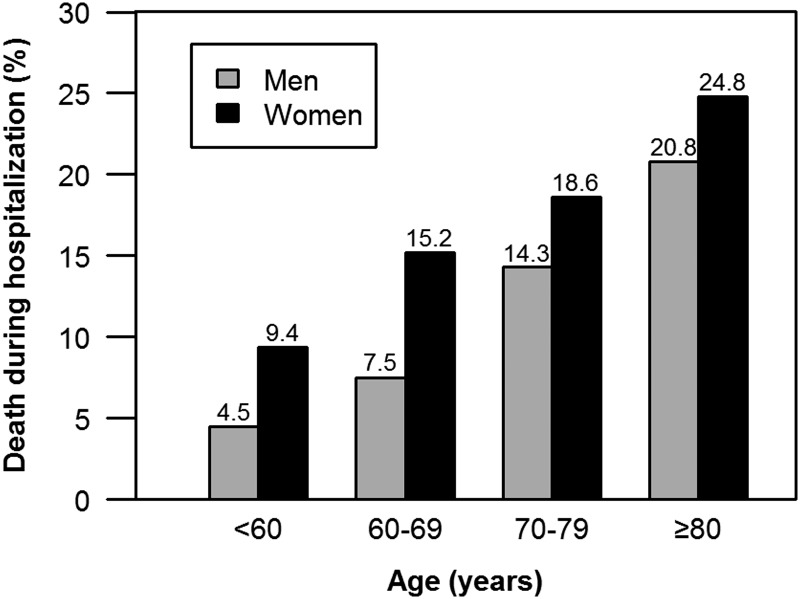
The overall in-hospital mortality rate was higher in women than in men (17.2% vs 9.1%, p<0.0001; unadjusted OR 2.07, 95% CI 1.85 to 2.33). In-hospital mortality following STEMI increased with age for both genders. Among younger women (<60 years), the in-hospital mortality rate was twofold higher compared with men (9.4% vs 4.5%, p<0.0001; unadjusted OR=2.20, 95% CI 1.59 to 3.04), and similarly for women aged 60–69 years (15.2% vs 7.5%, p<0.0001; unadjusted OR=2.21, 95% CI 1.74 to 2.79). A higher risk of death was also observed among older women 70–79 years of age (18.6% vs 14.3%, p=0.0006; unadjusted OR=1.37, 95% CI 1.15 to 1.65), but there was no difference in the risk of death in women over 80 years of age (24.8% vs 20.8%, p=0.09; unadjusted OR=1.25, 95% CI 0.97 to 1.63). When mortality rates were examined by age, a gender–age interaction was observed (p=0.0007) (figure 1). In addition, we also found that women aged 60–69, as well as ≥80 years of age, were more likely to die within 24 h of admission compared with their male peers (table 2).
Figure 1.
In-hospital mortality rate following ST-segment elevation myocardial infarction among women and men by age. The interaction between gender and age was statistically significant (p=0.0007).
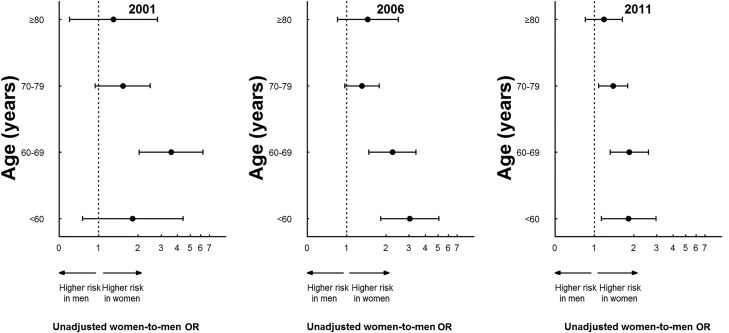
The gender–age–year interaction was not significant (p=0.38), indicating that the gender–age interaction did not change over time (figure 2). In addition, there was no gender–age–area (rural/urban) interaction (p=0.95), suggesting equally poor outcomes in women regardless of whether they were treated in urban or in rural hospitals.
Figure 2.
Unadjusted risk of in-hospital mortality comparing women with men according to age (2001, 2006 and 2011). The interaction among gender, age and year was not statistically significant (p=0.38).
Multivariable-adjusted in-hospital mortality
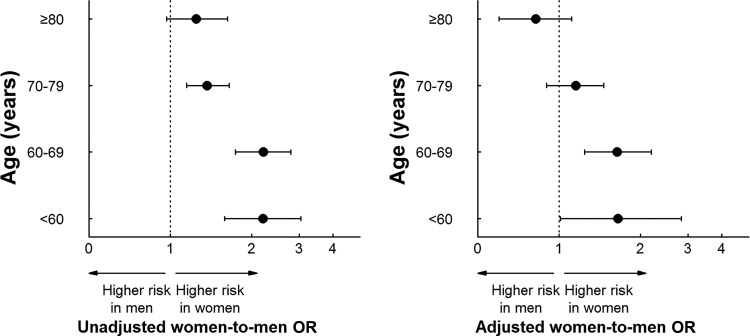
In the multivariable model, even after adjustment for differences in patient characteristics, hospital characteristics, length of stay and year of discharge, the interaction between gender and age for in-hospital mortality remained significant (p=0.0012). The adjusted OR for in-hospital mortality, comparing women with men, was 1.69 (95% CI 1.01 to 2.83), 1.64 (95% CI 1.24 to 2.19), 1.15 (95% CI 0.90 to 1.46) and 0.82 (95% CI 0.60 to 1.11) for age <60 years, age 60–69, age 70–79 and age ≥80 years, respectively. Women <70 years of age had an excess risk of mortality as compared with men; while there was no significant gender difference among patients ≥70 years old (figure 3).
Figure 3.
Unadjusted and adjusted risk of in-hospital mortality comparing women with men by age. In the unadjusted data (left panel), the interaction between gender and age was statistically significant (p=0.0009). After adjustment for differences in patient characteristics, hospital characteristics and year of discharge (right panel), the interaction between gender and age remained statistically significant (p=0.0012).
Discussion
In this first study to investigate gender differences in in-hospital mortality across the spectrum of age in a nationally representative population of patients with STEMI in China, women younger than 70 years had a >60% increased odds of dying in the hospital, compared with similarly aged men. This pattern of mortality was observed after accounting for differences in a wide range of patient and hospital characteristics. These mortality differences persisted despite changes in cardiovascular epidemiology over the last decade and differences in healthcare resources across rural and urban settings.
Our findings extend the previous literature, primarily based on US patients, by showing significant gender differences in outcomes by age in China, a low-income and middle-income country with a large, potentially very different patient population and with different challenges and inequalities in healthcare services than more highly resourced Western countries.3 5 15 Some differences between the USA and China do exist, however, in the USA, there was a twofold higher risk of death in the sub-group of women aged <50 years,15 while in the current study of Chinese patients the increased risk of death was observed among older patients as well, up to 70 years of age. The reasons for this international difference are unknown and worthy of further investigation. Based on the current data, and on our knowledge of gender differences in coronary heart disease, there are several potential explanations for the higher risk of mortality in younger women compared with similarly aged men.
Cardiovascular risk factors and co-morbidities have been considered as important contributors to gender-related mortality differences in younger patients.1 3 16 We speculated that, in our study, a higher burden of cardiovascular risk, such as diabetes, hypertension and chronic renal insufficiency, in younger women could contribute to the higher risk in mortality in this group. Women who develop atherosclerosis at a young age or in middle age may represent a distinct phenotype, whereby cardiovascular risk factors outweigh the protective effects of oestrogen and portend a higher risk of premature cardiovascular events and adverse outcomes following these events.1 In addition to considering the higher prevalence of these risk factors in younger women than their male peers, as well as their association with prognosis after AMI,17–19 moreover, in the INTERHEART study, such risk factors as diabetes and hypertension were more strongly associated with the development of myocardial infarction in women than in men, as well as in younger women (<60 years) compared with older (≥60 years) women.20 As such, several strategies, including prevention and the early identification and aggressive management of risk factors before the event, may be a target for future efforts to reduce the excess risk of mortality following STEMI among younger women, especially in China, a developing country with rapidly increasing cardiovascular risk factor burdens.21–24
Other possible explanations for the poor prognosis observed among younger women include a lack of awareness of symptoms, under-recognition at presentation and lower application of evidence-based medication and timely reperfusion therapy. In our study, women aged <60 years had a longer time from symptom onset to admission compared with other age groups, which may explain the less frequent use of early reperfusion and, ultimately, the higher risk of mortality in this age group. Delayed hospital presentation in younger women may be due to atypical symptom onset of AMI,25 which can also result in delayed detection of the condition after the patients arrive at hospital.26 In addition, less use of evidence-based therapies at admission may contribute to the higher mortality in younger women. Of note, the US studies have found that the gender difference in evidence-based treatment did not adequately account for the observed mortality disparity.2 27 However, in developing countries, addressing the aforementioned disparities in disease awareness,28 recognition and timely administration of evidence-based therapies could lead to meaningful improvements in the care of young women with AMI and should be a target of future interventions.
In our study, however, adjustment for differences in co-morbidities, clinical characteristics at admission, treatments, and hospital characteristics did not eliminate the gender–age interaction, suggesting the contribution of alternative mechanisms to this observation. Other clinical factors not measured in this study, such as door-to-balloon time and coronary angiographic characteristics, may be involved. Moreover, biological factors not typically measured, including plaque type, clotting mechanisms, coagulability states, hormonal status, and coronary spasm, may also be involved. Alternatively, in the specific context of China, the influence of socioeconomic, cultural, educational and psychological factors29 30 differentially affecting young women compared with men may be potential contributors, especially in rural areas. Investigation into these and other factors may have important implications for how we educate patients and physicians, as well as how we assess the risk and evaluate treatment strategies for younger women with AMI.
Limitations
Our findings should be interpreted within the context of several potential limitations. First, we lacked data on some important clinical variables, such as door-to-balloon time, coronary angiographic characteristics and other potential unmeasured factors, which may have influenced management. However, we were able to adjust for several potential confounders, including cardiovascular risk, severity of illness on presentation and practice management. Second, based on the random sampling design, we may have had inadequate sample size to detect important differences within specific age groups. For example, the proportion of premenopausal women was relatively low, which prevented us from having an adequate sample size to investigate patients <55 years of age. Finally, we had no information on pre-hospital death and long-term health outcomes of patients with STEMI.
Conclusions
Similar to findings in the USA and other developed countries, among a Chinese population with STEMI, gender differences in early mortality were age-dependent and greatest among the younger groups <70 years of age. This pattern persisted even after adjusting for a wide range of confounders, including patient characteristics and hospital characteristics. Further research is required to enhance the understanding and development of interventions to improve the outcomes of younger women, especially in low-income and middle-income countries undergoing epidemiological transition like China.
Key messages.
What is already known on this subject?
In studies from high-income areas such as the USA, Canada and Europe, gender differences in mortality following acute myocardial infarction (AMI) vary by age, with a higher risk of death observed in younger women compared with their male counterparts.
What might this study add?
In China, a low-income and middle-income country with a diverse population and healthcare system, we also observed significantly higher in-hospital mortality among younger women compared with men, following hospitalisation for ST-Segment Elevation Myocardial Infarction (STEMI). This pattern persisted after adjusting for a wide range of confounders, including patient- and hospital-level characteristics, and did not vary over time or across rural/urban areas. The gender differences were greatest in the younger groups <70 years of age.
How might this impact on clinical practice?
These findings indicate that the higher risk of death among younger women following AMI is a global health problem, found in high-income countries and in developing nations such as China. The findings identify young women as a vulnerable group for in-hospital mortality after STEMI and underscore the need for interventions to address this disparity.
Supplementary Material
Acknowledgments
We appreciate the multiple contributions made by study teams at the China Oxford Centre for International Health Research and the Yale-New Haven Hospital Center for Outcomes Research and Evaluation in the realms of study design and operations, particularly the data collection by Yi Pi, Jiamin Liu, Wuhanbilige Hundei, Haibo Zhang, Lijuan Zhan, Lihua Zhang, Xue Du and Wenchi Guan. We appreciate the advice by Yongfei Wang, Zhenqiu Lin, Shuxia Li and Haiqun Lin.
Footnotes
Contributors: HMK and LJ conceived the China PEACE study and take responsibility for all aspects of it. JL, XL, FAM, JAS, HMK and LJ designed the study. XZ wrote the first draft of the article, with further contributions from RPD, ESS, FAM, JAS, KN, XL, JL, SW, HMK and LJ. SH did statistical analysis, with support from XL. All authors interpreted data and approved the final version of the article.
Funding: This project was partly supported by the Research Special Fund for Public Welfare Industry of Health (201202025) from National Health and Family Planning Commission of China. Professor Krumholz is supported by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute.
Competing interests: None.
Ethics approval: The Central Ethics Committee of the China National Center for Cardiovascular Diseases.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: China PEACE Collaborative Group
References
- 1.Vaccarino V, Horwitz RI, Meehan TP, et al. Sex differences in mortality after myocardial infarction: evidence for a sex–age interaction. Arch Intern Med 1998;158:2054–62. [DOI] [PubMed] [Google Scholar]
- 2.Vaccarino V, Parsons L, Every NR, et al. Sex-based differences in early mortality after myocardial infarction. N Engl J Med 1999;341:217–25. [DOI] [PubMed] [Google Scholar]
- 3.Champney KP, Frederick PD, Bueno H, et al. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart 2009;95:895–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon T, Mary-Krause M, Cambou JP, et al. Impact of age and gender on in-hospital and late mortality after acute myocardial infarction: increased early risk in younger women: results from the French nation-wide USIC registries. Eur Heart J 2006;27:1282–8. [DOI] [PubMed] [Google Scholar]
- 5.Berger JS, Brown DL. Gender–age interaction in early mortality following primary angioplasty for acute myocardial infarction. Am J Cardiol 2006;98:1140–3. [DOI] [PubMed] [Google Scholar]
- 6.Bangalore S, Fonarow GC, Peterson ED, et al. Age and gender differences in quality of care and outcomes for patients with ST-segment elevation myocardial infarction. Am J Med 2012;125:1000–9. [DOI] [PubMed] [Google Scholar]
- 7.Otten AM, Maas AH, Ottervanger JP, et al. Is the difference in outcome between men and women treated by primary percutaneous coronary intervention age dependent? Gender difference in STEMI stratified on age. Eur Heart J Acute Cardiovasc Care 2013;2:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izadnegahdar M, Singer J, Lee MK, et al. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J Womens Health 2014;23:10–17. [DOI] [PubMed] [Google Scholar]
- 9.Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2013;381:1987–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Li X, Wang Q, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Jiang L, Smith M, et al. Sex differences in hospital mortality following acute myocardial infarction in China: findings from a study of 45 852 patients in the COMMIT/CCS-2 study. Heart Asia 2011;3:104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dharmarajan K, Li J, Li X, et al. The China patient-centered evaluative assessment of cardiac events (China PEACE) retrospective study of acute myocardial infarction: study design. Circ Cardiovasc Qual Outcomes 2013;6:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health of the People's Republic of China. The qualification standard of tertiary general hospital (version 2011). http://www.nhfpc.gov.cn/zhuzhan/wsbmgz/201304/b98329ec713a4e8d812b23a56d13f94f.shtml (accessed 6 Mar 2014).
- 14.Ministry of Health of the People's Republic of China. The qualification standard of secondary general hospital (version 2012). http://www.nhfpc.gov.cn/yzygj/s3586q/201201/b8dda05b1d23413c94150b5c17b5cc6f.shtml (accessed 6 Mar 2014).
- 15.Zhang Z, Fang J, Gillespie C, et al. Age-specific gender differences in in-hospital mortality by type of acute myocardial infarction. Am J Cardiol 2012;109:1097–103. [DOI] [PubMed] [Google Scholar]
- 16.Vaccarino V, Parsons L, Peterson ED, et al. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med 2009;169:1767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fresco C, Avanzini F, Bosi S, et al. Prognostic value of a history of hypertension in 11483 patients with acute myocardial infarction treated with thrombolysis. J Hypertens 1996;14:743–50. [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Li L, Shang X-M, et al. Analysis of factors related to short-term prognosis in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Exp Ther Med 2013;5:1206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae EH, Lim SY, Cho KH, et al. GFR and cardiovascular outcomes after acute myocardial infarction: results from the Korea acute myocardial infarction registry. Am J Kidney Dis 2012;59:795–802. [DOI] [PubMed] [Google Scholar]
- 20.Anand SS, Islam S, Rosengren A, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 2008;29:932–40. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–22. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–59. [DOI] [PubMed] [Google Scholar]
- 23.Celermajer DS, Chow CK, Marijon E, et al. Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol 2012;60:1207–16. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Meng Q, Sun X, et al. Prevalence, awareness, treatment, and control of hypertension in rural China: results from Shandong Province. J Hypertens 2010;28:432–8. [DOI] [PubMed] [Google Scholar]
- 25.Canto JG, Goldberg RJ, Hand MM, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med 2007;167:2405–13. [DOI] [PubMed] [Google Scholar]
- 26.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163–70. [DOI] [PubMed] [Google Scholar]
- 27.Ani C, Pan D, Martins D, et al. Age- and sex-specific in-hospital mortality after myocardial infarction in routine clinical practice. Cardiol Res Pract 2010;2010:752765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canto JG, Rogers WJ, Goldberg RJ, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012;307:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu M-Y, Sarri R. Women's health status and gender inequality in China. Soc Sci Med 1997;45:1885–98. [DOI] [PubMed] [Google Scholar]
- 30.Hannum E. Market transition, educational disparities, and family strategies in rural China: new evidence on gender stratification and development. Demography 2005;42:275–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.