Abstract
We previously demonstrated that resveratrol and clofarabine elicited a marked cytotoxicity on malignant mesothelioma (MM) MSTO-211H cells but not on the corresponding normal mesothelial MeT-5A cells. Little is known of the possible molecules that could be used to predict preferential chemosensitivity on MSTO-211H cells. Resveratrol and clofarabine induced down-regulation of Mcl-1 protein level in MSTO-211H cells. Treatment of cells with cycloheximide in the presence of proteasome inhibitor MG132 suggested that Mcl-1 protein levels were regulated at the post-translational step. The siRNA-based knockdown of Mcl-1 in MSTO-211H cells triggered more growth-inhibiting and apoptosis-inducing effects with the resultant cleavages of procaspase-3 and its substrate PARP, increased caspase-3/7 activity, and increased percentage of apoptotic propensities. However, the majority of the observed changes were not shown in MeT-5A cells. Collectively, these studies indicate that the preferential activation of caspase cascade in malignant cells might have important applications as a therapeutic target for MM. [BMB Reports 2015; 48(3): 166-171]
Keywords: Chemosensitivity, Clofarabine, MeT-5A, MSTO-211H, Resveratrol
INTRODUCTION
Malignant mesothelioma (MM) is a lethal asbestos-associated neoplasm that arises from the mesothelial surface of the pleura and peritoneum. MM is characterized by poor responsiveness to current chemotherapeutic drugs, often attributable to evasion of apoptosis (1, 2). The resistance to apoptosis is a major problem that limits the effectiveness of chemotherapies used to treat MM. Therefore, establishment of new therapeutic strategies to activate the pro-apoptotic signals in cancer cells or to deprive cancer cells of the anti-apoptotic signals that sustain them is essential to overcoming chemoresistance in MM.
Apoptosis is triggered by various intracellular and extracellular signals, and is executed by recruiting proteases of the caspase family. There are two apoptotic pathways: the intrinsic pathway regulated by Bcl-2 and related proteins and the extrinsic pathway activated by the tumor necrosis factor or the Fas ligand (3). Whichever pathway is taken, both usually lead to the activation of caspases. The intrinsic apoptotic pathway is tightly regulated by the interplay between pro-apoptotic proteins (e.g. Bax, Bak and Bid) of the Bcl-2 family and anti-apoptotic proteins (e.g. Bcl-2, Mcl-1 and Bcl-xL). In most MM cell lines and tumor tissues, Bcl-2 expression is nearly undetectable, whereas Mcl-1 and Bcl-xL are frequently overexpressed (4, 5). These findings thus suggest that the resistance to apoptosis in MM could rather be related to Mcl-1 and/or Bcl-xL than to Bcl-2.
Mcl-1, a key anti-apoptotic member of the Bcl-2 family, inhibits cell death by suppressing the release of cytochrome c from mitochondria via binding and sequestering the pro-apoptotic protein Bak on the outer mitochondrial membrane (6). An increase in Mcl-1 expression has been shown in a number of human malignancies including leukemia (7, 8). Certain in vitro and in vivo studies have demonstrated that down-regulating Mcl-1 can sensitize human tumor cells to a variety of anticancer drugs including dacarbazine and sorafenib (9, 10). Likewise, the use of Mcl-1 antisense oligonucleotides to down-regulate Mcl-1 expression promoted apoptosis of the HA14-1 nonresponsive ovarian carcinoma cells in response to anticancer agent HA14-1 (11). Furthermore, Mcl-1 overexpression has actually been associated with poor response to chemotherapy, particularly malignant hematopoietic cells (12). Based on the available information, enhanced Mcl-1 expression appears to be an important factor in the cell survival and drug resistance. These findings suggest that inhibition of Mcl-1 may be an effective therapeutic strategy in the anticancer therapy.
We previously demonstrated that a naturally occurring phytochemical resveratrol and anticancer drug clofarabine synergistically caused a marked cytotoxicity on mesothelioma cells but not on the corresponding normal mesothelial cells, and exhibited their cytotoxic effects via suppression of multi-targets including PI3-kinase, Sp1, and Nrf2 (13, 14). This indicates that simultaneous targeting of multiple biological pathways is required for the efficient suppression of MM cell survival. However, the precise molecular mechanism of how this combination treatment exerts a preferential apoptosis-activating effect on MM cells has not been clearly determined. It can provide a clue to predict systematic toxicity, and the responsiveness of MM cells to certain apoptosis-inducing therapies. In the present study, we report that simultaneously treating with resveratrol and clofarabine efficiently elicited apoptosis by down-regulating Mcl-1 protein levels and activating caspasesdependent pathway in MM MSTO-211H cells, whereas MeT-5A cells had resistance to apoptosis induction by up-regulating Mcl-1 and suppressing caspase activation.
RESULTS
Resveratrol and clofarabine decreases Mcl-1 protein level in MSTO-211H cells
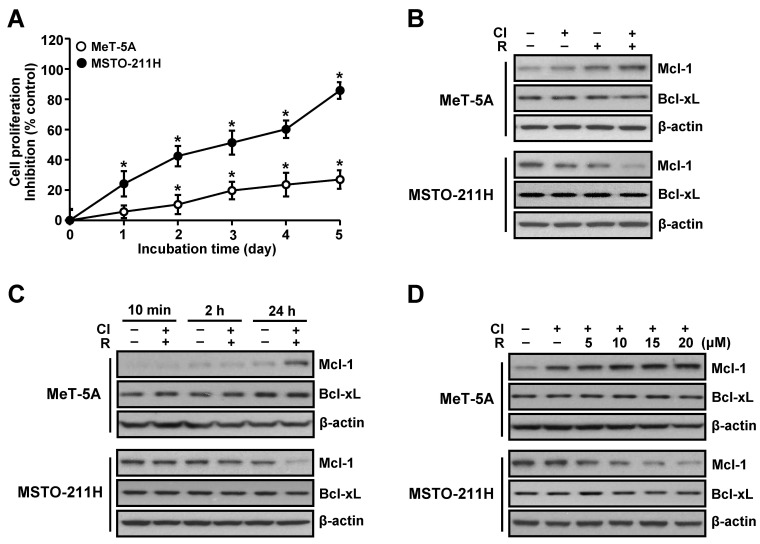
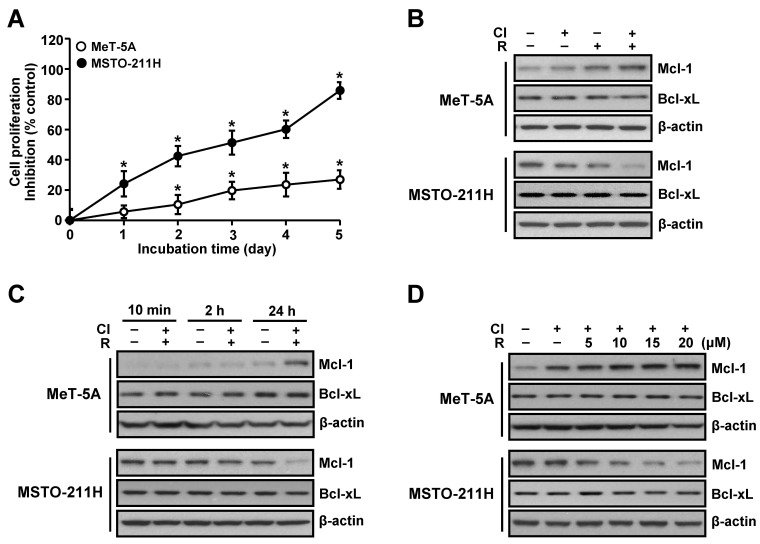
To ascertain whether differential effect of co-treatment with resveratrol and clofarabine on chemosensitivity in normal mesothelial MeT-5A cells and MM MSTO-211H cells is a result of dysregulation of anti-apopototic proteins, we initially measured the levels of Mcl-1 and Bcl-xL proteins. Interestingly, the Mcl-1 level was inversely correlated with chemosensitivity. MeT-5A cells, which showed resistance to this combination treatment, exhibited increased level of Mcl-1 protein, whereas MSTO-211H cells, which were highly sensitive to this combination treatment, exhibited reduced level of Mcl-1 protein (Fig. 1A and B). A time-response experiment showed that the effect of the combination treatment on Mcl-1 level was evident at 24 h treatment (Fig. 1C) and also further confirmed in a dose-dependent experiment (Fig. 1D). Of note, no significant changes on Bcl-xL levels were noted in these conditions.
Fig. 1. Effects of resveratrol and clofarabine on Mcl-1 and Bcl-xL protein levels in MeT-5A and MSTO-211H cells. (A) Cells were co-treated with resveratrol (15 μM) and clofarabine (40 nM) from 1 to 5 days, and the percentage of viable cells was determined by using the MTT assay. (B) Cells were treated with resveratrol (15 μM) and clofarabine (40 nM), alone or in combination, for 24 h. (C) Cells were cotreated with resveratrol (15 μM) and clofarabine (40 nM) for the indicated times. (D) Cells were co-treated with resveratrol (0, 5, 10, 15, and 20 μM) and clofarabine (40 nM) for 24 h. Whole cell lysates were analyzed by Western blot analysis using antibodies against Mcl-1, Bcl-xL, and β-actin. Error bars represent the mean ± SEM for three independent experiments. Cl, clofarabine; R, resveratrol. *P < 0.05 compared to respective controls.
Mcl-1 level is regulated at posttranslational step
The basal level of Mcl-1 protein was much higher in MSTO-211H cells than in MeT-5A cells, while that of Bcl-xL was lower in MSTO-211H cells (Fig. 2A). However, there were no detectable changes in the Mcl-1 mRNA levels in any of MeT-5A and MSTO-211H cells in response to resveratrol and clofarabine (Fig. 2B). In contrast, the amount of Mcl-1 protein upon the combined treatment was significantly increased by the pretreatment with proteasome inhibitor MG132 (Fig. 2C). Next, we analyzed protein turnover in cycloheximide (CHX) chase experiments in the presence of MG132. The Mcl-1 level was gradually declined over 160 min of treatment with CHX alone, with more rapidity in the basal turnover of MeT-5A cells compared to MSTO-211H cells (Fig. 2D). This effect remained unchanged following co-treatment with resveratrol and clofarabine, while MG132 treatment could restore the decrease of Mcl-1 level mediated by CHX to approximately basal level. For the decay curve, the half-lives of approximately 16.7 min and 68.1 min for Mcl-1 were calculated in MeT-5A and MSTO-211H cells, respectively.
Fig. 2. Effect of proteasome inhibitor on Mcl-1 downregulation mediated by resveratrol and clofarabine in MeT-5A and MSTO-211H cells. (A) Cells were seeded in 6-well culture plate and were incubated for 48 h. (B) Cells were cotreated with resveratrol (15 μM) and clofarabine (40 nM) for the indicated times, after which the total RNA was isolated and subjected to RT-PCR analysis. GAPDH primers were used to amplify as an internal standard. (C) Cells were treated with MG132 (10 μM) for 2 h prior to incubation with resveratrol (15 μM) and clofarabine (40 nM) for 24 h. (D) Cells were pretreated with 0.1 μM cycloheximide (CHX) alone, CHX plus 10 μM MG132, or CHX plus co-treatment with resveratrol (15 μM) and clofarabine (40 nM) for varying intervals as indicated. Whole cell lysates were obtained and subjected to Western blot analysis using antibodies against Mcl-1 and β-actin. Normalized intensity of Mcl-1 versus β-actin was presented as the mean value from three independent experiments. Cl/R, clofarabine plus resveratrol. *P < 0.05 compared to respective controls.
Reduction of Mcl-1 levels contributes to more selective sensitization of MSTO-211H to resveratrol and clofarabine
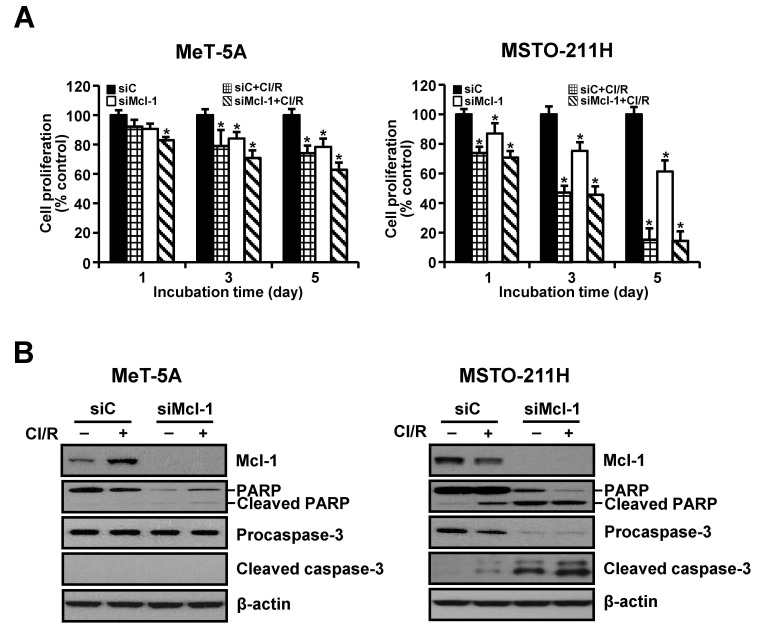
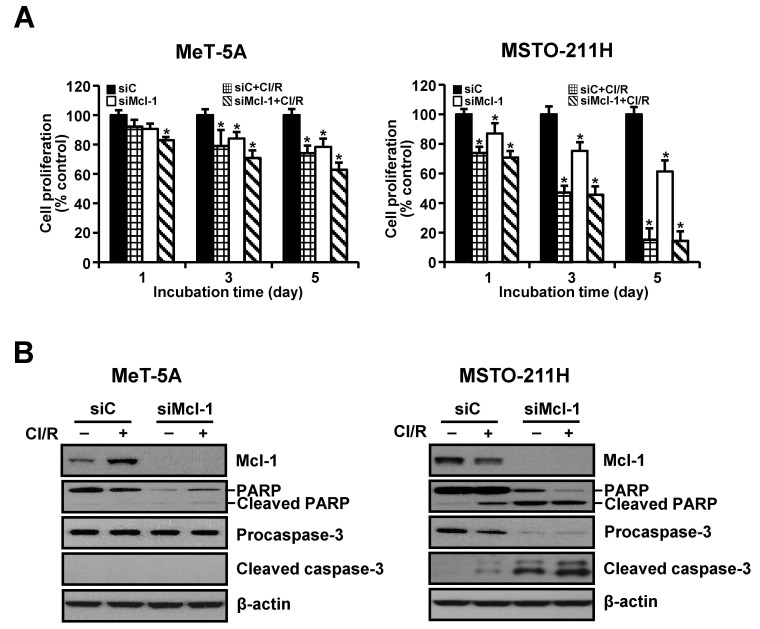
The siRNA-mediated silencing experiments were used to determine whether down-regulation of Mcl-1 was sufficient to sensitize cells to resveratrol and clofarabine. Transfection with Mcl-1-specific siRNA gradually decreased cell proliferation over the experimental period. This effect was markedly augmented following the combination treatment in MSTO-211H cells (Fig. 3A). MeT-5A cells were relatively resistant to Mcl-1 knockdown alone as well as co-treatment with resveratrol and clofarabine, compared to MSTO-211H cells. We then examined whether growth-inhibiting effects by Mcl-1 down-regulation were mediated by activation of caspase-3. As shown in Fig. 3B, Mcl-1 knockdown strongly induced cleavages of procaspase-3 and its substrate PARP in MSTO-211H cells, whereas no discernible cleaved products of procaspase-3 were detected in MeT-5A cells.
Fig. 3. Effects of Mcl-1 knockdown on proliferation of MeT-5A and MSTO-211H cells. Cells were transfected with 10 nM Mcl-1-targeting siRNA (siMcl-1) or Stealth RNAi control (siC) for 24 h. (A) Cells were incubated with resveratrol (15 μM) and clofarabine (40 nM) for 1, 3, and 5 days, after which they were processed for MTT assay. (B) Cells were incubated with resveratrol (15 μM) and clofarabine (40 nM) for 24 h. Whole cell lysates were obtained and subjected to Western blot analysis using antibodies against Mcl-1, PARP, procaspase-3, cleaved caspase-3, and β-actin. Representative results from one of three independent experiments were shown. Error bars represent the mean ± SEM for three independent experiments. Cl/R, clofarabine plus resveratrol. *P < 0.05 compared to respective controls.
Knockdown of Mcl-1 with siRNA was accompanied by an increase in the percentage of apoptotic propensities in annexin V binding (Fig. 4A). In the absence of Z-VAD, Mcl-1 knockdown increased sensitivity to apoptosis caused by co-treatment with resveratrol and clofarabine in both cell types. In the presence of Z-VAD, the percentage of apoptotic cells was significantly restored in MSTO-211H cells, but not completely. In MeT-5A cells, however, significant changes in the percentage of apoptotic cells were not observed. Consistently, Mcl-1 knockdown in MSTO-211H cells induced more caspase-3/7 activity than those of the control siRNA. This effect was augmented by the presence of resveratrol and clofarabine, whereas pan-caspase inhibitor Z-VAD suppressed caspase-3/7 activity. However, small changes were observed in MeT-5A cells transfected with Mcl-1 siRNA (Fig. 4B).
Fig. 4. Effects of Mcl-1 knockdown and pan-caspase inhibitor on activation of caspase-3 in MeT-5A and MSTO-211H cells. Cells were transfected with 10 nM Mcl-1-targeting siRNA (siMcl-1) or Stealth RNAi control (siC) for 24 h, after which cells were incubated with resveratrol (15 μM) and clofarabine (40 nM) for 48 h in the presence or absence of Z-VAD (10 μM). (A) The percentage of apoptotic cells after annexin V binding was analyzed by a Muse cell analyzer. (B) Caspase-3/7 activity was measured by ApoToxGloTM Triplex Assay. Error bars represent the mean ± SEM for three independent experiments. Cl/R, clofarabine plus resveratrol. *P < 0.05 compared to respective controls.
DISCUSSION
Investigating the underlying mechanism(s) of differential sensitivity to resveratrol and clofarabine between MSTO-211H and MeT-5A cells, we were able to define two major factors. First, we identified Mcl-1 as an anti-apoptotic factor executing a central role in the apoptosis signaling pathway. Secondly, the cell death induced by the combination treatment was mainly caspase-dependent in MSTO-211H cells, whereas it proceeded independently of caspase activation in MeT-5A cells.
Some members of Bcl-2 protein family, including Bcl-2 itself, Mcl-1, and Bcl-xL, are principle anti-apoptotic proteins that block activities of pro-apoptotic proteins Bax and Bak, and they work to keep cytochrome c penning up in the mitochondrion (15). The nature of upstream activating signals that lead to changes in expression and/or activation of various Bcl-2 family members vary depending on cell type and the stresses on the cells, such as hypoxia, depleted survival factors, or damages on DNA. In this study, down-regulation of Mcl-1 protein started within 2 h after the exposure to resveratrol and clofarabine in MSTO-211H cells. In contrast, MeT-5A cells exhibited upregulation of Mcl-1 without any effect on Bcl-xL level in this condition. This finding implies that differential sensitivity to resveratrol and clofarabine may be related to Mcl-1 level. Intracellular levels of Mcl-1 protein are known to be regulated through several different mechanisms at the transcriptional, translational, and posttranslational levels depending on the cell types and culture conditions (16). The ability of cells to regulate Mcl-1 level at multiple steps may serve as a mechanism for controlling the fate of the cells, as the means of allowing the production of appropriate numbers of cells and preventing excessive accumulations. In the present study, the combined treatment of resveratrol and clofarabine up-regulated Mcl-1 level in MeT-5A cells and down-regulated it in MSTO-211H cells, whereas Mcl-1 mRNA levels remained unchanged. The translational inhibition of Mcl-1 was unlikely to be relevant in our model, because the combination treatment did not affect the decline of Mcl-1 proteins after the inhibition of protein synthesis by CHX. In contrast, MG132 treatment followed by the combination treatment prevented degradation of Mcl-1 protein and promoted its accumulation to approximately basal level. Proteasome inhibitor MG132 was previously shown to block degradation of Mcl-1 in apoptosis mediated by UV irradiation (17), and to slow down its proapoptotic effects by inducing Mcl-1 accumulation (18). These findings suggest that resveratrol and clofarabine, in combination, regulate Mcl-1 levels through the proteasome system rather than at the transcriptional and/or translational levels in MSTO-211H and MeT-5A cells. The half-life of Mcl-1 protein in MSTO-211H cells are much longer than that of MeT-5A cells. Therefore, it is possible that stabilization of Mcl-1 protein may influence its kinetics and turnover, resulting in the delay of Mcl-1 degradation and the maintaining of higher basal level of Mcl-1 in MSTO-211H cells.
Gene silencing of Mcl-1 with siRNA induced growth inhibition and spontaneous apoptosis of MeT-5A and MSTO-211H cells. Similar findings have also been observed in several studies using different cellular models (8, 19). Mcl-1 has also been reported to be down-regulated during apoptosis by various stresses, including ultraviolet irradiation, cytotoxic agents, and interleukin-3 withdrawal (17, 20). In this study, MSTO-211H cells, which exhibit a high basal level in Mcl-1 protein, were more sensitive to spontaneous apoptosis due to Mcl-1 knockdown than in MeT-5A cells. This finding indicates that the reduction of Mcl-1 level may also partially contribute to selective sensitization of MSTO-211H cells. Besides down-regulation of Mcl-1, another possible mechanism by which a massive cell death could be caused, upon the combined treatment of resveratrol and clofarabine in MSTO-211H cells, is activation of caspase cascade. Caspase activation plays an important role in the classical apoptosis pathway. It is well known that executioner procaspases (caspases-3, -6, and -7) are proteolytically activated by initiator caspases (caspases-8, -9, and -10), and they specifically cleave several cellular proteins such as PARP and inhibitor of caspase-activated DNase, resulting in apoptosis. Of these, cleaved caspase-3 has been used as a distinct biochemical marker of the activation of apoptosis pathway. In this study, the mechanism of cell death induction in MSTO-211H cells was essentially different from that of MeT-5A cells. The combined treatment of MSTO-211H cells with resveratrol and clofarabine increased caspase-3/7 activity with the resultant cleavages of caspase-3 and PARP. Furthermore, apoptosis-inducing effect by the combination treatment is caspase-dependent. In contrast, the cleaved form of caspase-3 and the caspase-3/7 activity in MeT-5A cells did not significantly changed after the combination treatment. Also, the apoptosis observed was not reversed even when the cells were pretreated with pan-caspase inhibitor Z-VAD. This indicates that apoptosis mediated by resveratrol and clofarabine in MeT-5A cells proceeded independently of caspase activation. Previous studies have shown that resveratrol or its structural analog induces caspase-independent cell death in several different types of cancer cells. For example, Pozo-Guisado et al. reported that resveratrol do not activate caspases to induce cell death in MCF-7 breast cancer cells (21). Pterostilbene, a natural 3,5-dimethoxy analog of trans-resveratrol, also induced caspase-independent cell death in leukemia cells (22). This may be a unique characteristic of resveratrol, depending on the cell type or cell context. Although we did not address specific mechanisms involved in caspase-independent apoptosis, processes that occurred in MeT-5A cells under the presence of resveratrol and clofarabine blocked activation of the caspase-3, provoking intrinsic resistance to cell death.
In conclusion, our results reveal that simultaneous treatment of resveratrol and clofarabine strongly induced apoptosis of MM cells by inducing enhanced caspase-3/7 activity and, at least in part, through the Mcl-1 down-regulation. Tumor cells frequently lose caspase activities during malignant progression, which makes them highly resistant toward anticancer therapies, such as chemotherapeutics or ionizing irradiation, and thus may affect prognosis and overall survival of the disease. In this regard, the preferential activation of caspase cascade might offer an alternative approach for treating MM with the potential advantages of specifically targeting neoplastic cells.
MATERIALS AND METHODS
Reagents and cell culture
Resveratrol, clofarabine, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrozolum bromide (MTT), phosphate buffered saline (PBS), and antibody to β-actin were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Anti-human Mcl-1, Bcl-xL, caspase-3, cleaved caspase-3, and poly (ADP-ribose) polymerase (PARP) antibodies were from Cell Signaling Technologies (Beverly, MA, USA). Trizol reagent, cell culture media and reagents were purchased from Invitrogen (Carlsbad, CA, USA). The human mesothelioma cell line MSTO-211H cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1 mM glutamine, 100 units of penicillin/ml and 100 μg of streptomycin/ml. The human normal mesothelial cell line MeT-5A cells were maintained in M199 (Welgene, Daegu, Korea) medium supplemented with 3.3 nM epidermal growth factor, 10% fetal bovine serum, 100 units of penicillin/ml and 100 μg of streptomycin/ml. Cells were grown to 50% confluence in a monolayer culture in this medium for 24 h before treatment.
Cell proliferation assay
The cells were seeded in 96-well microtiter plates, and were treated with drugs or chemicals at different concentrations for the indicated times, after which they were exposed to MTT (final 0.1 mg/ml) for additional 4 h. The formazan crystals, formed by the reduction of MTT in living cells, were solubilized in 200 μl of DMSO and were measured with spectrophotometry at 560 nm.
Western blot analysis
Western blot analyses were performed using cell lysate as described previously (16). Cell lysate containing 40 μg of protein was separated on NuPAGE 4-12% bis-tris polyacrylamide gels (Invitrogen, Carlsbad, CA, USA) and were electrophoretically transferred to Immuno-Blot PVDF membranes. The signal was visualized by an ECL detection kit.
Reverse transcription-polymerase chain reaction (RT-PCR)
One μg of the total RNA was converted to cDNA using the oligo(dT)15 primer and AMV reverse transcriptase (iNtRON Biotechnology, Seongnam, Korea). The PCR conditions were as follows; initial denaturation at 94℃ for 2 min was followed by 28 cycles of 94℃ for 40 s, 57℃ for 40 s and 72℃ for 40 s. Specific primers for RT-PCR amplification of various genes were as follows: (a) Mcl-1: sense 5’-cggtaatcggactcaacctc-3’ and anti-sense 5’-cctccttctccgtagccaa-3’, (b) GAPDH: sense 5’-acctgacctgccgtctagaa-3’ and antisense 5’-tccaccaccctgttgctgta-3’.
RNA interference
RNA interference of Mcl-1 was performed using a Mcl-1-targeting small interfering RNA (siRNA) duplex from Invitrogen (Oligo ID: HSS181041). The cells were seeded in 96-well or 6-well plates and were transfected at 40% confluency with siRNA duplex using lipofectamine RNAiMAX (Invitrogen) as described previously (16).
Caspase-3/7 activity
Activation of caspase-3/7 was quantified with the ApoToxGloTM Triplex Assay kit according to the manufacturer’s protocol (Promega, Medison, WI, USA). Caspase-3/7 activities were calculated after detection of luminescence by a GloMax-Multi Microplate Multimode Reader.
Apoptosis assay
The apoptotic cell distribution was determined using MuseTM Annexin V & Dead Cell kit according manufacturer’s protocol (Merck Milipore, Darmstadt, Germany). Briefly, cells were collected in culture medium, mixed with the Muse Annexin V and Dead Cell Reagent, and analyzed using a Muse Cell Analyzer (Merck Milipore).
Statistical analysis
Statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc correlation for multiple comparisons using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ± SEM. Significant differences were considered with values of P< 0.05
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. NRF-2012R1A1A4A01014255).
References
- 1.Montanaro F, Rosato R, Gangemi M, et al. Survival of pleural malignant mesothelioma in Italy: a population-based study. Int J Cancer. (2009);124:201–207. doi: 10.1002/ijc.23874. [DOI] [PubMed] [Google Scholar]
- 2.Barbone D, Cheung P, Battula S, et al. Vorinostat eliminates multicellular resistance of mesothelioma 3D spheroids via restoration of Noxa expression. PLoS One. (2012);7:e52753. doi: 10.1371/journal.pone.0052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm D, Wehland M, Pietsch J, Infanger M, Bauer J. Drugs interfering with apoptosis in breast cancer. Curr Pharm Des. (2011);17:272–283. doi: 10.2174/138161211795049723. [DOI] [PubMed] [Google Scholar]
- 4.Soini Y, Kinnula V, Kaarteenaho-Wiik R, Kurttila E, Linnainmaa K, Pääkkö P. Apoptosis and expression of apoptosis regulating proteins bcl-2, mcl-1, bcl-X, and bax in malignant mesothelioma. Clin Cancer Res. (1999);5:3508–3515. [PubMed] [Google Scholar]
- 5.O'Kane SL, Pound RJ, Campbell A, Chaudhuri N, Lind MJ, Cawkwell L. Expression of bcl-2 family members in malignant pleural mesothelioma. Acta Oncol. (2006);45:449–453. doi: 10.1080/02841860500468927. [DOI] [PubMed] [Google Scholar]
- 6.Shimazu T, Degenhardt K, Nur-E-Kamal A, et al. NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes Dev. (2007);21:929–941. doi: 10.1101/gad.1522007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann SH, Karp JE, Svingen PA, et al. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood. (1998);91:991–1000. [PubMed] [Google Scholar]
- 8.Moulding DA, Giles RV, Spiller DG, White MR, Tidd DM, Edwards SW. Apoptosis is rapidly triggered by antisense depletion of MCL-1 in differentiating U937 cells. Blood. (2000);96:1756–1763. [PubMed] [Google Scholar]
- 9.Thallinger C, Wolschek MF, Wacheck V, et al. Mcl-1 antisense therapy chemosensitizes human melanoma in a SCID mouse xenotransplantation model. J Invest Dermatol. (2003);120:1081–1096. doi: 10.1046/j.1523-1747.2003.12252.x. [DOI] [PubMed] [Google Scholar]
- 10.Augustine CK, Toshimitsu H, Jung SH, et al. Sorafenib, a multikinase inhibitor, enhances the response of melanoma to regional chemotherapy. Mol Cancer Ther. (2010);9:2090–2101. doi: 10.1158/1535-7163.MCT-10-0073. [DOI] [PubMed] [Google Scholar]
- 11.Simonin K, Brotin E, Dufort S, et al. Mcl-1 is an important determinant of the apoptotic response to the BH3-mimetic molecule HA14-1 in cisplatin-resistant ovarian carcinoma cells. Mol Cancer Ther. (2009);8:3162–3170. doi: 10.1158/1535-7163.MCT-09-0493. [DOI] [PubMed] [Google Scholar]
- 12.Kitada S, Anderson J, Akar S. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood. (1998);91:3379–3389. [PubMed] [Google Scholar]
- 13.Lee YJ, Lee YJ, Im JH, et al. Synergistic anti-cancer effects of resveratrol and chemotherapeutic agent clofarabine against human malignant mesothelioma MSTO-211H cells. Food Chem Toxicol. (2013);52:61–68. doi: 10.1016/j.fct.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 14.Lee YJ, Im JH, Lee DM, et al. Synergistic inhibition of mesothelioma cell growth by the combination of clofarabine and resveratrol involves Nrf2 downregulation. BMB Rep. (2012);45:647–652. doi: 10.5483/BMBRep.2012.45.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shroff EH, Snyder C, Chandel NS. Bcl-2 family members regulate anoxia-induced cell death. Antioxid Redox Signal. (2007);9:1405–1409. doi: 10.1089/ars.2007.1731. [DOI] [PubMed] [Google Scholar]
- 16.Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia. (2002);16:444–454. doi: 10.1038/sj.leu.2402416. [DOI] [PubMed] [Google Scholar]
- 17.Nijhawan D, Fang M, Traer E, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. (2003);17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nencioni A, Hua F, Dillon CP, et al. Evidence for a protective role of Mcl-1 in proteasome inhibitor-induced apoptosis. Blood. (2005);105:3255–3262. doi: 10.1182/blood-2004-10-3984. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Goldstein LA, Gastman BR. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem. (2006);281:10153–10163. doi: 10.1074/jbc.M510349200. [DOI] [PubMed] [Google Scholar]
- 20.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of Mcl-1. Mol. Cell. (2006);21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Pozo-Guisado E, Merino JM, Mulero-Navarro S, et al. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer. (2005);115:74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 22.Tolomeo M, Grimaudo S, Di Cristina A, et al. Pterostilbene and 3'-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol. (2005);37:1709–1726. doi: 10.1016/j.biocel.2005.03.004. [DOI] [PubMed] [Google Scholar]