Abstract
Objective
Several observational studies suggest that medroxyprogesterone acetate (MPA) injectable contraception may increase a woman’s risk of sexual HIV-1 acquisition. In vitro studies are conflicting, mainly due to differences in the type of progestin studied or activation status of the primary cells. We sought to determine if MPA increases infection of unstimulated peripheral blood mononuclear cells (PBMC).
Methods
Freshly isolated PBMC from normal blood donors were treated with physiologic MPA concentrations ranging from 0.003 ng/mL to 5 ng/mL and infected with GFP-tagged R5-tropic or X4-tropic HIV-1 pseudoviruses by spinoculation. The infection was limited to a single cycle. Cells were stained with CD3, CD8, and CD14. Infection was quantified as the percentage of GFP+ cells by flow cytometry.
Results
Absolute infection was greater among unstimulated MPA-treated CD3+CD8− T cells versus untreated cells across MPA concentrations of 0.003 to 3 ng/mL using R5 (P <0.003) and 0.03 to 0.3 ng/mL using X4 pseudovirus (P < 0.005). There was increased relative infection of CD3+CD8− T cells in MPA-treated whole PBMC cultures but not after monocytes were depleted (P<0.02). HIV-1 infection of stimulated PBMC showed no differences in R5 or X4 infection across all MPA concentrations (P > 0.5).
Conclusions
CD3+CD8− T cell population of MPA-treated unstimulated PBMC were more susceptible to HIV-1 infection than untreated cells. The increased infection was partly due to monocytes and was lost when PBMC were exogenously stimulated. These data provide confirmation of a biological association between MPA exposure and increased susceptibility to HIV-1 infection, particularly among women who inject drugs.
Keywords: depoprovera, medroxyprogesterone acetate, HIV, contraception, injectables, infectivity, HIV acquisition
INTRODUCTION
There are currently over 18 million women worldwide infected with HIV-1, many of whom are of reproductive age (1). These women require access to safe and effective contraception, which prevents maternal deaths, unintended pregnancy, unsafe abortions, and infant morbidity and mortality (2). However, several observational studies have suggested that hormonal contraception, particularly injectable medroxyprogesterone acetate (MPA), increases a woman’s susceptibility to sexual HIV-1 infection (3–7). In one large prospective study in HIV-1 serodiscordant couples, MPA was associated with a 2-fold (adjusted hazard ratio 2.05, 95% confidence interval 1.04–4.04) higher risk of HIV-1 acquisition compared to non-hormonal contraceptive methods (5). The impact of hormonal contraception on HIV acquisition among women who inject drugs has received little investigative attention (8).
MPA is different from other progestin contraceptives since it is a derivative of progesterone, similar to the progesterone rods used in macaque simian immunodeficiency virus transmission studies, while other progestin contraceptives are mostly derivatives of testosterone (9). Whereas all progestin contraceptives bind the progesterone receptor (PR) (10), MPA is one of relatively few progestins that is also a partial to full agonist of the glucocorticoid receptor (GR). Sex steroid hormones that signal via GR and PR may influence antigen presentation and T cell responses, and the number and function of immune cells (11–13). MPA binds to the GR, which is expressed on peripheral mononuclear blood cells (PBMC), with greater affinity than cortisol (14). Thus MPA could potentially impact susceptibility to HIV-1 infection.
Previous in vitro studies have given mixed results concerning the influence of MPA on HIV-1 infection. However, some of these studies focused on the effect of progesterone and other PR agonists, but not MPA (15, 16). Additionally, other studies used exogenous stimulation of primary cells prior to MPA exposure, which may not replicate normal physiologic responses. This exogenous stimulation activates CD4+T cells and renders them more efficient at HIV-1 infection and/or replication (17,18).
Hence, the purpose of this study is to determine the effect of MPA on HIV-1 infection in more clinically relevant conditions. We used freshly isolated and purified PBMC without exogenous stimulation (19), and concentrations of MPA similar to those measured in vivo after MPA injection to closely recapitulate susceptibility to HIV-1 infection in women using the drug (20). We used an assay with a wide dynamic range and sensitivity to detect differences in a single round of infection. Initially, we studied non-stimulated PBMC from male donors and later added female donors to determine if there were sex differences.
MATERIALS AND METHODS
Study participants
Blood was obtained from healthy female volunteers in the follicular-phase of the menstrual cycle who denied use of exogenous hormones and from healthy adult males. We selected the follicular-phase of the menstrual cycle when immune defenses are purportedly high to reduce any confounding effect of the hypothesized increased susceptibility to HIV infection that occurs in the luteal phase of the menstrual cycle (21,22). The Johns Hopkins Institutional Review Board approved this study. All donors provided written, informed consent.
Drug dilutions
MPA (Sigma-Aldrich, St. Louis, Missouri) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at a concentration of 600 ng/mL and then diluted in phenol-red free RPMI 1640 (Gibco Laboratories, Grand Island, NY) to concentrations ranging from 0.003 ng/mL to 5ng/mL. These concentrations represent physiologic serum concentrations after administration of either 150mg MPA intramuscularly or 104mg subcutaneously. Typically, serum MPA concentration plateaus at approximately 1.0 ng/mL for about 12 weeks, then it declines (20). Final concentration of DMSO was < 0.1%.
Cell cultures
Fresh PBMC were isolated from whole blood using Ficoll (GE Healthcare Biosciences, Pittsburgh, PA) density gradient centrifugation. Previous studies demonstrated that the combination of phytohemagglutinin (PHA) and interleukin (IL)-2 can activate PBMC populations via a pathway that requires monocytes or soluble monocyte products such as IL -1 and IL-6 (23, 24). Hence, for activation experiments, PBMC were cultured in medium containing 0.5 µg/mL PHA and 100 U/mL of IL-2 for 72-hrs. Otherwise, cells were cultured in phenol-red free RPMI 1640 supplemented with 10% charcoal stripped, heat-inactivated fetal bovine serum (FBS, Gibco) and 12mM HEPES. For infections, 6 × 105 PBMC or 1.5 × 105 CD4+T cells per well were incubated with MPA for 18-hrs before infection in round-bottom 96-well plates at 37°C in 5% CO2 in duplicate. Negative control wells contained DMSO. In one infection experiment, cells were washed twice with PBS to remove MPA from the culture media before pseudotyped virus was added; while MPA remained in the culture media for all other infection experiments.
For experiments involving isolation of CD14+ cells (monocytes), PBMC were stained with CD14-PE (phycoerythrin Becton Dickinson [BD], San Jose, CA) after incubation with Fc receptor blocker (Miltenyi, San Diego, CA) for 10 minutes at room temperature. The live CD14+ monocyte population was separated from the CD14− population using a FACSAria III cell sorter (BD). For experiments involving CD4+ T cells, CD4+T cells were negatively selected from stimulated or unstimulated PBMC using the Miltenyi human CD4+T cell Isolation Kit II (Gladbach, Germany) according to the manufacturer’s instructions. Purity was confirmed using flow cytometry with CD3-phycoerythrin Cyanine5 (PE Cy5; BD) and CD4-fluorescein isothiocynate (FITC; BD).
Infectivity assay
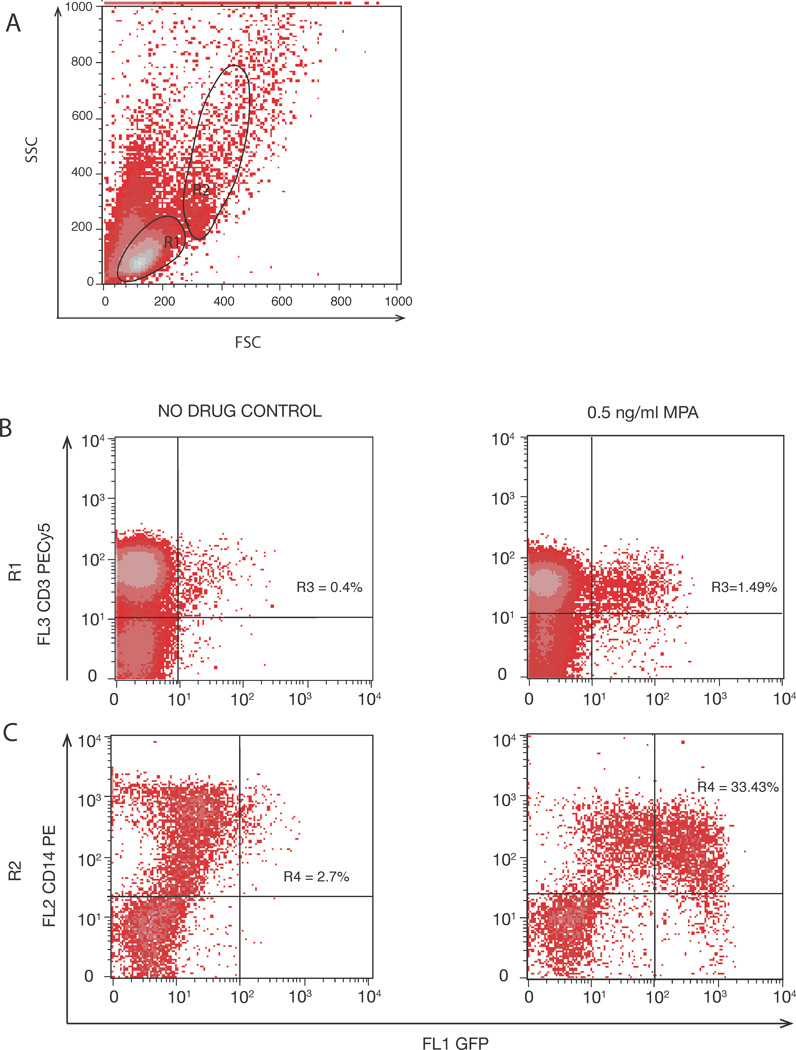
X4- and R5-tropic pseudoviruses were generated as previously described (25). Briefly, HEK293T cells were cotransfected with X4 orR5 env vectors and an NL43 plasmid in which green fluorescent protein (GFP) was inserted into the env open reading frame (NL43eGFP). The envelope was provided in trans by co-transfection with a CCR5-tropic or CXCR4-tropic envelope expression vector. The pseudotyped virus particles contain functional HIV-1 Env protein and infect target cells in a physiologic, Env–dependent manner; however the infection is limited to a single cycle because the pseudotyped virus does not express the env gene and thus the next round of replication results in non-functional virions. X4 or R5 pseudoviruses (50ng or 100ng p24, respectively) were added per well and infection was initiated through spinoculation at 1200 × g for 2 hours (26). After spinoculation, cultures were incubated at 37°C for 72-hrs. Cells were washed, treated with an Fc receptor blocking agent (Miltenyi) to minimize nonspecific binding, and stained with labeled CD3 (PE Cy5) and CD8 (APC) antibodies on ice for 25 minutes in the dark. CD4 expression was not analyzed because it is down regulated on infected cells (27, 28); instead CD4+ T cells were defined as CD3+CD8− cells. After incubation, plates were washed twice with PBS, centrifuged at 335 × g for 10 minutes, and fixed with 5% paraformaldehyde. Flow cytometry (BD FACSCalibur) was performed, and 100,000 – 200,000 events were acquired. Fluorescence emitted by the cells carrying GFP-expressing virus was quantified using CellQuest software (BD). To assess which cell populations were infected, we first gated on live populations of lymphocytes (R1) and monocytes and other cell populations (R2) (Figure 1). The percentage of infected cells in each experimental well was divided by the percentage of infected cells in MPA-free cultures and reported as relative infection.
FIGURE 1. Representative flow cytometry analysis showing gating strategy.
(A) Viable lymphocytes (R1) and non-lymphocytes (R2) in PBMC cultures. (B) Representative dot plots show an increase in percentage of CD3+ GFP+ cells in R1. (C) Representative dot plots show an increase in percentage of CD14+ GFP+ cells in R2 following treatment with MPA.
Entry assay
X4- and R5-tropic single-cycle, pseudoviruses that incorporate the enzyme β-lactamase fused to the viral accessory gene vpr were made as previously described (29). X4 and R5 pseudotyped viruses were used to infect 200,000 MPA-treated PBMC. After spinoculation, viruses were left to fuse with the target T cells for 2hr at 37°C. Cells were washed once and then incubated with CCF2-AM, a cell-permeating substrate (Invitrogen) for 1-hr at room temperature. 1µM T20 was added to stop any further fusion events. Cells were washed twice, then subsequently incubated overnight in CO2-Independent Medium (Invitrogen) supplemented with 10% charcoal stripped FBS. CCF2 is a fluorescence resonance energy transfer (FRET) substrate that emits at 520nm when excited by a 405nm laser. When the substrate is cleaved by the enzyme β-lactamase, the dyeemits at 447nm. This change in emission can be monitored by flow cytometry. After 12-hr, the cells were fixed with 5% paraformaldehyde and analyzed on FACSCalibur. Percentage of PBMC in MPA-containing cultures that had undergone a fusion event was divided by the percentage of PBMC in MPA-free cultures to give a value for relative fusion.
Immune activation markers and HIV-1 co-receptor expression
2 × 105 PBMC were incubated in MPA concentrations ranging from 0.003 ng/mL to 3 ng/mL. Cells were washed once, treated with Fc receptor blocking agent (Miltenyi), and stained with fluorochrome-conjugated antibodies (BD Biosciences) specific for the following cell surface markers: CD3 and CD4 or CD14; CCR5 (CD195 clone 3A9:APC) or CXCR4 (CD184; APC), CD25, CD69, HLA-DR, CD38, and CD16. PHA-IL2 activated cells and DMSO-treated cells were used as positive and negative controls, respectively. Cells were incubated on ice for 25 minutes in the dark, washed, and fixed with 5% paraformaldehyde. Percentage of CD3+CD4+ or CD3−CD14+ cells expressing surface markers, and median fluorescence intensity (MFI), were quantified using flow cytometry within 24hrs. At least 50,000 events were acquired.
Statistical analysis
Experimental replicates were averaged. Summary statistics were reported as median and interquartile range (IQR). Differences in absolute and relative infection between cells treated with varying MPA concentrations or between cell types were analyzed using a non-parametric test. Significance was defined as P<0.05. Bonferroni method was used to correct for multiple comparisons in some analyses. For immunofluorescence analyses, data from MPA-treated cells were pooled and compared to no-drug controls, and weighted means ± SEM were calculated. GraphPad Prism 6.0 (GraphPad Software, LaJolla, CA) was used to perform the analyses.
RESULTS
MPA increases infection of unstimulated PBMC by X4 and R5 pseudotyped HIV-1
The fraction of cells expressing GFP was measured 72-hrs after MPA-treated PBMC were infected. Lymphocyte viability was > 70% in all cultures. In unstimulated PBMC, the maximum infection was 1.43% in CD3+CD8− T cells infected with R5-tropic pseudovirus (n=21 unique donors), whereas the maximum absolute infection was 4.87% in T cells infected with X4-tropic pseudovirus (n=13 unique donors). Infection varied from donor to donor, probably due to differences in baseline activation. There were no differences in infection between male and female donors, hence the results are not stratified by sex.
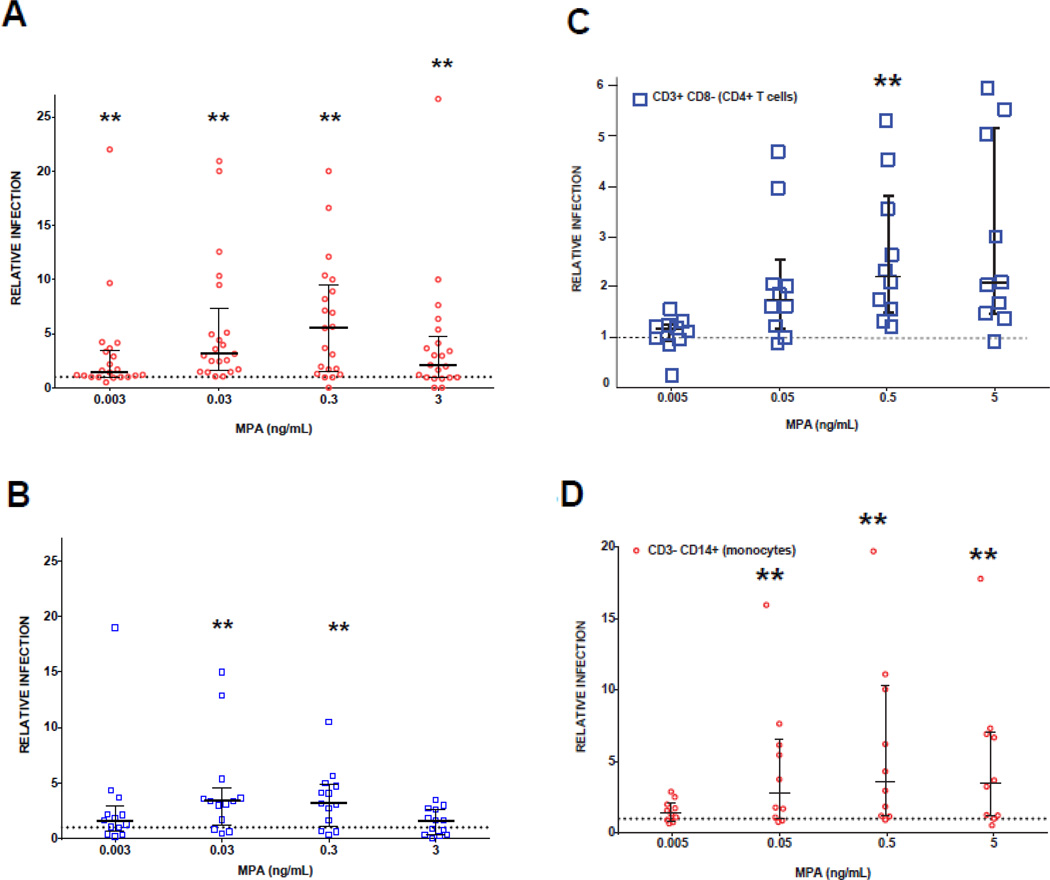
Absolute infection was greater among MPA-treated T cells versus untreated cells across all MPA concentrations (0.003 to 3 ng/mL) using R5 pseudovirus (P<0.003), and at concentrations 0.03 ng/mL and 0.3 ng/mL using X4 pseudovirus (P<0.005). Relative R5 and X4 infection of T cells is demonstrated in Figure 2A and B. Attypical in-vivo MPA concentrations (0.003, 0.03, 0.3, and 3ng/mL), relative R5 infection was 1.5–5.5 fold greater than control (median 1.42 IQR [1 – 3.49], 3.13 [1.59 – 7.3], 5.52 [1.5 – 9.46], 2.1 [0.95 – 4.75], respectively). Relative X4 infection of MPA-treated cells was also 1.6–3.8 fold greater than control (1.6 [0.68 – 2.95], 3.38 [1.25 – 4.5], 3.18 [1.14 – 4.87], 1.63 [0.36 – 2.66], respectively). Infection increased in a dose-response manner but decreased at the highest concentration, which showed decreased cell viability. Washing MPA from PBMC cultures before spinoculation did not change the results (data not shown).
FIGURE 2. Relative HIV-1 infection (compared to no drug controls) of MPA treated PBMC.
Lymphocyte population (R1) within PBMC were infected with (A) R5 pseudotyped virus (n=21 donors) and (B) X4 pseudotyped virus (n=13 donors). PBMC were stained to identify (C) CD4+ cells and (D) CD14+ cells (n=10 donors) after infection with R5 pseudotyped virus. Each symbol represents data from 600,000 PBMCs/well, in duplicate, from individual blood donors. Relative infection is defined as % GFP expression in MPA treated cells divided by % GFP expression in cells without MPA. Values > 1 represent increased infection and are plotted above the dotted reference line of 1 on the y -axis. Error bars are median and IQR. Wilcoxon signed rank test was done using Bonferroni correction for multiple comparisons. Each drug concentration is compared to a value of 1. **Significance is P < 0.0125
To determine the cell types infected within gates R1 and R2, we stained PBMC after infection. First, we looked for CD3+ cells expressing GFP that were negative for CD8 and identified these as infected CD4+ T cells (Figure 1B and C). We identified infected monocytes as CD3− CD14+ cells expressing GFP within the R2 population. Both CD4+T cells and CD3− CD14+ monocytes were found to be more susceptible to infection after pre-treatment with MPA (Figure 2C and D, n=10 unique donors). Relative infection of CD4+ T cells ranged from 1.1 – 2-fold higher than controls at varying MPA concentrations using R5 virus, and 1.4 – 3.6-fold higher in CD3− CD14+ monocytes from MPA-treated PBMC cultures than controls. There was a statistically significant increase in relative infection in MPA-treated cultures compared to no drug controls at 0.5ng/mL for CD4+T cells and 0.05–5ng/mL for CD3− CD14+ monocytes (P<0.0125 for all).
Increased PBMC infection in the presence of MPA may be mediated by CD14+ monocytes
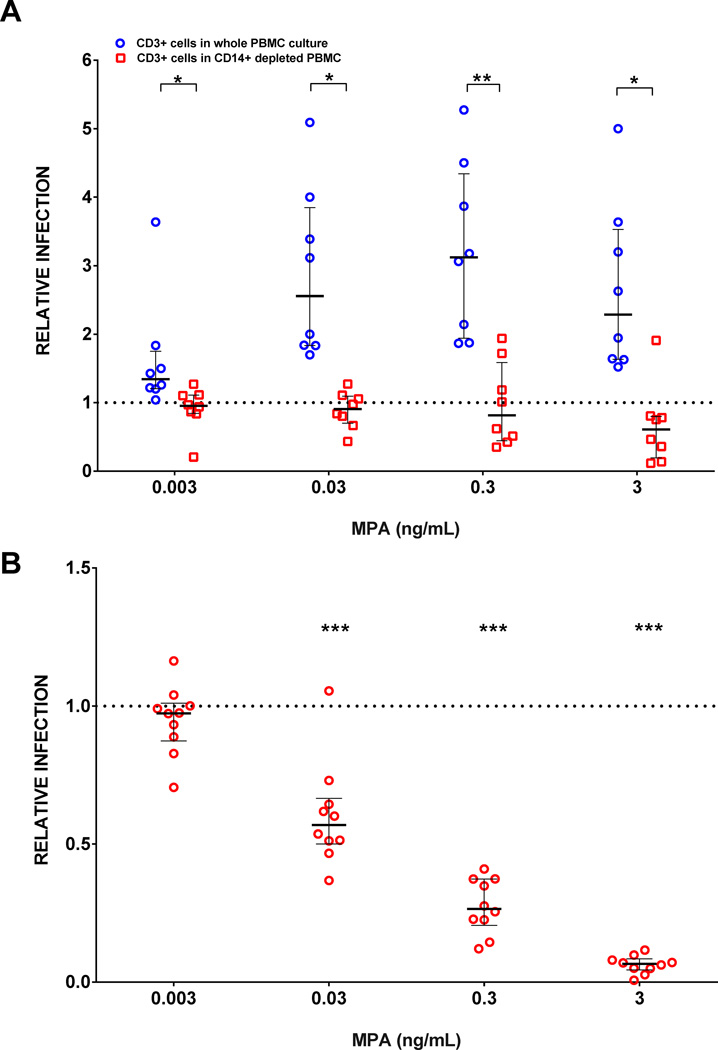
Because CD14+ cells showed greater relative infection compared to CD4+ T cells within the same PBMC culture, we performed paired experiments to determine the impact of depleting CD14+ cells from whole PBMC cultures. Relative R5 virus infection was compared between whole MPA-treated PBMC cultures to depleted MPA-treated PBMC of CD14+ cells. There was increased relative R5 virus infection of CD3+CD8− cells in whole PBMC cultures but not after CD3−CD14+ cells were depleted (Figure 3A, n=8 unique donors). Additionally, there was significantly greater relative R5 virus infection in whole PBMC cultures compared to CD14+ depleted PBMC cultures (P<0.02 for all concentrations).
FIGURE 3. Differences in CD3+CD8− cell infection between whole PBMC versus CD3−CD14+ depleted PBMC cultures, and isolated CD4+ T cells.
(A) Whole PBMC and CD14+ depleted PBMC cultures were treated with MPA and infected with R5-pseudotyped virus. Each circle and square represents data from 600,000 PBMCs/well, in duplicate, from individual blood donors in paired experiments (n=8). Relative infection of CD3+CD8− cells is defined as % GFP expression in MPA treated cultures divided by % GFP expression in cells without MPA. Values > 1 represent increased infection and are plotted above the dotted reference line of 1 on the y -axis. Error bars are median and IQR. Differences in CD3+CD8-T cell infection between whole and CD14+ depleted PBMC were compared at each drug concentration using Mann-Whitney test. *P < 0.009, **P<0.02. (B) CD4+ T cells were purified from fresh PBMC and infected with R5-pseudotyped virus. They were not stimulated with PHA/IL-2. Each circle represents data from 150,000 CD4+ T cells/well, in duplicate, from an individual blood donor (n=10). Relative infection is defined as % GFP expression in MPA treated cultures divided by % GFP expression in cells without MPA. Values > 1 represent increased infection and are plotted above the dotted reference line of 1 on the y -axis. Error bars are median and IQR. Wilcoxon signed rank test was done using Bonferroni correction for multiple comparisons. Each drug concentration is compared to a value of 1. ***Significance is P < 0.0125.
HIV infection of isolated CD4+ T cells decreases with MPA treatment
In contrast to the results of unfractionated PBMC, when CD4+ T cells were isolated by negative selection and incubated with MPA, R5 infection decreased in a dose-response manner (Figure 3B).
In vitro activation minimizes the effect of MPA
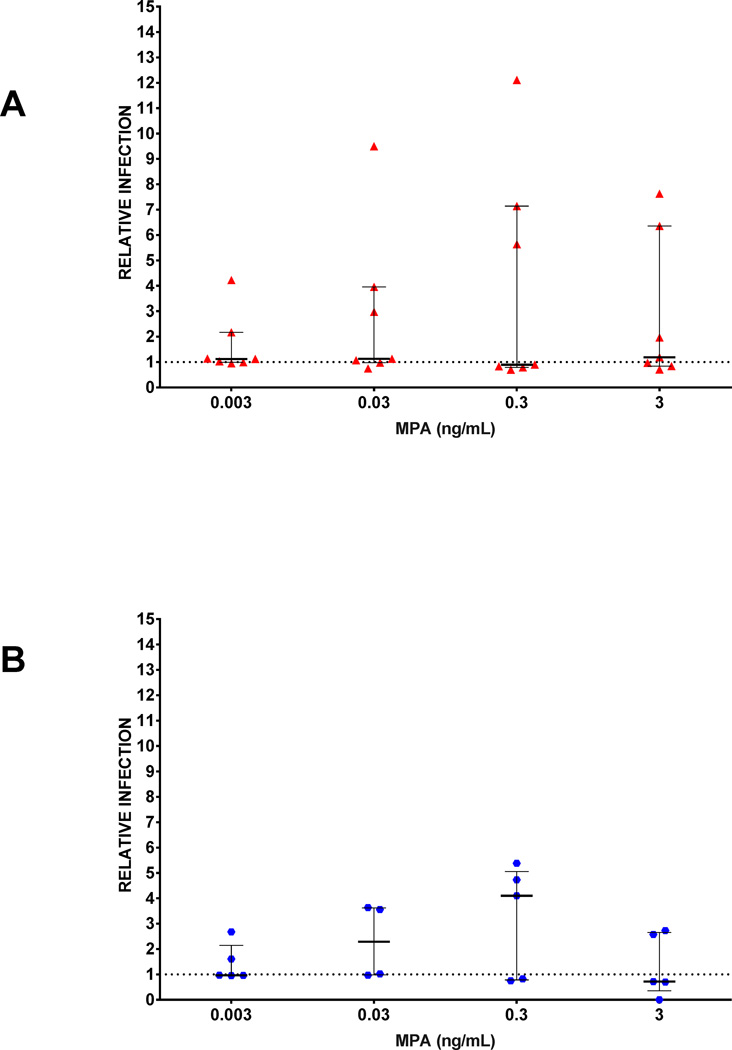
Because activation of cells modulates co-receptor density, which, in turn, affects susceptibility to infection (30–32), for some experiments, we activated PBMC using PHA/IL-2 for 72-hrs (Figure 4 [n=7 and n=5 unique donors for R5 and X4, respectively]). In stimulated PBMC, MPA infection was no different than control across varying concentrations of MPA (P > 0.5).
FIGURE 4. Effect of stimulation of PBMC with PHA/IL-2on susceptibility to infection.
PBMC were stimulated with PHA/IL-2 for 72 hours prior to infection with (A) R5 pseudotyped virus (n=7 donors) and (B)X4 pseudotyped virus (n=5 donors). Each symbol represents data from 600,000 PBMCs/well, in duplicate, from an individual blood donor. Relative infection is defined as % GFP expression in MPA treated cultures divided by % GFP expression in cells without MPA. Values > 1 represent increased infection and are plotted above the dotted reference line of 1 on the y -axis. Error bars are median and IQR. Wilcoxon signed rank test was done using Bonferroni correction for multiple comparisons. Each drug concentration is compared to a value of 1. All comparisons were NS.
MPA-mediated increase in HIV infection of PBMC occurs post-entry
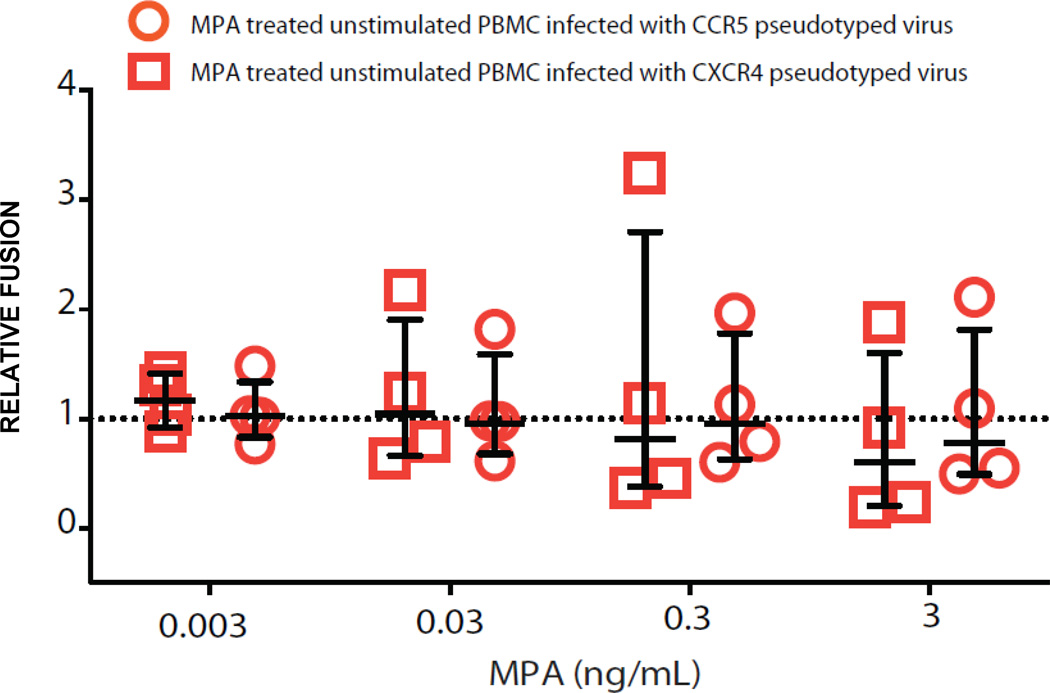
It is possible that the increase in infectivity observed may be a result of MPA-induced entry of the virus into PBMC. Hence, we used a fusion assay that makes use of a β-lactamase reporter to determine if MPA causes increased fusion of virus to target cells. We found that there are no differences in fusion of virus to MPA-treated target cells and controls (Figure 5, n=4 unique donors). This data suggests that the mechanism of increased infection with MPA most likely occurs following fusion within of the viral life cycle.
FIGURE 5. Fusion events of MPA-treated PBMC using R5 and X4-pseudotyped viruses.
A β-galactosidase fusion assay was performed to measure fusion events of HIV-1 to PBMC after MPA treatment compared to controls. Each circle and square represent data from 200,000 PBMCs/well, in duplicate, from individual blood donors (n=4). Relative fusion is defined as % of MPA treated PBMC that underwent a fusion event divided by the % of PBMC in MPA-free cultures. Values > 1 represent increased infection and are plotted above the dotted reference line of 1 on the y -axis. Error bars are median and IQR.
MPA does not have an effect on early or late immune activation markers, CXCR4, or CCR5 HIV co-receptor expression
Constitutive expression of activation markers and HIV-co-receptor expression was analyzed after incubation of PBMC samples for 24 and 48-hrs (n=8 unique donors). At 24-hrs, there were no differences between MPA-treated cultures at each drug dilution compared to no-drug controls in the percentage of CD3+CD4+ T cells expressing CD25 (1.47 ± 0.25 MPA vs. 2.18 ± 0.25 control, P=0.71), CD69 (8.56 ± 1.05 MPA vs. 9.52 ± 0.79 control, P=0.27), CD38 (1.52 ± 0.57 MPA vs. 2.03 ± 0.61 control, P=0.95), HLA-DR (40.1 ± 4.18% MPA vs. 41.9 ± 3.88 control, P=0.41), CCR5 (1.47 ± 0.41 MPA vs. 1.81 ± 0.42 control, P=0.6), or CXCR4 (94.48 ± 2.11 MPA vs. 87.19 ± 3.5 control, P=0.12). Additionally, treatment with MPA for 48-hrs did not have an effect on percentage of cells expressing activation markers or the MFI of staining of CD3+CD4+ T cells. For CD3−CD14+ monocytes, incubation with MPA for 24 or 48-hours altered neither expression of these activation markers nor the co receptors CCR5 and CXCR4. For this same subset, there was no difference in CD16 expression after 24 hours (data not shown). To capture earlier and later time points, experiments were repeated in two donors. Expression of activation markers and co-receptors in MPA treated cultures was not different from no-drug controls at 1, 2, 3, 6, 12, 72, and 96-hrs (data not shown).
DISCUSSION
To our knowledge, this is the first report of increased HIV-1 infection of MPA-treated, unstimulated PBMC in a dose dependent manner that required the presence of monocytes. The results indicate that PBMC exposed to MPA, even for 16–18 hours, are more susceptible to HIV-1 infection than untreated cells. Moreover, our data clearly demonstrate that this difference in susceptibility to infection is lost when cells are exogenously stimulated. Lastly, we observed a dose-response relationship for both CD4+ T cells and monocytes. These data provide confirmation of a biological association between MPA exposure and increased susceptibility to HIV-1 infection.
The precise molecular mechanism by which MPA increases susceptibility of PBMC to HIV-1 infection is yet to be determined. However, we eliminated several possibilities. First, increased infection was still observed when MPA was removed from the culture media by washing the cells, suggesting that MPA has an effect on the target cells rather than the virus. Second, direct quantitation of viral entry demonstrated no difference between MPA-treated PBMC and untreated control PBMC. Furthermore, immunofluorescence staining for CXCR4 and CCR5 showed no significant differences between MPA-treated PBMC and controls. Hence, our results suggest a mechanism of increased infectivity that predominantly affects post-entry steps in the virus life cycle. Resting CD4+ T cells are relatively non-permissive for HIV-1 infection, but can be rendered permissive by various forms of T cell activation (17). However, we did not detect significant differences between MPA-treated PBMC and controls in the expression of the activation markers CD25, CD69, CD38 and HLA-DR. Last, depletion experiments suggest that MPA’s action may be dependent upon the presence of monocytes. This hypothesis is supported by the observation that CD4+ T cells cultured alone did not show increased susceptibility to HIV-1 infection on exposure to MPA. Rather, a slight protection against HIV-1 infection was observed. This difference in susceptibility to infection suggests that CD4+ T cells cultured alone with hormones may behave differently compared to those cultured in the presence of other cell types such as monocytes present in the PBMC fraction. This observation is important, especially in the light of recent studies of CD4+ T cell cultures that concluded that estrogen confers protection against HIV-1 infection (33).
Our findings are in contrast to Vassiliadou, et al., who did not find increased HIV-1 replication in progesterone treated, PHA-stimulated PBMC (15). However, there are several major methodological differences. First, progesterone was used in Valissaidou’s experiments; this hormone is distinct from MPA. Not all progestogens are alike, and some bind different receptors, resulting in different biological effects (9,12). Second, in order to simulate physiologic conditions, we performed experiments with and without exogenous stimulation with PHA. Our results reveal that there are distinct differences in HIV-1 in-vitro infection between unstimulated and stimulated MPA-treated cell cultures. Last, we used a more sensitive HIV-1 infectivity assay that is able to quantify infection at the single-cell level.
Our findings are consistent with Hujbrets, et al., who demonstrated in stimulated PBMC that MPA increased HIV-1 replication (34). However, as our data and others have shown, stimulation of primary cells alters HIV-1 infection (17,18). Additionally, the authors suggest that MPA decreases production of IFN-γ, IL-2 and TNF-α, which leads to the suppression of innate and adaptive mechanisms of immune control and subsequent increased susceptibility to HIV-1 infection. Although we did not measure cytokines or chemokines, this proposed mechanism contrasts other studies. For example, higher systemic inflammation and innate immune activation were independently associated with HIV-1 seroconversion in one microbicide trial (35). Women who seroconverted had significantly higher levels of pro-inflammatory and T-cell cytokines TNF-α, IL-2, IL-7, and IL-12p70, and higher proportions of activated natural killer (NK) cells expressing HLA-DR and CD69, compared to women who did not seroconvert. Furthermore, decreased immune activation and cytokine production are associated with lower viremia and better outcomes in HIV-1 infection (33,36). The significance of these contrasting findings is unknown.
The strengths of our study include use of a simple, sensitive, quantitative, and physiologically sound assay. Second, to simulate physiologic conditions, we performed experiments without exogenous stimulation, and we demonstrated that use of exogenous stimulation can alter results. Without exogenous stimulation, we report lower levels of infection overall and greater inter-donor variation compared to exogenous stimulation using PHA, cytokines and/or other methods of cell surface receptor engagement, which might yield higher infection and less inter-donor variation. Third, experiments using unstimulated PBMC were repeated in more than 20 individual blood donors, which increased accuracy. Last, we tested our hypothesis using both CCR5 and CXCR4 pseudotyped viruses to assess differences in viral tropism.
However, there are limitations. We chose to use PBMC as opposed to genital tract cells, thus our findings are more applicable to systemic HIV-1 infection like injection drug use (IDU), than sexual HIV-1 infection. Although there may be phenotypic differences between peripheral and genital tract cells, there are no data demonstrating relevant functional differences. In fact, one study showed that vaginal macrophages are monocyte-like and permissive to HIV-1 replication (37). Additionally, while use of pseudotyped viruses provides a precise quantitative measurement of viral infection, it does not measure the events in the viral life cycle that occur after protein (GFP) expression. It is thus possible that there are differences in HIV-1 budding from CD4+ T cells from MPA-treated cultures versus controls.
The question of whether MPA increases susceptibility to HIV-1 acquisition has critical medical and public health ramifications. Over 100 million doses of MPA have been distributed worldwide, and the drug is commonly used by women of low socioeconomic status who often reside in areas of high HIV-1 prevalence (38). Some proponents have argued that the reported associations between MPA use and increased susceptibility to sexual HIV-1 infection may be artificial and attributable to confounding factors such as behavioral factors, i.e. sex without condoms. While this argument is reasonable, it is not supported by conclusive evidence, and does not explain why this association has not been consistently observed in women on oral contraceptive pills (39). Women at high risk for sexual HIV-1 infection should be made aware that MPA does not protect against STDs including HIV, and therefore barrier methods should be used. Importantly, our results suggest that future research is needed on the potential impact of MPA on HIV acquisition among women who inject drugs. Although provocative, it is unclear if our in vitro findings can be extrapolated to current users of injectable MPA. Further investigation is warranted.
ACKNOWLEDGEMENTS
We would like to thank the volunteers for their blood donations and Dr. Craig Hendrix for his critique of the manuscript.
FUNDING
This work was supported by Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Award (J.S.C.), NIH grant R01 1R01AI110371-01 (J.S.C.), NIH R01 AI081600 (R.F.S.) and the Howard Hughes Medical Institute (R.F.S.).
Footnotes
Author contribution: M.S., G.L., J.B., R.S., and J.C. conceived the experiments. M.S., G.L. and J.C performed experiments. M.S. and J.C. wrote the paper. J.B. and R.S. corrected the manuscript.
The authors have no conflict of interest.
REFERENCES
- 1.World Health Organization. Data and Statistics HD, ed. Geneva: 2013. Global Summary of the AIDS Epidemic. [Google Scholar]
- 2.Division P, editor. United Nation Department of Economic Social Affairs. World Contraceptive Use. New York: 2009. [Google Scholar]
- 3.Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21:1771–1777. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 4.Bulterys M, Chao A, Habimana P, Dushimimana A, Nawrocki P, Saah A. Incident HIV-1 infection in a cohort of young women in Butare, Rwanda. AIDS. 1994;8:1585–1591. doi: 10.1097/00002030-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapiga SH, Lyamuya EF, Lwihula GK, Hunter DJ. The incidence of HIV infection among women using family planning methods in Dar es Salaam, Tanzania. AIDS. 1998;12:75–84. doi: 10.1097/00002030-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Wang CC, McClelland RS, Overbaugh J, et al. The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS. 2004;18:205–209. doi: 10.1097/00002030-200401230-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kral AH, Bluthenthal RN, Lorvick J, Gee L, Bacchetti P, Edlin BR. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. Lancet. 2001;357:1397–1401. doi: 10.1016/S0140-6736(00)04562-1. [DOI] [PubMed] [Google Scholar]
- 9.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Koubovec D, Ronacher K, Stubsrud E, Louw A, Hapgood JP. Synthetic progestins used in HRT have different glucocorticoid agonist properties. Molecular and cellular endocrinology. 2005;242:23–32. doi: 10.1016/j.mce.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, Wira CR. Influence of the estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol. 1998;39(3):209–216. doi: 10.1111/j.1600-0897.1998.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 12.Kontula K, Paavonen T, Luukkainen T, Andersson LC. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem Pharmacol. 1983;32(9):1511–1518. doi: 10.1016/0006-2952(83)90474-4. [DOI] [PubMed] [Google Scholar]
- 13.Chandra N, Thurman AR, Anderson S, Cunningham TD, Yousefieh N, Mauck C, Doncel GF. Depot Medroxyprogesterone Acetate Increases Immune Cell Numbers and Activation Markers in Human Vaginal Mucosal Tissues. AIDS Research and Human Retroviruses. 2013:592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szekeres-Bartho J, Weill BJ, Mike G, Houssin D, Chaouat G. Progesterone receptors in lymphocytes of liver-transplanted and transfused patients. Immunology letters. 1989;22:259–261. doi: 10.1016/0165-2478(89)90162-4. [DOI] [PubMed] [Google Scholar]
- 15.Vassiliadou N, Tucker L, Anderson DJ. Progesterone-Induced Inhibition of Chemokine Receptor Expression on Peripheral Blood Mononuclear Cells Correlates with Reduced HIV-1 Infectability In Vitro. Journal of Immunology. 1999;162(12):7510–7518. [PubMed] [Google Scholar]
- 16.Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL. Estradiol and Progesterone Regulate HIV Type 1 Replication in Peripheral Blood Cells. AIDS Research and Human Retroviruses. 2008;24(5):701–716. doi: 10.1089/aid.2007.0108. [DOI] [PubMed] [Google Scholar]
- 17.McDougal JS, Mawle A, Cort SP, et al. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985;135:3151–3162. [PubMed] [Google Scholar]
- 18.O’Connell KA, Rabi SA, Siliciano RF, Blankson JN. Proc Natl Acad Sci U S A. 2011 Sep 13;108(37):E689–E698. doi: 10.1073/pnas.1108866108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabi SA, O'Connell KA, Nikolaeva D, Bailey JR, Jilek BL, Shen L, Page KR, Siliciano RF, Blankson JN. Unstimulated primary CD4+ T cells from HIV-1-positive elite suppressors are fully susceptible to HIV-1 entry and productive infection. J Virol. 2011;85(2):979–986. doi: 10.1128/JVI.01721-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishell DR., Jr Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med. 1996;41:381–390. [PubMed] [Google Scholar]
- 21.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, Kersh EN. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 1999;2011:261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 23.Unanue ER, Allen PM. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987;236:551. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- 24.Ceuppens, et al. Human T Cell Activation with Phytohemagglutinin. 1988. The Function of Il-6 as an Accessory Signal. The Journal of Immunology. 141(11):3868–3874. [PubMed] [Google Scholar]
- 25.Zhang H, Zhou Y, Alcock C, Kiefer T, Monie D, Siliciano J, Li Q, Pham P, Cofrancesco J, Persaud D, Siliciano RF. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol. 2004;78(4):1718–1729. doi: 10.1128/JVI.78.4.1718-1729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74(21):10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser P, Joos B, Niederost B, Weber R, Gunthard HF, Fischer M. Productive human immunodeficiency virus type 1 infection in peripheral blood predominantly takes place in CD4/CD8 double-negative T lymphocytes. J Virol. 2007 Sep;81(18):9693–9706. doi: 10.1128/JVI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia JV, Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350(6318):508–5011. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 29.Cavrois M, De Noronha C, Greene WC. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol. 2002;20(11):1151–1154. doi: 10.1038/nbt745. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Garcia M1, Biswas N, Patel MV, Barr FD, Crist SG, Ochsenbauer C, Fahey JV, Wira CR. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PLoS One. 2013;8(4):e62069. doi: 10.1371/journal.pone.0062069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll RG, et al. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 32.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced Human Immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 34.Huijbregts RP, Helton ES, Michel KG, et al. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154:1282–1295. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naranbhai V, Abdool Karim SS, Altfeld M, et al. Innate Immune Activation Enhances HIV Acquisition in Women, Diminishing the Effectiveness of Tenofovir Microbicide Gel. J Infect Dis. 2012;206:993–1001. doi: 10.1093/infdis/jis465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zangerle R, Steinhuber S, Sarcletti M, Dierich MP, Wachter H, Fuchs D, et al. Serum HIV-1 RNA levels compared to soluble markers of immune activation to predict disease progression in HIV-1-infected individuals. Int Arch Allergy Immunol. 1998;116(3):228–239. doi: 10.1159/000023949. [DOI] [PubMed] [Google Scholar]
- 37.Shen R, Richter H, Clements RH, Novak L, et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Vir April. 2009;83(7):3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keener T, editor. Efforts to Improve the Safety of Delivery of DMPA (Injectable Contraceptive) 2001. USAID Office of Population. [Google Scholar]
- 39.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect. Dis. 2013;13(9):797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]